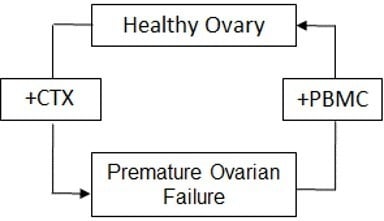
Intravenous Infusion of Nucleated Peripheral Blood Cells Restores Fertility in Mice with Chemotherapy-Induced Premature Ovarian Failure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Blood and Serum Preparation
2.3. Isolation of PBMC
2.4. Apoptosis Tunel Assay
2.5. Ovulation Rate and Maturational Stages of Ovulated Ova
2.6. Determination of Ovarian Follicle Reserve
2.7. Immunohistochemistry Staining and Image Analysis
2.8. mRNA Isolation and cDNA Preparation
2.9. Real Time PCR
2.10. Radioimmunoassay
2.11. Statistical Analysis
3. Results
3.1. Effect of Chemotherapy Treatment on Estrous Cycle and Hormone Production
3.2. Chemotherapy Induces Apoptosis of Granulosa Cells
3.3. Alteration of Expression of Steroidogenesis and Follicular Gap Junction Genes
3.4. Chemotherapy Decreases Oocytes Production after Ovarian Stimulation
3.5. Effect of CTX on Ovarian Follicle and Corpora Lutea Numbers
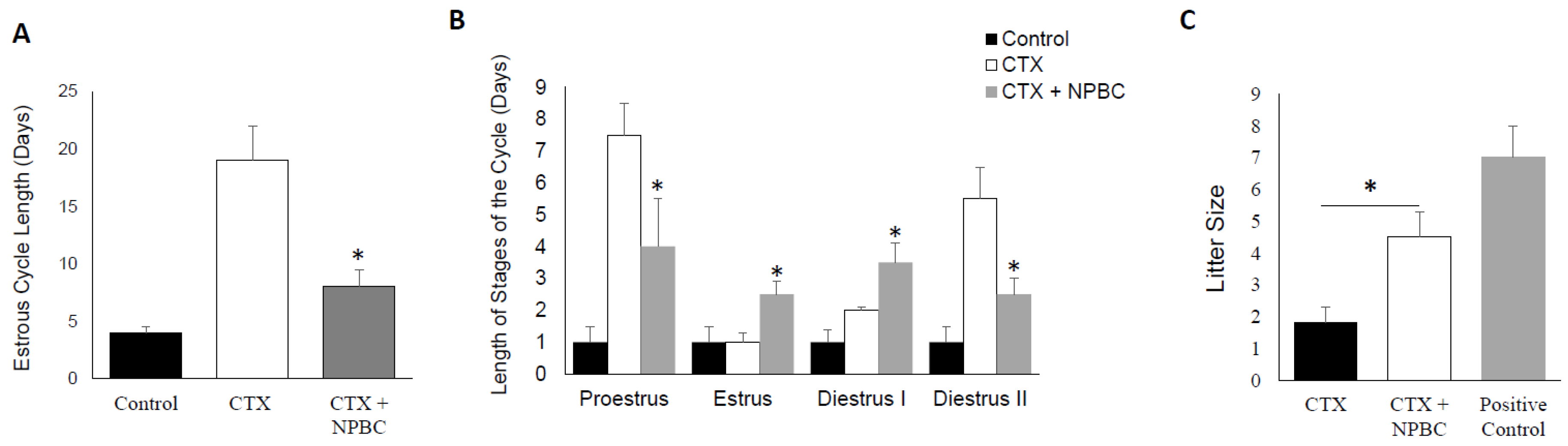
3.6. Nucleated Peripheral Blood Cells Infusion Restore Estrous Cycle Length in CXT-Treated Mice
3.7. Nucleated Peripheral Blood Cells Rescues the Negative Effect of CTX on Fertility
3.8. Nucleated Peripheral Blood Cells Treatment Promotes Stem Cell Markers in the Chemotherapy-Exposed Ovaries
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Espey, D.K.; Wu, X.C.; Swan, J.; Wiggins, C.; Jim, M.A.; Ward, E.; Wingo, P.A.; Howe, H.L.; Ries, L.A.; Miller, B.A.; et al. Annual report to the nation on the status of cancer, 1975–2004, featuring cancer in American Indians and Alaska Natives. Cancer 2007, 110, 2119–2152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, N.P.; Cheung, S.T.; Poon, R.T.; Fan, S.-T.; Luk, J.M. Genomic and proteomic biomarkers for diagnosis and prognosis of hepatocellular carcinoma. Biomark. Med. 2007, 1, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Angulo, A.M.; Morales-Vasquez, F.; Hortobagyi, G.N. Overview of resistance to systemic therapy in patients with breast cancer. Adv. Exp. Med. Biol. 2007, 608, 1–22. [Google Scholar] [PubMed]
- Ataya, K.; Moghissi, K. Chemotherapy-induced premature ovarian failure: Mechanisms and prevention. Steroids 1989, 54, 607–626. [Google Scholar] [CrossRef]
- Shalet, S.M.; Beardwell, C.G.; Jones, P.H.; Pearson, D.; Orrell, D.H. Ovarian failure following abdominal irradiation in childhood. Br. J. Cancer 1976, 33, 655–658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blumenfeld, Z.; Avivi, I.; Ritter, M.; Rowe, J.M. Preservation of fertility and ovarian function and minimizing chemotherapy-induced gonadotoxicity in young women. J. Soc. Gynecol. Investig. 1999, 6, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Schover, L.R.; Partridge, A.H.; Patrizio, P.; Wallace, W.H.; Hagerty, K.; Beck, L.N.; Brennan, L.V.; Oktay, K.; American Society of Clinical Oncology. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J. Clin. Oncol. 2006, 24, 2917–2931. [Google Scholar] [CrossRef] [PubMed]
- Aly, H.; Mohsen, L.; Badrawi, N.; Gabr, H.; Ali, Z.; Akmal, D. Viability and neural differentiation of mesenchymal stem cells derived from the umbilical cord following perinatal asphyxia. J. Perinatol. 2012, 32, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Selesniemi, K.; Lee, H.J.; Niikura, T.; Tilly, J.L. Young adult donor bone marrow infusions into female mice postpone age-related reproductive failure and improve offspring survival. Aging (Albany NY) 2008, 1, 49–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donahue, R.E.; Kessler, S.W.; Bodine, D.; McDonagh, K.; Dunbar, C.; Goodman, S.; Agricola, B.; Byrne, E.; Raffeld, M.; Moen, R.; et al. Helper virus induced T cell lymphoma in nonhuman primates after retroviral mediated gene transfer. J. Exp. Med. 1992, 176, 1125–1135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Averette, H.E.; Boike, G.M.; Jarrell, M.A. Effects of cancer chemotherapy on gonadal function and reproductive capacity. CA Cancer J. Clin. 1990, 40, 199–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plowchalk, D.R.; Mattison, D.R. Phosphoramide mustard is responsible for the ovarian toxicity of cyclophosphamide. Toxicol. Appl. Pharmacol. 1991, 107, 472–481. [Google Scholar] [CrossRef]
