Carollia perspicillata: A Small Bat with Tremendous Translational Potential for Studies of Brain Aging and Neurodegeneration
Abstract
:1. Introduction
2. Methods
3. Results
4. Discussion
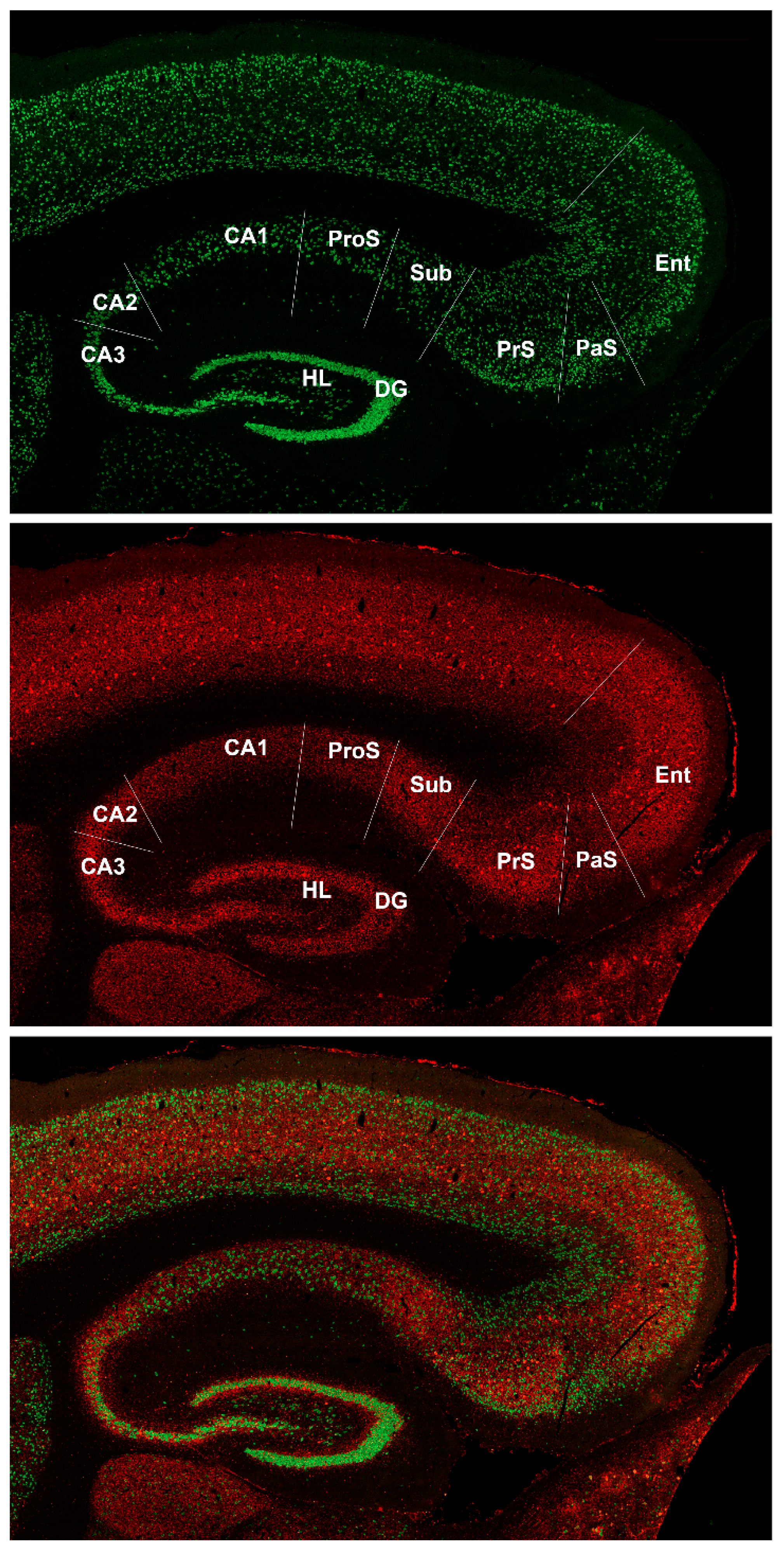
4.1. Prosubiculum in Bat Brain
4.2. Carollia as a New Animal Model of Brain Aging and Neurodegeneration
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beach, T.G. A Review of Biomarkers for Neurodegenerative Disease: Will They Swing Us Across the Valley? Neurol. Ther. 2017, 6, 5–13. [Google Scholar] [CrossRef] [Green Version]
- Ramachandran, A.K.; Das, S.; Joseph, A.; Shenoy, G.G.; Alex, A.T.; Mudgal, J. Neurodegenerative Pathways in Alzheimer’s Disease: A Review. Curr. Neuropharmacol. 2021, 19, 679–692. [Google Scholar] [CrossRef]
- Driscoll, I.; Sutherland, R.J. The aging hippocampus: Navigating between rat and human experiments. Rev. Neurosci. 2005, 16, 87–121. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M. And when I die… What time should I expect it? J. Physiol. 2021, 599, 1729–1730. [Google Scholar] [CrossRef] [PubMed]
- Trujillo-Estrada, L.; Davila, J.C.; Sanchez-Mejias, E.; Sanchez-Varo, R.; Gomez-Arboledas, A.; Vizuete, M.; Vitorica, J.; Gutierrez, A. Early neuronal loss and axonal/presynaptic damage is associated with accelerated amyloid-beta accumulation in AbetaPP/PS1 Alzheimer’s disease mice subiculum. J. Alzheimers’ Dis. 2014, 42, 521–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angulo, S.L.; Orman, R.; Neymotin, S.A.; Liu, L.; Buitrago, L.; Cepeda-Prado, E.; Stefanov, D.; Lytton, W.W.; Stewart, M.; Small, S.A.; et al. Tau and amyloid-related pathologies in the entorhinal cortex have divergent effects in the hippocampal circuit. Neurobiol. Dis. 2017, 108, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Carlesimo, G.A.; Piras, F.; Orfei, M.D.; Iorio, M.; Caltagirone, C.; Spalletta, G. Atrophy of presubiculum and subiculum is the earliest hippocampal anatomical marker of Alzheimer’s disease. Alzheimers’ Dement. 2015, 1, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Wang, G.Z.; Braak, H. Pathological changes of the retrosplenial cortex in senile dementia of Alzheimer type. Chin. Med. J. 1994, 107, 119–123. [Google Scholar]
- Pengas, G.; Williams, G.B.; Acosta-Cabronero, J.; Ash, T.W.; Hong, Y.T.; Izquierdo-Garcia, D.; Fryer, T.D.; Hodges, J.R.; Nestor, P.J. The relationship of topographical memory performance to regional neurodegeneration in Alzheimer’s disease. Front. Aging Neurosci. 2012, 4, 17. [Google Scholar] [CrossRef] [Green Version]
- Nestor, P.J.; Fryer, T.D.; Ikeda, M.; Hodges, J.R. Retrosplenial cortex (BA 29/30) hypometabolism in mild cognitive impairment (prodromal Alzheimer’s disease). Eur. J. Neurosci. 2003, 18, 2663–2667. [Google Scholar] [CrossRef]
