Effect of Probiotic Dose Escalation on Gut Microbiota and Clinical Outcomes in Preterm Infants—A Systematic Review
Abstract
:1. Introduction
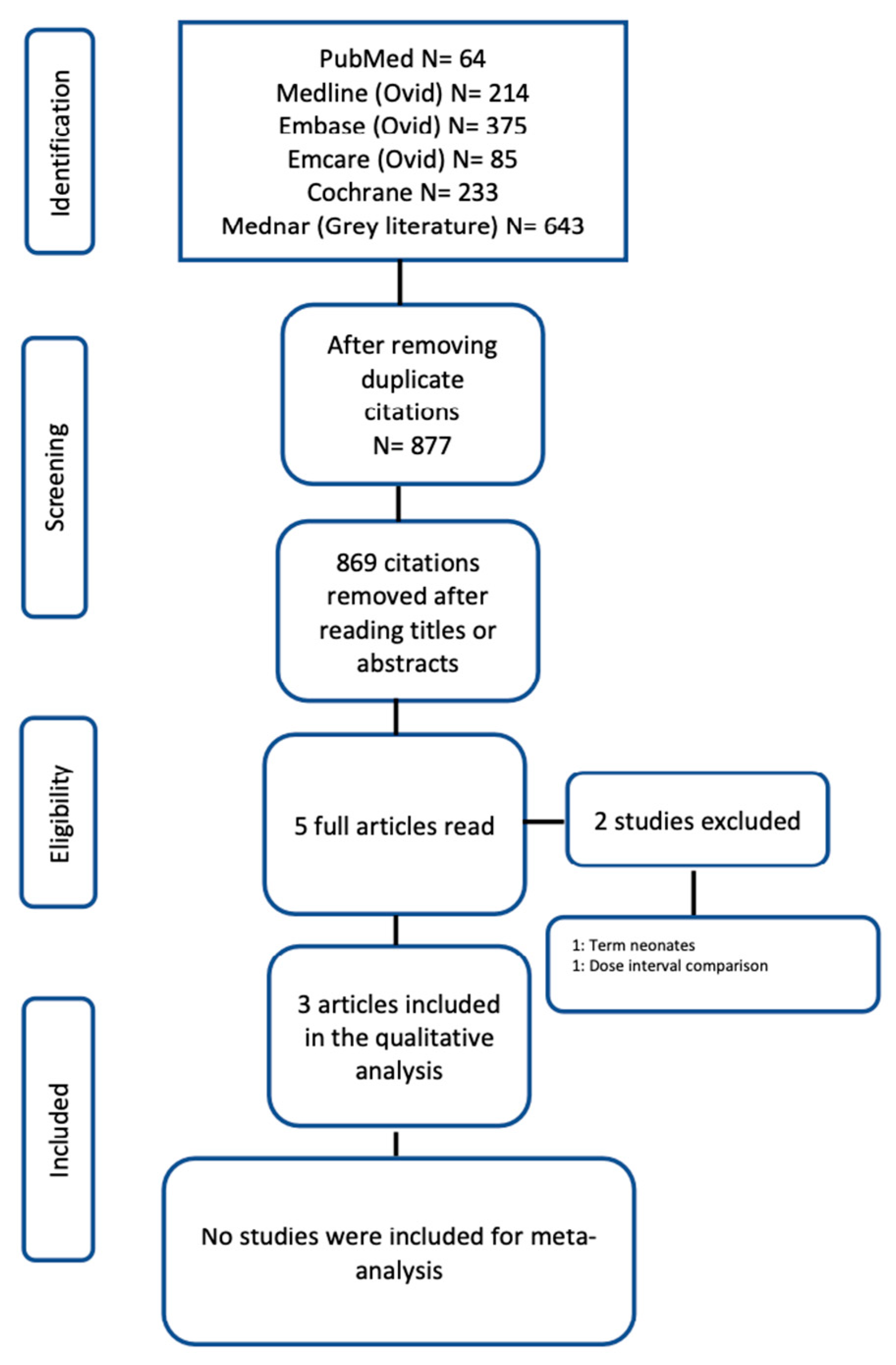
2. Materials and Methods
2.1. Data Sources and Searches
2.2. Study Selection
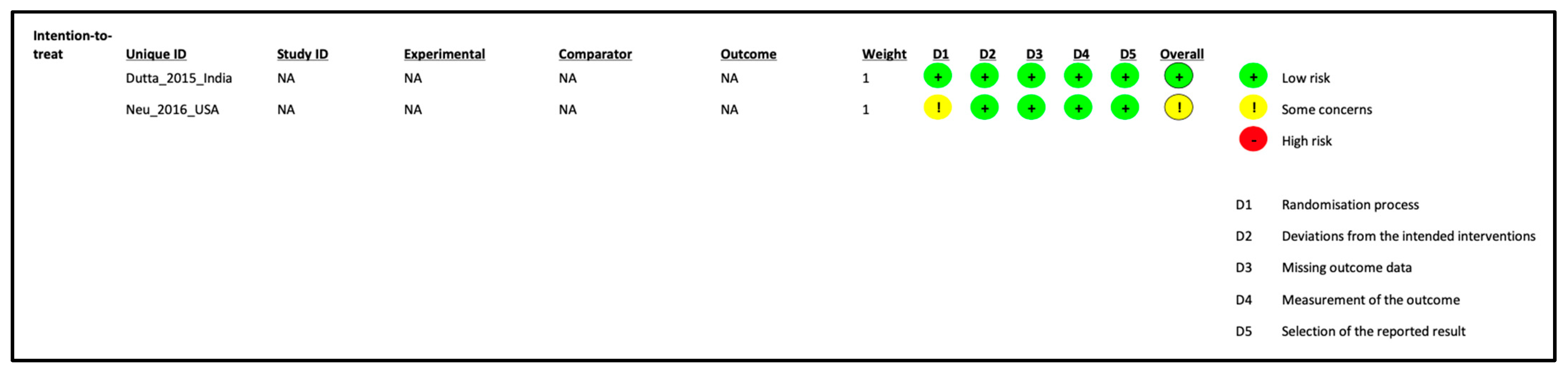
2.3. Data Extraction and Quality Assessment
2.4. Data Synthesis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cotten, C.M. Modifiable risk factors in necrotizing enterocolitis. Clin. Perinatol. 2019, 46, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Gowda, H.; Norton, R.; White, A.; Kandasamy, Y. Late-onset neonatal sepsis—A 10-year review from North Queensland, Australia. Pediatr. Infect. Dis. J. 2017, 36, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.H.; Johnson, M.J.; Leaf, A.A.; Vollmer, B. Nutrition and neurodevelopmental outcomes in preterm infants: A systematic review. Acta Paediatr. 2016, 105, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Ehrenkranz, R.A.; Dusick, A.M.; Vohr, B.R.; Wright, L.L.; Wrage, L.A.; Poole, W.K. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics 2006, 117, 1253–1261. [Google Scholar] [CrossRef]
- Lee, E.S.; Kim, E.K.; Shin, S.H.; Choi, Y.H.; Jung, Y.H.; Kim, S.Y.; Koh, J.W.; Choi, E.K.; Cheon, J.E.; Kim, H.S. Factors associated with neurodevelopment in preterm infants with systematic inflammation. BMC Pediatr. 2021, 21, 114. [Google Scholar] [CrossRef]
- Mondal, A.; Misra, D.; Al-Jabir, A.; Hubail, D.; Ward, T.; Patel, B. Necrotizing enterocolitis in neonates: Has the brain taken a hit 10 years later? J. Pediatr. Neurosci. 2021, 16, 30. [Google Scholar]
