HGF–Met Pathway in Regeneration and Drug Discovery
Abstract
:1. Background of Hepatocyte Growth Factor (HGF)–Met Pathway Leading to Drug Discovery


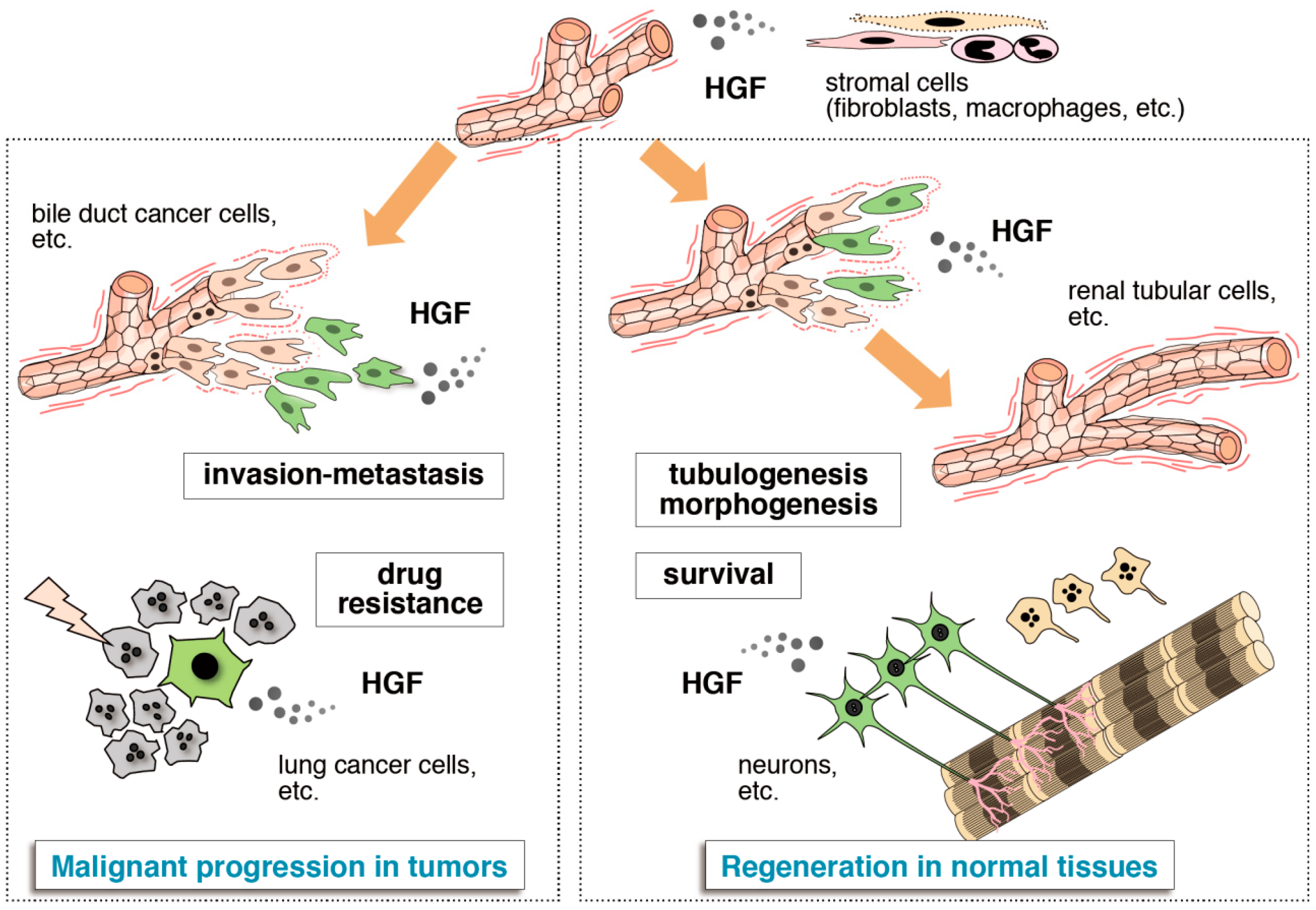
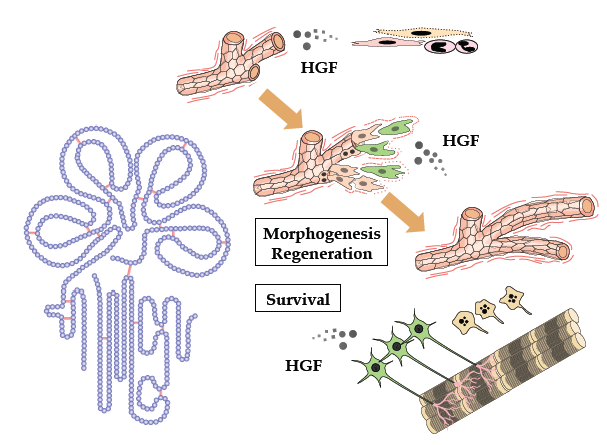
2. Tissue Regeneration/Protection Deduced from Met Disruption
| Met−/− Tissue/Cell Types | Characteristics | Ref. |
|---|---|---|
| Liver | ||
| Hepatocytes | Highly susceptible to apoptosis after liver injury | [22] |
| Impairment in recovery from liver necrosis after liver injury | ||
| Impairment in Erk1/2 activation and G2/M transition after liver injury | [23] | |
| Hepatocytes | Steatotic change of the liver in aged mice | [24] |
| Decrease in mitotic hepatocytes after partial hepatectomy | ||
| Delayed regeneration after partial hepatectomy | ||
| Hepatocytes | Promoted liver fibrosis after liver injury | [26] |
| Extensive necrosis and lower proliferation of hepatocytes after bile-duct ligation | [25] | |
| Enhanced susceptibility to liver fibrosis | ||
| Oval cells | Decrease in oval cell viability and more prone to apoptosis | [27] |
| Reduction in oval cell pool | [28] | |
| Impairment in migration and differentiation into hepatocytes | ||
| Kidney | ||
| Tubular cells | No appreciable defect in kidney morphology and function | [29] |
| Aggravated renal injury and inflammation after acute kidney injury | ||
| Podocytes | Neither albuminuria nor overt pathologic lesions | [30] |
| Severe podocyte injury and apoptosis, and albuminuria after toxic injury | ||
| Collecting duct | Increased fibrosis and tubular necrosis after unilateral ureteral obstruction | [31] |
| Reduced capacity in regeneration after release of the obstruction | ||
| Ureteric bud | Double knockout of Met and EGF receptor in ureteric bud | [32] |
| Decrease in branching and a reduction in final glomerular number | ||
| Skin | ||
| Keratinocytes | Lack of keratinocyte migration after skin wound | [33] |
| Severe impairment epidermal wound closure | ||
| Pancreas | ||
| β-Cell | Mild hyperglycemia, and decreased serum insulin levels at 6 months | [34] |
| Loss of acute-phase insulin secretion in response to glucose, and impaired glucose tolerance | ||
| Diminished glucose tolerance and reduced plasma insulin after a glucose challenge | [35] | |
| Normal glucose and β-cell homeostasis | [36] | |
| Susceptible to streptozotocin-induced diabetes | ||
| Nervous System | ||
| Ganglionic eminence | Increased numbers of striatal GABAergic interneurons in the lateral sensorimotor | [37] |
| Areas with distinct behavioral deficits | ||
| Delayed procedural learning | ||
| Cerebral cortex and hippocampus | Larger size in the rostral cortex, caudal hippocampus, dorsal striatum, thalamus, and corpus callosum | [38] |
| Dorsal pallial | Increases proximal and reduces distal apical dendritic branching of neocortical pyramidal neurons in post-pubertal period | [39] |
| Forebrain neurons | Reduced volume of cortical tissue | [40] |
| Increase in spine head volume, but no change in density of spines | ||
| Hyperconnectivity in circuit-specific intracortical neurons | ||
| Heart | ||
| Cardiomyocytes | Normal heart development | [41] |
| Cardiomyocyte hypertrophy and interstitial fibrosis by 6 months | ||
| Systolic cardiac dysfunction by 9 months | ||
| Immune System | ||
| Dendritic cells | Impaired emigration toward draining lymph nodes upon inflammation-induced activation | [42] |
| Impaired contact hypersensitivity reaction to contact allergens | ||
3. Neurotrophic Function and Involvement in Neuronal Disorder/Symptoms
4. HGF as a Biological Drug Candidate
| Tissues and Disease/Injury Models | References | |
|---|---|---|
| Liver | ||
| Acute hepatitis | [58,59,60,61,62,63] | |
| Chorestasis | [64] | |
| Fulminant hepatitis | [65,66] | |
| Liver fibrosis/cirrhosis | [67,68,69,70,71] | |
| Liver cirrhosis + surgery | [72] | |
| Alcoholic steatohepatitis | [73] | |
| Gastrointestinal | ||
| Ulcerative colitis | [74,75] | |
| Gastric ulcer | [76] | |
| Gastric injury | [77] | |
| Kidney | ||
| Acute kidney injury | [78,79,80,81,82,83] | |
| Acute renal inflammation | [84] | |
| Septic acute renal failure | [85] | |
| Diabetic nephropathy | [86] | |
| Chronic kidney disease | [87,88,89,90] | |
| Glomerulonephritis | [91] | |
| Chronic allograft nephropathy | [92] | |
| Cardiovascular | ||
| Critical limb ischemia | [93,94,95] | |
| Neointimal hyperplasia | [96] | |
| Coronary artery disease | [97] | |
| Myocardial infarction | [98,99] | |
| Cardiac allograft vasculopathy | [100] | |
| Dilated cardiomyopathy | [101] | |
| Respiratory | ||
| Acute lung injury | [102] | |
| Ischemia-reperfusion | [103] | |
| Lung fibrosis | [104,105,106] | |
| Pulmonary emphysema | [107] | |
| Left peumonectomy | [108] | |
| Allergic airway inflammation | [109] | |
| Vocal fold scarring | [110,111] | |
| Skin | ||
| Wounding | [112,113] | |
| Nervous System(s) | ||
| Cerebral ischemia | [114,115,116,117,118] | |
| Peripheral nerve injury | [119] | |
| Amyotrophic lateral sclerosis | [120] | |
| Hydrocephalus | [121] | |
| Retinal injury | [122] | |
| Photoreceotr degeneration | [123,124] | |
| Difficulty in hearing | [125] | |
| Musclosleletal | ||
| Articular cartilage injury | [126] | |
| Skeletal muscle injury | [127] | |
| Rheumatoid arthritis | [128] | |
| Ligament injury | [129] | |
5. Clinical Study and Drug Development
5.1. Chronic Leg Ulcer
5.2. Critical Limb Ischemia

5.3. Hepatitis and Acute Kidney Injury
5.4. Amyotrophic Lateral Sclerosis (ALS)
5.5. Spinal Cord Injury
6. Small-Molecule HGF-Inducers and Therapeutic Approaches
7. Conclusions
Acknowledgments
Conflicts of Interest
References
- Nakamura, T.; Nishizawa, T.; Hagiya, M.; Seki, T.; Shimonishi, M.; Sugimura, A.; Tashiro, K.; Shimizu, S. Molecular cloning and expression of human hepatocyte growth factor. Nature 1989, 342, 440–443. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, K.; Tsubouchi, H.; Naka, D.; Takahashi, K.; Okigaki, M.; Arakaki, N.; Nakayama, H.; Hirono, S.; Sakiyama, O.; Takahashi, K.; et al. Molecular cloning and sequence analysis of cDNA for human hepatocyte growth factor. Biochem. Biophys. Res. Commun. 1989, 163, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Stoker, M.; Gherardi, E.; Perryman, M.; Gray, J. Scatter factor is a fibroblast-derived modulator of epithelial cell mobility. Nature 1987, 327, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Weidner, K.M.; Behrens, J.; van der Kerckhove, J.; Birchmeier, W. Scatter factor: Molecular characteristics and effect on the invasiveness of epithelial cells. J. Cell Biol. 1990, 111, 2097–2108. [Google Scholar] [CrossRef] [PubMed]