- Robertson, R.T.; Baratta, J.; Yu, J.; LaFerla, F.M. Amyloid-beta expression in retrosplenial cortex of triple transgenic mice: Relationship to cholinergic axonal afferents from medial septum. Neuroscience 2009, 164, 1334–1346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, E.M.C.; Bannerman, D.M. Mouse models of neurodegeneration: Know your question, know your mouse. Sci. Transl. Med. 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Fuhrer, T.E.; Palpagama, T.H.; Waldvogel, H.J.; Synek, B.J.L.; Turner, C.; Faull, R.L.; Kwakowsky, A. Impaired expression of GABA transporters in the human Alzheimer’s disease hippocampus, subiculum, entorhinal cortex and superior temporal gyrus. Neuroscience 2017, 351, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Kwakowsky, A.; Calvo-Flores Guzman, B.; Pandya, M.; Turner, C.; Waldvogel, H.J.; Faull, R.L. GABAA receptor subunit expression changes in the human Alzheimer’s disease hippocampus, subiculum, entorhinal cortex and superior temporal gyrus. J. Neurochem. 2018, 145, 374–392. [Google Scholar] [CrossRef] [PubMed]
- Mikkonen, M.; Alafuzoff, I.; Tapiola, T.; Soininen, H.; Miettinen, R. Subfield- and layer-specific changes in parvalbumin, calretinin and calbindin-D28K immunoreactivity in the entorhinal cortex in Alzheimer’s disease. Neuroscience 1999, 92, 515–532. [Google Scholar] [CrossRef]
- Ahn, J.H.; Hong, S.; Park, J.H.; Kim, I.H.; Cho, J.H.; Lee, T.K.; Lee, J.C.; Chen, B.H.; Shin, B.N.; Bae, E.J.; et al. Immunoreactivities of calbindinD28k, calretinin and parvalbumin in the somatosensory cortex of rodents during normal aging. Mol. Med. Rep. 2017, 16, 7191–7198. [Google Scholar] [CrossRef] [Green Version]
- Freund, T.F.; Buzsaki, G. Interneurons of the hippocampus. Hippocampus 1996, 6, 347–470. [Google Scholar] [CrossRef]
- Petilla Interneuron Nomenclature, G.; Ascoli, G.A.; Alonso-Nanclares, L.; Anderson, S.A.; Barrionuevo, G.; Benavides-Piccione, R.; Burkhalter, A.; Buzsaki, G.; Cauli, B.; Defelipe, J.; et al. Petilla terminology: Nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat. Rev. Neurosci. 2008, 9, 557–568. [Google Scholar] [CrossRef] [Green Version]
- DeFelipe, J.; Lopez-Cruz, P.L.; Benavides-Piccione, R.; Bielza, C.; Larranaga, P.; Anderson, S.; Burkhalter, A.; Cauli, B.; Fairen, A.; Feldmeyer, D.; et al. New insights into the classification and nomenclature of cortical GABAergic interneurons. Nat. Rev. Neurosci. 2013, 14, 202–216. [Google Scholar] [CrossRef] [Green Version]
- Baimbridge, K.G.; Celio, M.R.; Rogers, J.H. Calcium-binding proteins in the nervous system. Trends Neurosci. 1992, 15, 303–308. [Google Scholar] [CrossRef]
- Eliav, T.; Geva-Sagiv, M.; Yartsev, M.M.; Finkelstein, A.; Rubin, A.; Las, L.; Ulanovsky, N. Nonoscillatory Phase Coding and Synchronization in the Bat Hippocampal Formation. Cell 2018, 175, 1119–1130.e15. [Google Scholar] [CrossRef] [Green Version]
- Gatome, C.W.; Mwangi, D.K.; Lipp, H.P.; Amrein, I. Hippocampal neurogenesis and cortical cellular plasticity in Wahlberg’s epauletted fruit bat: A qualitative and quantitative study. Brain Behav. Evol. 2010, 76, 116–127. [Google Scholar] [CrossRef] [Green Version]
- Cotter, J.R.; Laemle, L.K. Cholecystokinin (CCK)-like immunoreactivity in the brain of the little brown bat (Myotis lucifugus). J. Hirnforsch 1990, 31, 87–97. [Google Scholar]