- Plowchalk, D.R.; Meadows, M.J.; Mattison, D.R. Reproductive toxicity of cyclophosphamide in the C57BL/6N mouse: 2. Effects on uterine structure and function. Reprod. Toxicol. 1992, 6, 423–429. [Google Scholar] [CrossRef]
- Desmeules, P.; Devine, P.J. Characterizing the ovotoxicity of cyclophosphamide metabolites on cultured mouse ovaries. Toxicol. Sci. 2006, 90, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Lopez, S.G.; Luderer, U. Effects of cyclophosphamide and buthionine sulfoximine on ovarian glutathione and apoptosis. Free Radic. Biol. Med. 2004, 36, 1366–1377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blumenfeld, Z.; Haim, N. Prevention of gonadal damage during cytotoxic therapy. Ann. Med. 1997, 29, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Blumenfeld, Z. Gynaecologic concerns for young women exposed to gonadotoxic chemotherapy. Curr. Opin. Obstet. Gynecol. 2003, 15, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.H.; Linde, R.; Hainsworth, J.D.; Vale, W.; Rivier, J.; Stein, R.; Flexner, J.; Van Welch, R.; Greco, F.A. Effect of a luteinizing hormone releasing hormone agonist given during combination chemotherapy on posttherapy fertility in male patients with lymphoma: Preliminary observations. Blood 1985, 65, 832–836. [Google Scholar] [PubMed]
- Aliagas, E.; Torrejon-Escribano, B.; Lavoie, E.G.; de Aranda, I.G.; Sévigny, J.; Solsona, C.; Martín-Satué, M. Changes in expression and activity levels of ecto-5′-nucleotidase/CD73 along the mouse female estrous cycle. Acta Physiol. (Oxf.) 2010, 199, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, T.; Peters, H. Proposal for a classification of oocytes and follicles in the mouse ovary. J. Reprod. Fertil. 1968, 17, 555–557. [Google Scholar] [CrossRef] [PubMed]
- Archibong, A.E.; Inyang, F.; Ramesh, A.; Greenwood, M.; Nayyar, T.; Kopsombut, P.; Hood, D.B.; Nyanda, A.M. Alteration of pregnancy related hormones and fetal survival in F-344 rats exposed by inhalation to benzo(a)pyrene. Reprod. Toxicol. 2002, 16, 801–808. [Google Scholar] [CrossRef]
- Manna, P.R.; Stetson, C.L.; Slominski, A.T.; Pruitt, K. Role of the steroidogenic acute regulatory protein in health and disease. Endocrine 2016, 51, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis: A Crucial Target for Anti- and Pro-Angiogenic Therapies. Genes Cancer 2011, 2, 1097–1105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, K.E.; Wilgus, T.A. Vascular Endothelial Growth Factor and Angiogenesis in the Regulation of Cutaneous Wound Repair. Adv. Wound. Care 2014, 3, 647–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gheorghe, C.P.; Goyal, R.; Mittal, A.; Longo, L.D. Gene expression in the placenta: Maternal stress and epigenetic responses. Int. J. Dev. Biol. 2010, 54, 507–523. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.V.; Ricardo, S.D. Macrophages and CSF-1: Implications for development and beyond. Organogenesis 2013, 9, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Tung, J.J.; Tattersall, I.W.; Kitajewski, J. Tips, stalks, tubes: Notch-mediated cell fate determination and mechanisms of tubulogenesis during angiogenesis. Cold Spring Harb. Perspect. Med. 2012, 2, a006601. [Google Scholar] [CrossRef] [PubMed]