- Bottaro, D.P.; Rubin, J.S.; Faletto, D.L.; Chan, A.-M.I.; Kmiecik, T.E.; vande Woude, G.F.; Aaronson, S.A. Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science 1991, 251, 802–804. [Google Scholar] [CrossRef] [PubMed]
- Naldini, L.; Vigna, E.; Narcimham, R.P.; Gaudino, G.; Zarnegar, R.; Michalopoulos, G.K.; Comoglio, P.M. Hepatocyte growth factor stimulates the tyrosine kinase activity of the receptor encoded by the proto-oncogene c-MET. Oncogene 1991, 6, 501–504. [Google Scholar] [PubMed]
- Kataoka, H.; Miyata, S.; Uchinokura, S.; Itoh, H. Roles of hepatocyte growth factor (HGF) activator and HGF activator inhibitor in the pericellular activation of HGF/scatter factor. Cancer Metastasis Rev. 2003, 22, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Parr, C.; Jiang, W.G. Hepatocyte growth factor activation inhibitors (HAI-1 and HAI-2) regulate HGF-induced invasion of human breast cancer cells. Int. J. Cancer 2006, 119, 1176–1183. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Dean, M.; Kaul, K.; Braun, M.J.; Gonda, M.A.; vande Woude, G. Sequence of MET protooncogene cDNA has features characteristic of the tyrosine kinase family of growth-factor receptors. Proc. Natl. Acad. Sci. USA 1987, 84, 6379–6383. [Google Scholar] [CrossRef] [PubMed]
- Gherardi, E.; Sandin, S.; Petoukhov, M.V.; Finch, J.; Youles, M.E.; Ofverstedt, L.G.; Miguel, R.N.; Blundell, T.L.; vande Woude, G.F.; Skoglund, U.; et al. Structural basis of hepatocyte growth factor/scatter factor and MET signaling. Proc. Natl. Acad. Sci. USA 2006, 103, 4046–4051. [Google Scholar] [CrossRef] [PubMed]
- Basilico, C.; Arnesano, A.; Galluzzo, M.; Comoglio, P.M.; Michieli, P. A high affinity hepatocyte growth factor-binding site in the immunoglobulin-like region of Met. J. Biol. Chem. 2008, 283, 21267–21277. [Google Scholar] [CrossRef] [PubMed]
- Weidner, K.M.; di Cesare, S.; Sachs, M.; Brinkmann, V.; Behrens, J.; Birchmeier, W. Interaction between Gab1 and the c-Met receptor tyrosine kinase is responsible for epithelial morphogenesis. Nature 1996, 384, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Sachs, M.; Brohmann, H.; Zechner, D.; Müller, T.; Hülsken, J.; Walther, I.; Schaeper, U.; Birchmeier, C.; Birchmeier, W. Essential role of Gab1 for signaling by the c-Met receptor in vivo. J. Cell Biol. 2000, 150, 1375–1384. [Google Scholar] [PubMed]
- Liu, Y. Hepatocyte growth factor promotes renal epithelial cell survival by dual mechanisms. Am. J. Physiol. 1999, 277, F624–F633. [Google Scholar] [PubMed]
- Garcia-Ocana, A.; Takane, K.K.; Reddy, V.T.; Lopez-Talavera, J.C.; Vasavada, R.C.; Stewart, A.F. Adenovirus-mediated hepatocyte growth factor expression in mouse islets improves pancreatic islet transplant performance and reduces β-cell death. J. Biol. Chem. 2003, 278, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; DeFrances, M.C.; Dai, Y.; Pediaditakis, P.; Johnson, C.; Bell, A.; Michalopoulos, G.K.; Zarnegar, R. A mechanism of cell survival: Sequestration of Fas by the HGF receptor Met. Mol. Cell 2002, 9, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Peschard, P.; Fournier, T.M.; Lamorte, L.; Naujokas, M.A.; Band, H.; Langdon, W.Y.; Park, M. Mutation of the c-Cbl TKB domain binding site on the Met receptor tyrosine kinase converts it into a transforming protein. Mol. Cell 2001, 8, 995–1004. [Google Scholar] [CrossRef] [PubMed]
- Hashigasako, A.; Machide, M.; Nakamura, T.; Matsumoto, K.; Nakamura, T. Bi-directional regulation of Ser-985 phosphorylation of c-met via protein kinase-C and protein phosphatase 2A involves c-Met activation and cellular responsiveness to hepatocyte growth factor. J. Biol. Chem. 2004, 279, 26445–26452. [Google Scholar] [CrossRef] [PubMed]
- Uehara, Y.; Minowa, O.; Mori, C.; Shiota, K.; Kuno, J.; Noda, T.; Kitamura, N. Placental defect and embryonic lethality in mice lacking hepatocyte growth factor/scatter factor. Nature 1995, 373, 702–705. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.; Bladt, F.; Goedecke, S.; Brinkmann, V.; Zschiesche, W.; Sharpe, M.; Gherardi, E.; Birchmeier, C. Scatter factor/hepatocyte growth factor is essential for liver development. Nature 1995, 373, 699–702. [Google Scholar] [CrossRef] [PubMed]
- Bladt, F.; Riethmacher, D.; Isenmann, S.; Aguzzi, A.; Birchmeier, C. Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature 1995, 376, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Huh, C.G.; Factor, V.M.; Sanchez, A.; Uchida, K.; Conner, E.A.; Thorgeirsson, S.S. Hepatocyte growth factor/c-met signaling pathway is required for efficient liver regeneration and repair. Proc. Natl. Acad. Sci. USA 2004, 101, 4477–4482. [Google Scholar] [CrossRef] [PubMed]
- Borowiak, M.; Garratt, A.N.; Wustefeld, T.; Strehle, M.; Trautwein, C.; Birchmeier, C. Met provides essential signals for liver regeneration. Proc. Natl. Acad. Sci. USA 2004, 101, 10608–10613. [Google Scholar] [CrossRef] [PubMed]
- Factor, V.M.; Seo, D.; Ishikawa, T.; Kaposi-Novak, P.; Marquardt, J.U.; Andersen, J.B.; Conner, E.A.; Thorgeirsson, S.S. Loss of c-Met disrupts gene expression program required for G2/M progression during liver regeneration in mice. PLoS One 2010, 5, e12739. [Google Scholar] [CrossRef] [PubMed]
- Giebeler, A.; Boekschoten, M.V.; Klein, C.; Borowiak, M.; Birchmeier, C.; Gassler, N.; Wasmuth, H.E.; Müller, M.; Trautwein, C.; Streetz, K.L. c-Met confers protection against chronic liver tissue damage and fibrosis progression after bile duct ligation in mice. Gastroenterology 2009, 137, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Marquardt, J.U.; Seo, D.; Gómez-Quiroz, L.E.; Uchida, K.; Gillen, M.C.; Kitade, M.; Kaposi-Novak, P.; Conner, E.A.; Factor, V.M.; Thorgeirsson, S.S. Loss of c-Met accelerates development of liver fibrosis in response to CCl4 exposure through deregulation of multiple molecular pathways. Biochim. Biophys. Acta 2012, 1822, 942–951. [Google Scholar] [CrossRef] [PubMed]