- Schlapbach, L.J.; Aebischer, M.; Adams, M.; Natalucci, G.; Bonhoeffer, J.; Latzin, P.; Nelle, M.; Bucher, H.U.; Latal, B.; Network, S.N.; et al. Impact of sepsis on neurodevelopmental outcome in a Swiss National Cohort of extremely premature infants. Pediatrics 2011, 128, e348–e357. [Google Scholar] [CrossRef]
- Strunk, T.; Inder, T.; Wang, X.; Burgner, D.; Mallard, C.; Levy, O. Infection-induced inflammation and cerebral injury in preterm infants. Lancet Infect. Dis. 2014, 14, 751–762. [Google Scholar] [CrossRef]
- Zozaya, C.; Shah, J.; Pierro, A.; Zani, A.; Synnes, A.; Lee, S.; Shah, P.S.; Network, C.N. Neurodevelopmental and growth outcomes of extremely preterm infants with necrotizing enterocolitis or spontaneous intestinal perforation. J. Pediatr. Surg. 2021, 56, 309–316. [Google Scholar] [CrossRef]
- Deshmukh, M.; Patole, S. Prophylactic probiotic supplementation for preterm neonates—A systematic review and meta-analysis of nonrandomized studies. Adv. Nutr. 2021, 12, 1411–1423. [Google Scholar] [CrossRef]
- Morgan, R.L.; Preidis, G.A.; Kashyap, P.C.; Weizman, A.V.; Sadeghirad, B.; Chang, Y.; Florez, I.D.; Foroutan, F.; Shahid, S.; Zeraatkar, D. Probiotics reduce mortality and morbidity in preterm, low-birth-weight infants: A systematic review and network meta-analysis of randomized trials. Gastroenterology 2020, 159, 467–480. [Google Scholar] [CrossRef] [PubMed]
- Sharif, S.; Meader, N.; Oddie, S.J.; Rojas-Reyes, M.X.; McGuire, W. Probiotics to prevent necrotising enterocolitis in very preterm or very low birth weight infants. Cochrane Database Syst. Rev. 2020. [Google Scholar] [CrossRef]