- Orman, R.; Kollmar, R.; Stewart, M. Claustrum of the short-tailed fruit bat, Carollia perspicillata: Alignment of cellular orientation and functional connectivity. J. Comp. Neurol. 2017, 525, 1459–1474. [Google Scholar] [CrossRef]
- Scalia, F.; Rasweiler, J.J.; Scalia, J.; Orman, R.; Stewart, M. Forebrain Atlas of the Short-Tailed Fruit Bat, Carollia perpicillata; Springer: New York, NY, USA, 2013; p. 1. [Google Scholar]
- Blatt, G.J.; Rosene, D.L. Organization of direct hippocampal efferent projections to the cerebral cortex of the rhesus monkey: Projections from CA1, prosubiculum, and subiculum to the temporal lobe. J. Comp. Neurol. 1998, 392, 92–114. [Google Scholar] [CrossRef]
- Braak, H.; Del Tredici, K. From the Entorhinal Region via the Prosubiculum to the Dentate Fascia: Alzheimer Disease-Related Neurofibrillary Changes in the Temporal Allocortex. J. Neuropathol. Exp. Neurol. 2020, 79, 163–175. [Google Scholar] [CrossRef]
- Ding, S.L. Comparative anatomy of the prosubiculum, subiculum, presubiculum, postsubiculum, and parasubiculum in human, monkey, and rodent. J. Comp. Neurol. 2013, 521, 4145–4162. [Google Scholar] [CrossRef] [PubMed]
- Marshall, G.A.; Kaufer, D.I.; Lopez, O.L.; Rao, G.R.; Hamilton, R.L.; DeKosky, S.T. Right prosubiculum amyloid plaque density correlates with anosognosia in Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 2004, 75, 1396–1400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Podlutsky, A.J.; Khritankov, A.M.; Ovodov, N.D.; Austad, S.N. A new field record for bat longevity. J. Gerontol. A Biol. Sci. Med. Sci. 2005, 60, 1366–1368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ball, H.C.; Levari-Shariati, S.; Cooper, L.N.; Aliani, M. Comparative metabolomics of aging in a long-lived bat: Insights into the physiology of extreme longevity. PLoS ONE 2018, 13, e0196154. [Google Scholar] [CrossRef]
- Brunet-Rossinni, A.K. Reduced free-radical production and extreme longevity in the little brown bat (Myotis lucifugus) versus two non-flying mammals. Mech. Ageing Dev. 2004, 125, 11–20. [Google Scholar] [CrossRef]
- Huang, Z.; Jebb, D.; Teeling, E.C. Blood miRNomes and transcriptomes reveal novel longevity mechanisms in the long-lived bat, Myotis myotis. BMC Genom. 2016, 17, 906. [Google Scholar] [CrossRef] [Green Version]
- Seim, I.; Fang, X.; Xiong, Z.; Lobanov, A.V.; Huang, Z.; Ma, S.; Feng, Y.; Turanov, A.A.; Zhu, Y.; Lenz, T.L.; et al. Genome analysis reveals insights into physiology and longevity of the Brandt’s bat Myotis brandtii. Nat. Commun. 2013, 4, 2212. [Google Scholar] [CrossRef] [Green Version]
- Rasweiler, J.J.t.; Badwaik, N.K. Improved procedures for maintaining and breeding the short-tailed fruit bat (Carollia perspicillata) in a laboratory setting. Lab. Anim. 1996, 30, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Rasweiler Iv, J.J.; Badwaik, N.K. The laboratory environment for maintaining and breeding some bats in the Family Phyllostomidae. In Bats in Captivity, 1st ed.; Barnard, S.M., Ed.; Logos Press: Washington, DC, USA, 2009; pp. 345–356. [Google Scholar]