- Del Castillo, G.; Factor, V.M.; Fernández, M.; Alvarez-Barrientos, A.; Fabregat, I.; Thorgeirsson, S.S.; Sánchez, A. Deletion of the Met tyrosine kinase in liver progenitor oval cells increases sensitivity to apoptosis in vitro. Am. J. Pathol. 2008, 172, 1238–1247. [Google Scholar] [CrossRef]
- Ishikawa, T.; Factor, V.M.; Marquardt, J.U.; Raggi, C.; Seo, D.; Kitade, M.; Conner, E.A.; Thorgeirsson, S.S. Hepatocyte growth factor/c-met signaling is required for stem-cell-mediated liver regeneration in mice. Hepatology 2012, 55, 1215–1226. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Tan, R.J.; Lin, L.; Zhou, L.; Liu, Y. Activation of hepatocyte growth factor receptor, c-met, in renal tubules is required for renoprotection after acute kidney injury. Kidney Int. 2013, 84, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Saleem, M.A.; Holzman, L.B.; Mathieson, P.; Liu, Y. Hepatocyte growth factor signaling ameliorates podocyte injury and proteinuria. Kidney Int. 2010, 77, 962–973. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Saenko, M.; Opuko, A.; Togawa, A.; Soda, K.; Marlier, A.; Moeckel, G.W.; Cantley, L.G.; Ishibe, S. Deletion of the Met receptor in the collecting duct decreases renal repair following ureteral obstruction. Kidney Int. 2009, 76, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Ishibe, S.; Karihaloo, A.; Ma, H.; Zhang, J.; Marlier, A.; Mitobe, M.; Togawa, A.; Schmitt, R.; Czyczk, J.; Kashgarian, M.; et al. Met and the epidermal growth factor receptor act cooperatively to regulate final nephron number and maintain collecting duct morphology. Development 2009, 136, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Chmielowiec, J.; Borowiak, M.; Morkel, M.; Stradal, T.; Munz, B.; Werner, S.; Wehland, J.; Birchmeier, C.; Birchmeier, W. c-Met is essential for wound healing in the skin. J. Cell Biol. 2007, 177, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Huh, C.G.; Thorgeirsson, S.S.; Liu, Y. β-Cell-specific ablation of the hepatocyte growth factor receptor results in reduced islet size, impaired insulin secretion, and glucose intolerance. Am. J. Pathol. 2005, 167, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Roccisana, J.; Reddy, V.; Vasavada, R.C.; Gonzalez-Pertusa, J.A.; Magnuson, M.A.; Garcia-Ocaña, A. Targeted inactivation of hepatocyte growth factor receptor c-met in β-cells leads to defective insulin secretion and GLUT-2 downregulation without alteration of β-cell mass. Diabetes 2005, 54, 2090–2102. [Google Scholar] [CrossRef] [PubMed]
- Mellado-Gil, J.; Rosa, T.C.; Demirci, C.; Gonzalez-Pertusa, J.A.; Velazquez-Garcia, S.; Ernst, S.; Valle, S.; Vasavada, R.C.; Stewart, A.F.; Alonso, L.C.; et al. Disruption of hepatocyte growth factor/c-Met signaling enhances pancreatic β-cell death and accelerates the onset of diabetes. Diabetes 2011, 60, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Martins, G.J.; Shahrokh, M.; Powell, E.M. Genetic disruption of Met signaling impairs GABAergic striatal development and cognition. Neuroscience 2011, 176, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.M.; Xu, J.; Powell, E.M. Age dependent forebrain structural changes in mice deficient in the autism associated gene Met tyrosine kinase. Neuroimage Clin. 2012, 1, 66–74. [Google Scholar] [CrossRef]
- Judson, M.C.; Eagleson, K.L.; Wang, L.; Levitt, P. Evidence of cell-nonautonomous changes in dendrite and dendritic spine morphology in the met-signaling-deficient mouse forebrain. J. Comp. Neurol. 2010, 518, 4463–4478. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Anderson, C.T.; Levitt, P.; Shepherd, G.M. Circuit-specific intracortical hyperconnectivity in mice with deletion of the autism-associated Met receptor tyrosine kinase. J. Neurosci. 2011, 31, 5855–5864. [Google Scholar] [CrossRef] [PubMed]
- Arechederra, M.; Carmona, R.; González-Nuñez, M.; Gutiérrez-Uzquiza, A.; Bragado, P.; Cruz-González, I.; Cano, E.; Guerrero, C.; Sánchez, A.; López-Novoa, J.M.; et al. Met signaling in cardiomyocytes is required for normal cardiac function in adult mice. Biochim. Biophys. Acta 2013, 1832, 2204–2215. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.H.; Birchmeier, C.; Zenke, M.; Hieronymus, T. The HGF receptor/Met tyrosine kinase is a key regulator of dendritic cell migration in skin immunity. J. Immunol. 2012, 189, 1699–1707. [Google Scholar] [CrossRef] [PubMed]
- Funakoshi, F.; Nakamura, T. Hepatocyte growth factor (HGF): Neurotrophic functions and therapeutic implications for neuronal injury/diseases. Curr. Signal. Transduct. Ther. 2011, 6, 156–167. [Google Scholar] [CrossRef]
- Ohya, W.; Funakoshi, H.; Kurosawa, T.; Nakamura, T. Hepatocyte growth factor (HGF) promotes oligodendrocyte progenitor cell proliferation and inhibits its differentiation during postnatal development in the rat. Brain Res. 2007, 1147, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Campbell, D.B.; Sutcliffe, J.S.; Ebert, P.J.; Militerni, R.; Bravaccio, C.; Trillo, S.; Elia, M.; Schneider, C.; Melmed, R.; Sacco, R.; et al. A genetic variant that disrupts MET transcription is associated with autism. Proc. Natl. Acad. Sci. USA 2006, 103, 16834–16839. [Google Scholar] [CrossRef] [PubMed]
- Campbell, D.B.; Li, C.; Sutcliffe, J.S.; Persico, A.M.; Levitt, P. Genetic evidence implicating multiple genes in the MET receptor tyrosine kinase pathway in autism spectrum disorder. Autism Res. 2008, 1, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Sousa, I.; Clark, T.G.; Toma, C.; Kobayashi, K.; Choma, M.; Holt, R.; Sykes, N.H.; Lamb, J.A.; Bailey, A.J.; Battaglia, A.; et al. MET and autism susceptibility: Family and case-control studies. Eur. J. Hum. Genet. 2009, 17, 749–758. [Google Scholar] [PubMed]
- Powell, E.M.; Mars, W.M.; Levitt, P. Hepatocyte growth factor/scatter factor is a motogen for interneurons migrating from the ventral to dorsal telencephalon. Neuron 2001, 30, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Powell, E.M.; Campbell, D.B.; Stanwood, G.D.; Davis, C.; Noebels, J.L.; Levitt, P. Genetic disruption of cortical interneuron development causes region- and GABA cell type-specific deficits, epilepsy, and behavioral dysfunction. J. Neurosci. 2003, 23, 622–631. [Google Scholar] [PubMed]