- James, A.C.; Szot, J.O.; Iyer, K.; Major, J.A.; Pursglove, S.E.; Chapman, G.; Dunwoodie, S.L. Notch4 reveals a novel mechanism regulating Notch signal transduction. Biochim. Biophys. Acta 2014, 1843, 1272–1284. [Google Scholar] [CrossRef] [PubMed]
- Leal, A.M.; Blount, A.L.; Donaldson, C.J.; Bilezikjian, L.M.; Vale, W.W. Regulation of follicle-stimulating hormone secretion by the interactions of activin-A, dexamethasone and testosterone in anterior pituitary cell cultures of male rats. Neuroendocrinology 2003, 77, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Sidney, L.E.; Branch, M.J.; Dunphy, S.E.; Dua, H.S.; Hopkinson, A. Concise review: Evidence for CD34 as a common marker for diverse progenitors. Stem Cells 2014, 32, 1380–1389. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Kajitani, T.; Uchida, H.; Arase, T.; Oda, H.; Uchida, S.; Ota, K.; Nagashima, T.; Masuda, H.; Miyazaki, K.; et al. CD34 and CD49f Double-Positive and Lineage Marker-Negative Cells Isolated from Human Myometrium Exhibit Stem Cell-Like Properties Involved in Pregnancy-Induced Uterine Remodeling. Biol. Reprod. 2015, 93, 37. [Google Scholar] [CrossRef] [PubMed]
- Heissig, B.; Hattori, K.; Dias, S.; Friedrich, M.; Ferris, B.; Hackett, N.R.; Crystal, R.G.; Besmer, P.; Lyden, D.; Moore, M.A.; et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell 2002, 109, 625–637. [Google Scholar] [CrossRef]
- Shivalingappa, H.; Satyanarayan, N.D.; Purohit, M.G.; Sharanabasappa, A.; Patil, S.B. Effect of ethanol extract of Rivea hypocrateriformis on the estrous cycle of the rat. J. Ethnopharmacol. 2002, 82, 11–17. [Google Scholar] [CrossRef]
- Roy, K.S.; Prakash, B.S. Plasma progesterone, oestradiol-17beta and total oestrogen profiles in relation to oestrous behaviour during induced ovulation in Murrah buffalo heifers. J. Anim. Physiol. Anim. Nutr. (Berl.) 2009, 93, 486–495. [Google Scholar] [CrossRef] [PubMed]
- McGee, E.A.; Perlas, E.; LaPolt, P.S.; Tsafriri, A.; Hsueh, A.J. Follicle-stimulating hormone enhances the development of preantral follicles in juvenile rats. Biol. Reprod. 1997, 57, 990–998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chun, S.Y.; Hsueh, A.J. Paracrine mechanisms of ovarian follicle apoptosis. J. Reprod. Immunol. 1998, 39, 63–75. [Google Scholar] [CrossRef]
- Robker, R.L.; Richards, J.S. Hormone-induced proliferation and differentiation of granulosa cells: A coordinated balance of the cell cycle regulators cyclin D2 and p27Kip1. Mol. Endocrinol. 1998, 12, 924–940. [Google Scholar] [CrossRef] [PubMed]
- Waxman, J.H.; Ahmed, R.; Smith, D.; Wrigley, P.F.M.; Gregory, W.; Shalet, S.; Crowther, D.; Rees, L.H.; Besser, G.M.; Malpas, J.S.; Lister, T.A. Failure to preserve fertility in patients with Hodgkin′s disease. Cancer Chemother. Pharmacol. 1987, 19, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Makabe, S.; Naguro, T.; Stallone, T. Oocyte-follicle cell interactions during ovarian follicle development, as seen by high resolution scanning and transmission electron microscopy in humans. Microsc. Res. Tech. 2006, 69, 436–449. [Google Scholar] [CrossRef] [PubMed]
- Eppig, J.J. Maintenance of meiotic arrest and the induction of oocyte maturation in mouse oocyte-granulosa cell complexes developed in vitro from preantral follicles. Biol. Reprod. 1991, 45, 824–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ackert, C.L.; Gittens, J.E.; O′Brien, M.J.; Eppig, J.J.; Kidder, G.M. Intercellular communication via connexin43 gap junctions is required for ovarian folliculogenesis in the mouse. Dev. Biol. 2001, 233, 258–570. [Google Scholar] [CrossRef] [PubMed]