- Razak, A.; Patel, R.M.; Gautham, K.S. Use of probiotics to prevent necrotizing enterocolitis: Evidence to clinical practice. JAMA Pediatr. 2021, 175, 773–774. [Google Scholar] [CrossRef] [PubMed]
- FAO/WHO. Evaluation of Health and Nutritional Properties of Powder Milk and Live Lactic Acid Bacteria; Joint FAO/WHO Expert Consultation: Cordoba, Argentina, 2001; pp. 1–34.
- Deshpande, G.C.; Rao, S.C.; Keil, A.D.; Patole, S.K. Evidence-based guidelines for use of probiotics in preterm neonates. BMC Med. 2011, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Depoorter, L.; Vandenplas, Y. Probiotics in Pediatrics. A Review and Practical Guide. Nutrients 2021, 13, 2176. [Google Scholar] [CrossRef] [PubMed]
- Ouwehand, A. A review of dose-responses of probiotics in human studies. Benef. Microbes 2017, 8, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- Stoll, C.R.T.; Izadi, S.; Fowler, S.; Green, P.; Suls, J.; Colditz, G.A. The value of a second reviewer for study selection in systematic reviews. Res. Synth. Methods 2019, 10, 539–545. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. 2000. Available online: https://www.semanticscholar.org/paper/The-Newcastle-Ottawa-Scale-(NOS)-for-Assessing-the-Wells-Wells/c293fb316b6176154c3fdbb8340a107d9c8c82bf (accessed on 1 August 2023).
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Schunemann, H.; Brozek, J.; Guyatt, G.; Oxman, A. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations; The GRADE Working Group: Austin, TX, USA, 2013; Available online: http://gdt.guidelinedevelopment.org/app/handbook/handbook.html (accessed on 1 August 2023).