- Ieraci, A.; Forni, P.E.; Ponzetto, C. Viable hypomorphic signaling mutant of the Met receptor reveals a role for hepatocyte growth factor in postnatal cerebellar development. Proc. Natl. Acad. Sci. USA 2002, 99, 15200–15205. [Google Scholar] [CrossRef] [PubMed]
- Palmen, S.J.; van Engeland, H.; Hof, P.R.; Schmitz, C. Neuropathological findings in autism. Brain 2004, 127, 2572–2583. [Google Scholar] [CrossRef] [PubMed]
- Courchesne, E.; Redcay, E.; Kennedy, D.P. The autistic brain: Birth through adulthood. Curr. Opin. Neurol. 2004, 17, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Burdick, K.E.; DeRosse, P.; Kane, J.M.; Lencz, T.; Malhotra, A.K. Association of genetic variation in the MET proto-oncogene with schizophrenia and general cognitive ability. Am. J. Psychiatr. 2010, 167, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Schultz, J.M.; Khan, S.N.; Ahmed, Z.M.; Riazuddin, S.; Waryah, A.M.; Chhatre, D.; Starost, M.F.; Ploplis, B.; Buckley, S.; Velásquez, D.; et al. Noncoding mutations of HGF are associated with nonsyndromic hearing loss, DFNB39. Am. J. Hum. Genet. 2009, 85, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Mizuno, S. The discovery of hepatocyte growth factor (HGF) and its significance for cell biology, life sciences and clinical medicine. Proc. Jpn. Acad. B 2010, 86, 588–610. [Google Scholar] [CrossRef] [PubMed]
- Rutella, S.; Bonanno, G.; Procoli, A.; Mariotti, A.; de Ritis, D.G.; Curti, A.; Danese, S.; Pessina, G.; Pandolfi, S.; Natoni, F.; et al. Hepatocyte growth factor favors monocyte differentiation into regulatory interleukin (IL)-10++IL-12l°w/neg accessory cells with dendritic-cell features. Blood 2006, 108, 218–227. [Google Scholar] [CrossRef]
- Benkhoucha, M.; Santiago-Raber, M.L.; Schneiter, G.; Chofflon, M.; Funakoshi, H.; Nakamura, T.; Lalive, P.H. Hepatocyte growth factor inhibits CNS autoimmunity by inducing tolerogenic dendritic cells and CD25+Foxp3+ regulatory T cells. Proc. Natl. Acad. Sci. USA 2010, 107, 6424–6429. [Google Scholar] [CrossRef] [PubMed]
- Ishiki, Y.; Ohnishi, H.; Muto, Y.; Matsumoto, K.; Nakamura, T. Direct evidence that hepatocyte growth factor is a hepatotrophic factor for liver regeneration and for potent antihepatitis effect in vivo. Hepatology 1992, 16, 1227–1235. [Google Scholar]
- Roos, F.; Terrell, T.G.; Godowski, P.J.; Chamow, S.M.; Schwall, R.H. Reduction of α-naphthylisothiocyanate-induced hepatotoxicity by recombinant human hepatocyte growth factor. Endocrinology 1992, 131, 2540–2544. [Google Scholar] [PubMed]
- Masunaga, H.; Fujise, N.; Shiota, A.; Ogawa, H.; Sato, Y.; Imai, E.; Yasuda, H.; Higashio, K. Preventive effects of the deleted form of hepatocyte growth factor against various liver injuries. Eur. J. Pharmacol. 1998, 342, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Sakakura, Y.; Kaibori, M.; Oda, M.; Okumura, T.; Kwon, A.H.; Kamiyama, Y. Recombinant human hepatocyte growth factor protects the liver against hepatic ischemia and reperfusion injury in rats. J. Surg. Res. 2000, 92, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Oe, S.; Hiros, T.; Fujii, H.; Yasuchika, K.; Nishio, T.; Iimuro, Y.; Morimoto, T.; Nagao, M.; Yamaoka, Y. Continuous intravenous infusion of deleted form of hepatocyte growth factor attenuates hepatic ischemia-reperfusion injury in rats. J. Hepatol. 2001, 34, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, A.; Kaido, T.; Seto, S.; Yamaoka, S.; Sato, M.; Ishii, T.; Imamura, M. Hepatocyte growth factor promotes liver regeneration with prompt improvement of hyperbilirubinemia in hepatectomized cholestatic rats. J. Surg. Res. 1998, 78, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Mizuno, S.; Nakamura, T. Antinecrotic and antiapoptotic effects of hepatocyte growth factor on cholestatic hepatitis in a mouse model of bile-obstructive diseases. Am. J. Physiol. 2007, 292, G639–G646. [Google Scholar] [CrossRef]
- Kosai, K.; Matsumoto, K.; Nagata, S.; Tsujimoto, Y.; Nakamura, T. Hepatocyte growth factor abrogates Fas-induced lethal liver apoptosis in mice. Biochem. Biophys. Res. Commun. 1998, 244, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Kosai, K.; Matsumoto, K.; Nakamura, T. Hepatocyte growth factor prevents endotoxin-induced lethal hepatic failure in mice. Hepatology 1999, 30, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, Y.; Matsumoto, K.; Ichida, T.; Nakamura, T. Hepatocyte growth factor prevents liver cirrhosis caused by dimethylnitrosamine in rats. J. Biochem. 1995, 118, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, Y.; Matsumoto, K.; Yamada, A.; Ichida, T.; Asakura, H.; Komoriya, K.; Nishiyama, E.; Nakamura, T. Preventive and therapeutic effects in rat of hepatocyte growth factor infusion on liver fibrosis/cirrhosis. Hepatology 1997, 26, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Oe, S.; Fukunaka, Y.; Hirose, T.; Yamaoka, Y.; Tabata, Y. A trial on regeneration therapy of rat liver cirrhosis by controlled release of hepatocyte growth factor. J. Control. Release 2003, 88, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.H.; Matsumoto, K.; Bessho, K.; Nakamura, T. Growth inhibition and apoptosis in liver myofibroblasts promoted by hepatocyte growth factor leads to resolution from liver cirrhosis. Am. J. Pathol. 2005, 166, 1017–1028. [Google Scholar] [CrossRef] [PubMed]
- Kusumoto, K.; Ido, A.; Moriuchi, A.; Katsura, T.; Kim, I.; Takahama, Y.; Numata, M.; Kodama, M.; Hasuike, S.; Nagata, K.; Uto, H.; Inui, K.; Tsubouchi, H. Repeated intravenous injection of recombinant human hepatocyte growth factor ameliorates liver cirrhosis but causes albuminuria in rats. Int. J. Mol. Med. 2006, 17, 503–509. [Google Scholar] [PubMed]