- Skrinyer, A.J.; Faure, P.A.; Dannemiller, S.; Ball, H.C.; Delaney, K.H.; Orman, R.; Stewart, M.; Cooper, L.N. Care and husbandry of bats, the world’s only flying mammals. Lab. Anim. Sci. Pro. 2017, 5, 24–27. [Google Scholar]
- Rasweiler, J.J.t.; Badwaik, N.K.; Mechineni, K.V. Ovulation, fertilization, and early embryonic development in the menstruating fruit bat, Carollia perspicillata. Anat. Rec. 2011, 294, 506–519. [Google Scholar] [CrossRef] [Green Version]
- Rasweiler, J.J.t.; Cretekos, C.J.; Behringer, R.R. The short-tailed fruit bat Carollia perspicillata: A model for studies in reproduction and development. Cold Spring Harb. Protoc. 2009, 2009, pdb emo118. [Google Scholar] [CrossRef]
- Smith, J.B.; Alloway, K.D.; Hof, P.R.; Orman, R.; Reser, D.H.; Watakabe, A.; Watson, G.D.R. The relationship between the claustrum and endopiriform nucleus: A perspective towards consensus on cross-species homology. J. Comp. Neurol. 2019, 527, 476–499. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Franklin, K.B.J. The Mouse Brain in Stereotaxic Coordinates, 2nd ed.; Elsevier Academic Press: Amsterdam, The Netherlands; Boston, MA, USA, 2004. [Google Scholar]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 6th ed.; Academic Press/Elsevier: Amsterdam, The Netherlands; Boston, MA, USA, 2007. [Google Scholar]
- Ding, S.L.; Van Hoesen, G.W. Organization and Detailed Parcellation of Human Hippocampal Head and Body Regions Based on a Combined Analysis of Cyto- and Chemoarchitecture. J. Comp. Neurol. 2015, 523, 2233–2253. [Google Scholar] [CrossRef] [PubMed]
- Orman, R. Claustrum: A case for directional, excitatory, intrinsic connectivity in the rat. J. Physiol. Sci. 2015, 65, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Rasweiler, J.J.t.; Cretekos, C.J.; Behringer, R.R. Feeding short-tailed fruit bats (Carollia perspicillata). Cold Spring Harb. Protoc. 2009, 2009, pdb-prot5159. [Google Scholar] [CrossRef]
- Jebb, D.; Huang, Z.; Pippel, M.; Hughes, G.M.; Lavrichenko, K.; Devanna, P.; Winkler, S.; Jermiin, L.S.; Skirmuntt, E.C.; Katzourakis, A.; et al. Six reference-quality genomes reveal evolution of bat adaptations. Nature 2020, 583, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Zeidman, P.; Maguire, E.A. Anterior hippocampus: The anatomy of perception, imagination and episodic memory. Nat. Rev. Neurosci. 2016, 17, 173–182. [Google Scholar] [CrossRef]
- Taube, J.S. Electrophysiological properties of neurons in the rat subiculum in vitro. Exp. Brain Res. 1993, 96, 304–318. [Google Scholar] [CrossRef] [PubMed]
- Witter, M.P.; Groenewegen, H.J. The subiculum: Cytoarchitectonically a simple structure, but hodologically complex. Prog. Brain Res. 1990, 83, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Harris, E.; Witter, M.P.; Weinstein, G.; Stewart, M. Intrinsic connectivity of the rat subiculum: I. Dendritic morphology and patterns of axonal arborization by pyramidal neurons. J. Comp. Neurol. 2001, 435, 490–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunitake, A.; Kunitake, T.; Stewart, M. Differential modulation by carbachol of four separate excitatory afferent systems to the rat subiculum in vitro. Hippocampus 2004, 14, 986–999. [Google Scholar] [CrossRef] [PubMed]