- Davis, W.L.; Crawford, L.A.; Cooper, O.J.; Farmer, G.R.; Thomas, D.; Freeman, B.L. Generation of radical oxygen species by neural crest cells treated in vitro with isotretinoin and 4-oxo-isotretinoin. J. Craniofac. Genet. Dev. Biol. 1990, 10, 295–310. [Google Scholar] [PubMed]
- Sherizly, I.; Galiani, D.; Dekel, N. Regulation of oocyte maturation: Communication in the rat cumulus-oocyte complex. Hum. Reprod. 1988, 3, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Chuai, Y.; Xu, X.; Wang, A. Preservation of fertility in females treated for cancer. Int. J. Biol. Sci. 2012, 8, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- Bottai, D.; Cigognini, D.; Nicora, E.; Moro, M.; Grimoldi, M.G.; Adami, R.; Abrignani, S.; Marconi, A.M.; Di Giulio, A.M.; Gorio, A. Third trimester amniotic fluid cells with the capacity to develop neural phenotypes and with heterogeneity among sub-populations. Restor. Neurol. Neurosci. 2012, 30, 55–68. [Google Scholar] [PubMed]
- Solchaga, L.A.; Penick, K.; Porter, J.D.; Goldberg, V.M.; Caplan, A.I.; Welter, J.F. FGF-2 enhances the mitotic and chondrogenic potentials of human adult bone marrow-derived mesenchymal stem cells. J. Cell Physiol. 2005, 203, 398–409. [Google Scholar] [CrossRef] [PubMed]





| Gene | Fold Change | Function | References | p Value |
|---|---|---|---|---|
| CD34 | 26.8 | Mediate the attachment of stem cells to the bone marrow extracellular matrix or directly to stromal cells. Act as a scaffold for the attachment of lineage specific glycans, allowing stem cells to bind to lectins expressed by stromal cells or other marrow components. (enhance stem cell activity) | [33,34,35] | <0.05 |
| Csf1 | 60.2 | The protein encoded by this gene is involved in development of the placenta. It plays a role in fertility and pregnancy. (blood cell differentiation) | [28,29] | <0.05 |
| Inhba | 2.2 | Beta A subunit form a pituitary FSH secretion inhibitor effectively regulating gonadal stromal cell proliferation negatively but when joined with Beta B subunit it stimulate FSH secretion | [32] | <0.05 |
| Kitl | 24.7 | Play a role in cell migration, cell-cell adhesion and augment proliferation of myeloid progenitors in bone marrow culture. (blood cell differentiation) | [33,34,35] | <0.05 |
| Notch4 | 20.0 | Functions as a receptor for membrane-bound ligands to regulate cell fate determination by working as a signaling network between adjacent cells. Regulate branching morphogenesis in the developing vascular system.Increase blood supply to stem cells | [30,31] | <0.05 |
| Vegfa | 143.1 | Encodes a protein that mediate increased vascular permeability, including angiogenesis, vasculogenesis and endothelial cell growth and in effect inhibits apoptosis. Increase blood supply to stem cells | [26,27] | <0.05 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Andaloussi, A.; Igboeli, P.; Amer, A.; Al-Hendy, A. Intravenous Infusion of Nucleated Peripheral Blood Cells Restores Fertility in Mice with Chemotherapy-Induced Premature Ovarian Failure. Biomedicines 2018, 6, 93. https://doi.org/10.3390/biomedicines6030093
El Andaloussi A, Igboeli P, Amer A, Al-Hendy A. Intravenous Infusion of Nucleated Peripheral Blood Cells Restores Fertility in Mice with Chemotherapy-Induced Premature Ovarian Failure. Biomedicines. 2018; 6(3):93. https://doi.org/10.3390/biomedicines6030093
Chicago/Turabian StyleEl Andaloussi, Abdeljabar, Prosper Igboeli, Amero Amer, and Ayman Al-Hendy. 2018. "Intravenous Infusion of Nucleated Peripheral Blood Cells Restores Fertility in Mice with Chemotherapy-Induced Premature Ovarian Failure" Biomedicines 6, no. 3: 93. https://doi.org/10.3390/biomedicines6030093