- Kaido, T.; Yoshikawa, A.; Seto, S.; Yamaoka, S.; Sato, M.; Ishii, T.; Imamura, M. Portal branch ligation with a continuous hepatocyte growth factor supply makes extensive hepatectomy possible in cirrhotic rats. Hepatology 1998, 28, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Tahara, M.; Matsumoto, K.; Nukiwa, T.; Nakamura, T. Hepatocyte growth factor leads to recovery from alcohol-induced fatty liver in rats. J. Clin. Investig. 1999, 103, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Tahara, Y.; Ido, A.; Yamamoto, S.; Miyata, Y.; Uto, H.; Hori, T.; Hayashi, K.; Tsubouchi, H. Hepatocyte growth factor facilitates colonic mucosal repair in experimental ulcerative colitis in rats. J. Pharmacol. Exp. Ther. 2003, 307, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Numata, M.; Ido, A.; Moriuchi, A.; Kim, I.; Tahara, Y.; Yamamoto, S.; Hasuike, S.; Nagata, K.; Miyata, Y.; Uto, H.; Tsubouchi, H. Hepatocyte growth factor facilitates the repair of large colonic ulcers in 2,4,6-trinitrobenzene sulfonic acid-induced colitis in rats. Inflamm. Bowel Dis. 2005, 11, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Schmassmann, A.; Stettler, C.; Poulsom, R.; Tarasova, N.; Hirschi, C.; Flogerzi, B.; Matsumoto, K.; Nakamura, T.; Halter, F. Roles of hepatocyte growth factor and receptor c-Met during gastric ulcer healing in rats. Gastroenterology 1997, 113, 1858–1872. [Google Scholar] [CrossRef] [PubMed]
- Nakahira, R.; Mizuno, S.; Yoshimine, T.; Nakamura, T. The loss of local HGF, an endogenous gastrotrophic factor, leads to mucosal injuries in the stomach of mice. Biochem. Biophys. Res. Commun. 2006, 341, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Kawaida, K.; Matsumoto, K.; Shimazu, H.; Nakamura, T. Hepatocyte growth factor prevents acute renal failure and accelerate renal regeneration in mice. Proc. Natl. Acad. Sci. USA 1994, 91, 4357–4361. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.B.; Martin, D.R.; Kissane, J.; Hammerman, M.R. Hepatocyte growth factor accelerates recovery from acute ischemic renal injury in rats. Am. J. Physiol. 1994, 266, F129–F134. [Google Scholar] [PubMed]
- Amaike, H.; Matsumoto, K.; Oka, T.; Nakamura, T. Preventive effect of hepatocyte growth factor on acute side effects of cyclosporin A in mice. Cytokine 1996, 8, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, N.; Nagano, T.; Mori-Kudo, I.; Tsuchida, A.; Kawamura, T.; Seki, H.; Taiji, M.; Noguchi, H. Hepatocyte growth factor protects functional and histological disorders of HgCl2-induced acute renal failure mice. Nephron 2002, 90, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Nagano, T.; Mori-Kudo, I.; Tsuchida, A.; Kawamura, T.; Taiji, M.; Noguchi, H. Ameliorative effect of hepatocyte growth factor on glycerol-induced acute renal failure with acute tubular necrosis. Nephron 2002, 91, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Takada, S.; Takahara, S.; Nishimura, K.; Ichimaru, N.; Hongsi, J.; Kokado, Y.; Matsumiya, K.; Matsumoto, K.; Nakamura, T.; Okuyama, A. Effect of hepatocyte growth factor on tacrolimus-induced nephrotoxicity in spontaneously hypertensive rats. Transplant. Intern. 1999, 12, 27–32. [Google Scholar] [CrossRef]
- Gong, R.; Rifai, A.; Dworkin, L.D. Hepatocyte growth factor suppresses acute renal inflammation by inhibition of endothelial E-selectin. Kidney Int. 2006, 69, 1166–1174. [Google Scholar] [CrossRef] [PubMed]
- Kamimoto, M.; Mizuno, S.; Matsumoto, K.; Nakamura, T. Hepatocyte growth factor prevents multiple organ injuries in endotoxemic mice through a heme oxygenase-1-dependent mechanism. Biochem. Biophys. Res. Commun. 2009, 380, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, S.; Nakamura, T. Suppressions of chronic glomerular injuries and TGF-β1 production by HGF in attenuation of murine diabetic nephropathy. Am. J. Physiol. 2004, 286, F134–F143. [Google Scholar]
- Mizuno, S.; Kurosawa, T.; Matsumoto, K.; Mizuno-Horikawa, Y.; Okamoto, M.; Nakamura, T. Hepatocyte growth factor prevents renal fibrosis and dysfunction in a mouse model of chronic renal disease. J. Clin. Investig. 1998, 101, 1827–1834. [Google Scholar] [CrossRef] [PubMed]
- Gong, R.; Rifai, A.; Dworkin, L.D. Anti-inflammatory effect of hepatocyte growth factor in chronic kidney disease: targeting the inflamed vascular endothelium. J. Am. Soc. Nephrol. 2006, 17, 2464–2473. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, S.; Matsumoto, K.; Wen, J.; Nakamura, T. Hepatocyte growth factor suppresses interstitial fibrosis in a mouse model of obstructive nephropathy. Kidney Int. 2001, 59, 1304–1314. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, Y. Delayed administration of hepatocyte growth factor reduces renal fibrosis in obstructive nephropathy. Am. J. Physiol. 2003, 284, F349–F357. [Google Scholar] [CrossRef]
- Bessho, K.; Mizuno, S.; Matsumoto, K.; Nakamura, T. Counteractive effects of HGF on PDGF-induced mesangial cell proliferation in a rat model of glomerulonephritis. Am. J. Physiol. 2003, 284, F1171–F1180. [Google Scholar]
- Azuma, H.; Takahara, S.; Matsumoto, K.; Ichimaru, N.; Wang, J.; Moriyama, T.; Wagga, A.; Kitamura, M.; Otsuki, Y.; Okuyama, A.; et al. Hepatocyte growth factor prevents development of chronic allograft nephropathy in an experimental rat transplant model. J. Am. Soc. Nephrol. 2001, 12, 1280–1292. [Google Scholar] [PubMed]
- Van Belle, E.; Witzenbichler, B.; Chen, D.; Silver, M.; Chang, L.; Schwall, R.; Isner, J.M. Potentiated angiogenic effect of scatter factor/hepatocyte growth factor via induction of vascular endothelial growth factor: The case for paracrine amplification of angiogenesis. Circulation 1998, 97, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Morishita, R.; Nakamura, S.; Hayashi, S.; Taniyama, Y.; Moriguchi, A.; Nagano, T.; Taiji, M.; Noguchi, H.; Takeshita, S.; Matsumoto, K.; et al. Therapeutic angiogenesis induced by human recombinant hepatocyte growth factor in rabbit hind limb ischemia model as cytokine supplement therapy. Hypertension 1999, 33, 1379–1384. [Google Scholar] [CrossRef] [PubMed]