- Orman, R.; Von Gizycki, H.; Lytton, W.W.; Stewart, M. Local axon collaterals of area CA1 support spread of epileptiform discharges within CA1, but propagation is unidirectional. Hippocampus 2008, 18, 1021–1033. [Google Scholar] [CrossRef] [Green Version]
- Naggar, I.; Stewart, M.; Orman, R. High Frequency Oscillations in Rat Hippocampal Slices: Origin, Frequency Characteristics, and Spread. Front. Neurol. 2020, 11, 326. [Google Scholar] [CrossRef] [Green Version]
- Ding, S.L.; Yao, Z.; Hirokawa, K.E.; Nguyen, T.N.; Graybuck, L.T.; Fong, O.; Bohn, P.; Ngo, K.; Smith, K.A.; Koch, C.; et al. Distinct Transcriptomic Cell Types and Neural Circuits of the Subiculum and Prosubiculum along the Dorsal-Ventral Axis. Cell Rep. 2020, 31, 107648. [Google Scholar] [CrossRef]
- Bienkowski, M.S.; Sepehrband, F.; Kurniawan, N.D.; Stanis, J.; Korobkova, L.; Khanjani, N.; Clark, K.; Hintiryan, H.; Miller, C.A.; Dong, H.W. Homologous laminar organization of the mouse and human subiculum. Sci. Rep. 2021, 11, 3729. [Google Scholar] [CrossRef] [PubMed]

| Target | Species | Immunogen | Clonality | Isotype | Working Dilution | Source (Cat. #) | RRID |
|---|---|---|---|---|---|---|---|
| PRIMARY ANTIBODIES | |||||||
| Calbindin | Rabbit | recombinant rat calbindin D-28k | Poly | (antiserum) | 1/3000 | Swant (CB 38) | AB_10000340 |
| Calretinin | Rabbit | recombinant human calretinin containing a 6-his tag at the N-terminal | Poly | (antiserum) | 1/3000 | Swant (CR7697) | AB_2619710 |
| NeuN | Mouse | purified cell nuclei from mouse brain | Mono | IgG1 | 1/2000 | Millipore (MAB377) | AB_2298772 |
| Parvalbumin | Rabbit | recombinant rat parvalbumin | Poly | (antiserum) | 1/3000 | Swant (PV 27) | AB_2631173 |
| SECONDARY ANTIBODIES | |||||||
| Mouse IgG | Goat | Poly | IgG | 1/500 | Jackson (115-545-003) | AB_2338840 | |
| Rabbit IgG | Goat | Poly | IgG | 1/500 | Jackson (111-295-003) | AB_2338022 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stewart, M.; Morello, T.; Kollmar, R.; Orman, R. Carollia perspicillata: A Small Bat with Tremendous Translational Potential for Studies of Brain Aging and Neurodegeneration. Biomedicines 2021, 9, 1454. https://doi.org/10.3390/biomedicines9101454
Stewart M, Morello T, Kollmar R, Orman R. Carollia perspicillata: A Small Bat with Tremendous Translational Potential for Studies of Brain Aging and Neurodegeneration. Biomedicines. 2021; 9(10):1454. https://doi.org/10.3390/biomedicines9101454
Chicago/Turabian StyleStewart, Mark, Timothy Morello, Richard Kollmar, and Rena Orman. 2021. "Carollia perspicillata: A Small Bat with Tremendous Translational Potential for Studies of Brain Aging and Neurodegeneration" Biomedicines 9, no. 10: 1454. https://doi.org/10.3390/biomedicines9101454