- Marui, A.; Kanematsu, A.; Yamahara, K.; Doi, K.; Kushibiki, T.; Yamamoto, M.; Itoh, H.; Ikeda, T.; Tabata, Y.; Komeda, M. Simultaneous application of basic fibroblast growth factor and hepatocyte growth factor to enhance the blood vessels formation. J. Vasc. Surg. 2005, 41, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, S.; Noguchi, T.; Gohda, M.; Arai, T.; Tsutsui, N.; Matsuda, T.; Nonogi, H. Single low-dose administration of human recombinant hepatocyte growth factor attenuates intimal hyperplasia in a balloon-injured rabbit iliac artery model. Circulation 2000, 101, 2546–2549. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Sawa, Y.; Miyamoto, Y.; Takahashi, T.; Jau, C.C.; Ahmet, I.; Nakamura, T.; Matsuda, H. Therapeutic angiogenesis induced by injecting hepatocyte growth factor in ischemic canine hearts. Surg. Today 2005, 35, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Mizuno, S.; Matsumoto, K.; Sawa, Y.; Matsuda, H.; Nakamura, T. Myocardial protection from ischemia/reperfusion injury by endogenous and exogenous HGF. J. Clin. Investig. 2000, 106, 1511–1519. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Yang, R.; Li, W.; Ogasawara, A.K.; Schwall, R.; Eberhard, D.A.; Zheng, Z.; Kahn, D.; Paoni, N.F. Early treatment with hepatocyte growth factor improves cardiac function in experimental heart failure induced by myocardial infarction. J. Pharmacol. Exp. Ther. 2003, 304, 54–60. [Google Scholar] [CrossRef]
- Yamaura, K.; Ito, K.; Tsukioka, K.; Wada, Y.; Makiuchi, A.; Sakaguchi, M.; Akashima, T.; Fujimori, M.; Sawa, Y.; Morishita, R.; et al. Suppression of acute and chronic rejection by hepatocyte growth factor in a murine model of cardiac transplantation: Induction of tolerance and prevention of cardiac allograft vasculopathy. Circulation 2004, 110, 1650–1657. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Matsumoto, K.; Mizuno, S.; Sawa, Y.; Matsuda, H.; Nakamura, T. Hepatocyte growth factor prevents tissue fibrosis, remodeling, and dysfunction in cardiomyopathic hamster hearts. Am. J. Physiol. 2005, 288, H2131–H2139. [Google Scholar]
- Ohmichi, H.; Matsumoto, K.; Nakamura, T. In vivo mitogenic action of hepatocyte growth factor on lung epithelial cells: Pulmotrophic role in lung regeneration. Am. J. Physiol. 1996, 270, L1031–L1039. [Google Scholar]
- Makiuchi, A.; Yamaura, K.; Mizuno, S.; Matsumoto, K.; Nakamura, T.; Amano, J.; Ito, K. Hepatocyte growth factor prevents pulmonary ischemia-reperfusion injury in mice. J. Heart Lung Transplant. 2007, 26, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Yaekashiwa, M.; Nakayama, S.; Ohnuma, K.; Sakai, T.; Abe, T.; Satoh, K.; Matsumoto, K.; Nakamura, T.; Takahashi, T.; Nukiwa, T. Simultaneous or delayed administration of hepatocyte growth factor (HGF) equally repress the fibrotic change in murine lung injury induced by bleomycin: A morphologic study. Am. J. Resp. Crit. Care Med. 1997, 156, 1937–1944. [Google Scholar] [CrossRef] [PubMed]
- Dohi, M.; Hasegawa, T.; Yamamoto, K.; Marshall, B.C. Hepatocyte growth factor attenuates collagen accumulation in a murine model of pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2000, 162, 2302–2307. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, S.; Matsumoto, K.; Li, M.Y.; Nakamura, T. HGF reduces advancing lung fibrosis in mice: a potential role for MMP-dependent myofibroblast apoptosis. FASEB J. 2005, 19, 580–582. [Google Scholar] [PubMed]
- Ishizawa, K.; Kubo, H.; Yamada, M.; Kobayashi, S.; Suzuki, T.; Mizuno, S.; Nakamura, T.; Sasaki, H. Hepatocyte growth factor induces angiogenesis in injured lungs through mobilizing endothelial progenitor cells. Biochem. Biophys. Res. Commun. 2004, 324, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Sakamaki, Y.; Matsumoto, K.; Mizuno, S.; Miyoshi, S.; Matsuda, H.; Nakamura, T. Hepatocyte growth factor stimulates proliferation of respiratory epithelial cells during postneumonectomy compensatory lung growth in mice. Am. J. Respir. Cell Mol. Biol. 2002, 26, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Ito, W.; Kanehiro, A.; Matsumoto, K.; Hirano, A.; Ono, K.; Maruyama, H.; Kataoka, M.; Nakamura, T.; Gelfand, E.W.; Tanimoto, M. Hepatocyte growth factor attenuates airway hyperresponsiveness, inflammation, and remodeling. Am. J. Respir. Cell Mol. Biol. 2005, 32, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Ohno, T.; Hirano, S.; Kanemaru, S.; Yamashita, M.; Umeda, H.; Suehiro, A.; Tamura, Y.; Nakamura, T.; Ito, J.; Tabata, Y. Drug delivery system of hepatocyte growth factor for the treatment of vocal fold scarring in a canine model. Ann. Otol. Rhinol. Laryngol. 2007, 116, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, Y.; Hirano, S.; Kitani, Y.; Suehiro, A.; Umeda, H.; Tateya, I.; Kanemaru, S.; Tabata, Y.; Ito, J. Chronic vocal fold scar restoration with hepatocyte growth factor hydrogel. Laryngoscope 2010, 120, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Bevan, D.; Gherardi, E.; Fan, T.P.; Edwards, D.; Warn, R. Diverse and potent activities of HGF/SF in skin wound repair. J. Pathol. 2004, 203, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Matsumoto, K.; Tomioka, D.; Bessho, K.; Itami, S.; Yoshikawa, K.; Nakamura, T. Recombinant hepatocyte growth factor accelerates cutaneous wound healing in a diabetic mouse model. Growth Factors 2004, 22, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, T.; Matsumoto, K.; Ohmichi, H.; Yamashima, T.; Chigasaki, H.; Nakamura, T. Protection of hippocampal neurons from ischemia-induced delayed neuronal death by hepatocyte growth factor: A novel neurotrophic factor. J. Celebral. Blood Flow Metab. 1998, 18, 345–348. [Google Scholar] [CrossRef]
- Tsuzuki, N.; Miyazawa, T.; Matsumoto, K.; Nakamura, T.; Shima, K. Hepatocyte growth factor reduces the infarct volume after transient focal cerebral ischemia in rats. Neurol. Res. 2001, 23, 417–424. [Google Scholar] [PubMed]
- Date, I.; Takagi, N.; Takagi, K.; Kago, T.; Matsumoto, K.; Nakamura, T.; Takeo, S. Hepatocyte growth factor improved learning and memory dysfunction of microsphere-embolized rats. J. Neurosci. Res. 2004, 78, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Niimura, M.; Takagi, N.; Takagi, K.; Mizutani, R.; Tanonaka, K.; Funakoshi, H.; Matsumoto, K.; Nakamura, T.; Takeo, S. The protective effect of hepatocyte growth factor against cell death in the hippocampus after transient forebrain ischemia is related to the improvement of apurinic/apyrimidinic endonuclease/redox factor-1 level and inhibition of NADPH oxidase activity. Neurosci. Lett. 2006, 407, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Deguchi, K.; Yamashita, T.; Ohta, Y.; Zhang, H.; Morimoto, N.; Liu, N.; Zhang, X.; Tian, F.; Matsuura, T.; et al. Antiapoptotic and antiautophagic effects of glial cell line-derived neurotrophic factor and hepatocyte growth factor after transient middle cerebral artery occlusion in rats. J. Neurosci. Res. 2010, 88, 2197–2206. [Google Scholar] [CrossRef] [PubMed]
- Okura, Y.; Arimoto, H.; Tamura, N.; Matsumoto, K.; Nakamura, T.; Yamashita, T.; Miyazawa, T.; Matsumoto, Y. Analysis of neurotrophic effects of hepatocyte growth factor in the adult hypoglossal nerve axotomy model. Eur. J. Neurosci. 1999, 11, 4130–4144. [Google Scholar] [CrossRef]
- Ishigaki, A.; Aoki, M.; Nagai, M.; Warita, H.; Kato, S.; Kato, M.; Nakamura, T.; Funakoshi, H.; Itoyama, Y. Intrathecal delivery of hepatocyte growth factor from amyotrophic lateral sclerosis onset suppresses disease progression in rat amyotrophic lateral sclerosis model. J. Neuropathol. Exp. Neurol. 2007, 66, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Tada, T.; Zhan, H.; Tanaka, Y.; Hongo, K.; Matsumoto, K.; Nakamura, T. Intraventricular administration of hepatocyte growth factor treats mouse communicating hydrocephalus induced by transforming growth factor-b1. Neurobiol. Disease 2006, 21, 576–586. [Google Scholar] [CrossRef]
- Shibuki, H.; Katai, N.; Kuroiwa, S.; Kurokawa, T.; Arai, J.; Matsumoto, K.; Nakamura, T.; Yoshimura, N. Expression and neuroprotective effect of hepatocyte growth factor in retinal ischemia-reperfusion injury. Invest. Ophthalmol. Vis. Sci. 2002, 43, 528–536. [Google Scholar] [PubMed]
- Ohtaka, K.; Machida, S.; Ohzeki, T.; Tanaka, M.; Kurosaka, D.; Masuda, T.; Ishii, T. Protective effect of hepatocyte growth factor against degeneration of the retinal pigment epithelium and photoreceptor in sodium iodate-injected rats. Curr. Eye Res. 2006, 31, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Machida, S.; Tanaka, M.; Ishii, T.; Ohtaka, K.; Takahashi, T.; Tazawa, Y. Neuroprotective effect of hepatocyte growth factor against photoreceptor degeneration in rats. Investig. Ophthalmol. Vis. Sci. 2004, 45, 4174–4182. [Google Scholar] [CrossRef]
- Inaoka, T.; Nakagawa, T.; Kikkawa, Y.S.; Tabata, Y.; Ono, K.; Yoshida, M.; Tsubouchi, H.; Ido, A.; Ito, J. Local application of hepatocyte growth factor using gelatin hydrogels attenuates noise-induced hearing loss in guinea pigs. Acta Otolaryngol. 2009, 129, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Wakitani, S.; Imoto, T.; Kimura, T.; Ochi, T.; Matsumoto, K.; Nakamura, T. Hepatocyte growth factor facilitates cartilage repair: full thickness articular cartilage defect studied in rabbit knee. Acta Orthop. Stand. 1997, 68, 474–480. [Google Scholar] [CrossRef]
- Miller, K.J.; Thaloor, D.; Matteson, S.; Pavlath, G.K. Hepatocyte growth factor affects satellite cell activation and differentiation in regenerating skeletal muscle. Am. J. Physiol. 2000, 278, C174–C181. [Google Scholar]
- Okunishi, K.; Dohi, M.; Nakagome, K.; Tanaka, R.; Mizuno, S.; Matsumoto, K.; Miyazaki, J.; Nakamura, T.; Yamamoto, K. A novel role of hepatocyte growth factor as an immune regulator through suppressing dendritic cell function. J. Immunol. 2005, 175, 4745–4753. [Google Scholar] [CrossRef] [PubMed]
- Nakase, J.; Kitaoka, K.; Matsumoto, K.; Tomita, K. Facilitated tendon-bone healing by local delivery of recombinant hepatocyte growth factor in rabbits. Arthroscopy 2010, 26, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Noma, S.; Ohya-Shimada, W.; Kanai, M.; Ueda, K.; Nakamura, T.; Funakoshi, H. Overexpression of HGF attenuates the degeneration of Purkinje cells and Bergmann glia in a knockin mouse model of spinocerebellar ataxia type 7. Neurosci. Res. 2012, 773, 115–121. [Google Scholar] [CrossRef]
- Bai, L.; Lennon, D.P.; Caplan, A.I.; DeChant, A.; Hecker, L.; Kranso, J.; Zaremba, A.; Miller, R.H. Hepatocyte growth factor mediates mesenchymal stem cell–induced recovery in multiple sclerosis models. Nat. Neurosci. 2012, 15, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Benkhoucha, M.; Molnarfi, N.; Dunand-Sauthier, I.; Merkler, D.; Schneiter, G.; Bruscoli, S.; Riccardi, C.; Tabata, Y.; Funakoshi, H.; Nakamura, T.; et al. Hepatocyte growth factor limits autoimmune neuroinflammation via GILZ expression in dendritic cells. J. Immunol. 2014, 193, 2743–2752. [Google Scholar] [CrossRef] [PubMed]
- Buchstein, N.; Hoffmann, D.; Smola, H.; Lang, S.; Paulsson, M.; Niemann, C.; Krieg, T.; Eming, S.A. Alternative proteolytic processing of hepatocyte growth factor during wound repair. Am. J. Pathol. 2009, 174, 2116–2128. [Google Scholar] [CrossRef] [PubMed]
- Nayeri, F.; Strömberg, T.; Larsson, M.; Brudin, L.; Söderström, C.; Forsberg, P. Hepatocyte growth factor may accelerate healing in chronic leg ulcers: A pilot study. J. Dermatol. Treat. 2002, 13, 81–86. [Google Scholar] [CrossRef]
- Rosen, E.M.; Jaken, S.; Carley, W.; Luckett, P.M.; Setter, E.; Bhargava, M.; Goldberg, I.D. Regulation of motility in bovine brain endothelial cells. J. Cell Physiol. 1991, 146, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, A.; Okamura, K.; Hamanaka, R.; Sato, Y.; Shima, N.; Higashio, K.; Kuwano, M. Hepatocyte growth factor modulates migration and proliferation of human microvascular endothelial cells in culture. Biochem. Biophys. Res. Commun. 1991, 179, 1042–1049. [Google Scholar] [CrossRef] [PubMed]
- Bussolino, F.; di Renzo, M.F.; Ziche, M.; Bocchietto, E.; Olivero, M.; Naldini, L.; Gaudino, G.; Tamagnone, L.; Coffer, A.; Comoglio, P.M. Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J. Cell Biol. 1992, 119, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Morishita, R.; Aoki, M.; Hashiya, N.; Makino, H.; Yamasaki, K.; Azuma, J.; Sawa, Y.; Matsuda, H.; Kaneda, Y.; Ogihara, T. Safety evaluation of clinical gene therapy using hepatocyte growth factor to treat peripheral arterial disease. Hypertension 2004, 44, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Shigematsu, H.; Yasuda, K.; Iwai, T.; Sasajima, T.; Ishimaru, S.; Ohashi, Y.; Yamaguchi, T.; Ogihara, T.; Morishita, R. Randomized, double-blind, placebo-controlled clinical trial of hepatocyte growth factor plasmid for critical limb ischemia. Gene Ther. 2010, 17, 1152–1161. [Google Scholar] [CrossRef] [PubMed]
- Shigematsu, H.; Yasuda, K.; Sasajima, T.; Takano, T.; Miyata, T.; Ohta, T.; Tanemoto, K.; Obitsu, Y.; Iwai, T.; Ozaki, S.; et al. Transfection of human HGF plasmid DNA improves limb salvage in Buerger’s disease patients with critical limb ischemia. Int. Angiol. 2011, 30, 140–149. [Google Scholar] [PubMed]
- Nakamura, T.; Sakai, K.; Nakamura, T.; Matsumoto, K. Hepatocyte growth factor twenty years on: Much more than a growth factor. J. Gastroenterol. Hepatol. 2011, 26, 188–202. [Google Scholar] [CrossRef] [PubMed]
- Ido, A.; Moriuchi, A.; Numata, M.; Murayama, T.; Teramukai, S.; Marusawa, H.; Yamaji, N.; Setoyama, H.; Kim, I.D.; Chiba, T.; et al. Safety and pharmacokinetics of recombinant human hepatocyte growth factor (rh-HGF) in patients with fulminant hepatitis: A phase I/II clinical trial, following preclinical studies to ensure safety. J. Transl. Med. 2011, 9, 55. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Funakoshi, H.; Nakamura, T. Overexpression of HGF retards disease progression and prolongs life span in a transgenic mouse model of ALS. J. Neurosci. 2002, 22, 6537–6548. [Google Scholar] [PubMed]
- Kadoyama, K.; Funakoshi, H.; Ohya, W.; Nakamura, T. Hepatocyte growth factor (HGF) attenuates gliosis and motoneuronal degeneration in the brainstem motor nuclei of a transgenic mouse model of ALS. Neurosci. Res. 2007, 59, 446–456. [Google Scholar] [CrossRef]
- Kitamura, K.; Fujiyoshi, K.; Yamane, J.; Toyota, F.; Hikishima, K.; Nomura, T.; Funakoshi, H.; Nakamura, T.; Aoki, M.; Toyama, Y.; et al. Human hepatocyte growth factor promotes functional recovery in primates after spinal cord injury. PLoS One 2011, 6, e27706. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Okazaki, H.; Nakamura, T. Novel function of prostaglandins as inducers of gene expression of HGF and putative mediators of tissue regeneration. J. Biochem. 1995, 117, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Casado, M.; Mollá, B.; Roy, R.; Fernández-Martínez, A.; Cucarella, C.; Mayoral, R.; Boscá, L.; Martín-Sanz, P. Protection against Fas-induced liver apoptosis in transgenic mice expressing cyclooxygenase 2 in hepatocytes. Hepatology 2007, 45, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Cheng, L.; Langenbach, R.; Ju, C. Prostaglandin I2 and E2 mediate the protective effects of cyclooxygenase-2 in a mouse model of immune-mediated liver injury. Hepatology 2007, 45, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Nagamatsu, T.; Oka, T.; Suzuki, Y. Modulation of anti-glomerular basement membrane nephritis in rats by ONO-1301, a non-prostanoid prostaglandin I2 mimetic compound with inhibitory activity against thromboxane A2 synthase. Jpn. J. Pharmacol. 1997, 73, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Nakayama, M.; Suzuki, Y.; Sakai, K.; Nakamura, T.; Sakai, Y.; Matsumoto, K. Suppression of acute hepatic injury by a synthetic prostacyclin agonist through hepatocyte growth factor expression. Am. J. Physiol. 2012, 302, G420–G429. [Google Scholar]
- Uchida, T.; Hazekawa, M.; Yoshida, M.; Matsumoto, K.; Sakai, Y. A novel long-acting prostacyclin agonist (ONO-1301) with an angiogenic effect: Promoting synthesis of hepatocyte growth factor and increasing cyclic AMP concentration via IP receptor signaling. J. Pharmacol. Sci. 2013, 123, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Nasu, T.; Kinomura, M.; Tanabe, K.; Yamasaki, H.; Htay, S.L.; Saito, D.; Hinamoto, N.; Watatani, H.; Ujike, H.; Suzuki, Y.; et al. A sustained-release prostacyclin analog ONO-1301 ameliorates tubulointerstitial alterations in a mouse obstructive nephropathy model. Am. J. Physiol. Renal Physiol. 2012, 302, F1616–F1629. [Google Scholar] [CrossRef] [PubMed]
- Hirata, Y.; Kurobe, H.; Uematsu, E.; Yagi, S.; Soeki, T.; Yamada, H.; Fukuda, D.; Shimabukuro, M.; Nakayama, M.; Matsumoto, K.; et al. Beneficial effect of a synthetic prostacyclin agonist, ONO-1301, in rat autoimmune myocarditis model. Eur. J. Pharmacol. 2013, 699, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Yamabayashi, C.; Koya, T.; Kagamu, H.; Kawakami, H.; Kimura, Y.; Furukawa, T.; Sakagami, T.; Hasegawa, T.; Sakai, Y.; Matsumoto, K.; et al. A novel prostacyclin agonist protects to airway hyperresponsiveness and remodeling in mice. Am. J. Respir. Cell Mol. Biol. 2012, 47, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Nagaya, N.; Obata, H.; Sakai, K.; Sakai, Y.; Yoshikawa, M.; Hamada, K.; Matsumoto, K.; Kimura, H. Oral administration of a novel long-acting prostacyclin agonist with thromboxane synthase inhibitory activity for pulmonary arterial hypertension. Circ. J. 2013, 77, 2127–2133. [Google Scholar] [CrossRef] [PubMed]
- Comoglio, P.M.; Giordano, S.; Trusolino, L. Drug development of MET inhibitors: Targeting oncogene addiction and expedience. Nat. Rev. Drug Discov. 2008, 7, 504–516. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.A.; Jiang, W.G. Hepatocyte growth factor and its receptor signalling complex as targets in cancer therapy. Anticancer Agent Med. Chem. 2010, 10, 2–6. [Google Scholar] [CrossRef]
- Cecchi, F.; Rabe, D.C.; Bottaro, D.P. Targeting the HGF/Met signaling pathway in cancer therapy. Expert Opin. Ther. Targets 2012, 16, 553–572. [Google Scholar] [CrossRef] [PubMed]
- Gherardi, E.; Birchmeier, W.; Birchmeier, C.; Vande Woude, G.F. Targeting MET in cancer: Rationale and progress. Nat. Rev. Cancer 2012, 12, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Giordano, S.; Columbano, A. Met as a therapeutic target in HCC: Facts and hopes. J. Hepatol. 2014, 60, 442–452. [Google Scholar] [CrossRef]
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsumoto, K.; Funakoshi, H.; Takahashi, H.; Sakai, K. HGF–Met Pathway in Regeneration and Drug Discovery. Biomedicines 2014, 2, 275-300. https://doi.org/10.3390/biomedicines2040275
Matsumoto K, Funakoshi H, Takahashi H, Sakai K. HGF–Met Pathway in Regeneration and Drug Discovery. Biomedicines. 2014; 2(4):275-300. https://doi.org/10.3390/biomedicines2040275
Chicago/Turabian StyleMatsumoto, Kunio, Hiroshi Funakoshi, Hisaaki Takahashi, and Katsuya Sakai. 2014. "HGF–Met Pathway in Regeneration and Drug Discovery" Biomedicines 2, no. 4: 275-300. https://doi.org/10.3390/biomedicines2040275
APA StyleMatsumoto, K., Funakoshi, H., Takahashi, H., & Sakai, K. (2014). HGF–Met Pathway in Regeneration and Drug Discovery. Biomedicines, 2(4), 275-300. https://doi.org/10.3390/biomedicines2040275