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (Updated February 2022); Cochrane: Chichester, UK, 2022. [Google Scholar]
- Dutta, S.; Ray, P.; Narang, A. Comparison of stool colonization in premature infants by three dose regimes of a probiotic combination: A randomized controlled trial. Am. J. Perinatol. 2015, 32, 733–740. [Google Scholar]
- Underwood, M.A.; Kalanetra, K.M.; Bokulich, N.A.; Lewis, Z.T.; Mirmiran, M.; Tancredi, D.J.; Mills, D.A. A comparison of two probiotic strains of bifidobacteria in premature infants. J. Pediatr. 2013, 163, 1585–1591.e1589. [Google Scholar] [CrossRef] [PubMed]
- Phase II: Safety and tolerability of IBP-9414–2016. Infant Bacterial Therapeutics AB. 2016. Available online: https://ibtherapeutics.com/science/ibp-9414/ (accessed on 10 August 2023).
- Baglatzi, L.; Gavrili, S.; Stamouli, K.; Zachaki, S.; Favre, L.; Pecquet, S.; Benyacoub, J.; Costalos, C. Effect of Infant Formula Containing a Low Dose of the Probiotic Bifidobacterium lactis CNCM I-3446 on Immune and Gut Functions in C-Section Delivered Babies: A Pilot Study. Clin. Med. Insights Pediatr. 2016, 10, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Watkins, C.; Murphy, K.; Dempsey, E.M.; O’Shea, C.A.; Murphy, B.P.; O’Toole, P.W.; Ross, R.P.; Stanton, C.; Ryan, C.A. Dose-interval study of a dual probiotic in preterm infants. Arch. Dis. Child. Fetal. Neonatal. Ed. 2019, 104, F159–F164. [Google Scholar] [CrossRef] [PubMed]
- Samuels, N.; van de Graaf, R.; Been, J.V.; De Jonge, R.C.; Hanff, L.M.; Wijnen, R.M.; Kornelisse, R.F.; Reiss, I.K.; Vermeulen, M.J. Necrotising enterocolitis and mortality in preterm infants after introduction of probiotics: A quasi-experimental study. Sci. Rep. 2016, 6, 31643. [Google Scholar] [CrossRef] [PubMed]
- Fortmann, I.; Marißen, J.; Siller, B.; Spiegler, J.; Humberg, A.; Hanke, K.; Faust, K.; Pagel, J.; Eyvazzadeh, L.; Brenner, K.; et al. Lactobacillus Acidophilus/Bifidobacterium Infantis Probiotics Are Beneficial to Extremely Low Gestational Age Infants Fed Human Milk. Nutrients 2020, 12, 850. [Google Scholar] [CrossRef] [PubMed]
- Mihi, B.; Good, M. Impact of Toll-Like Receptor 4 Signaling in Necrotizing Enterocolitis: The State of the Science. Clin. Perinatol. 2019, 46, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Cho, J.Y.; Chung, C.; Oh, S.H.; Do, H.-j.; Seo, J.-H.; Lim, J.Y.; Park, C.-H.; Woo, H.-O.; Youn, H.-S. Dynamic Changes of Fecal Calprotectin and Related Clinical Factors in Neonates. Front. Pediatr. 2020, 8, 326. [Google Scholar] [CrossRef]
- Petschow, B.W.; Figueroa, R.; Harris, C.L.; Beck, L.B.; Ziegler, E.; Goldin, B. Effects of feeding an infant formula containing Lactobacillus GG on the colonization of the intestine: A dose-response study in healthy infants. J. Clin. Gastroenterol. 2005, 39, 786–790. [Google Scholar] [CrossRef]
- Guo, Q.; Goldenberg, J.Z.; Humphrey, C.; El Dib, R.; Johnston, B.C. Probiotics for the prevention of pediatric antibiotic—Associated diarrhea. Cochrane Database Syst. Rev. 2019, 4, CD004827. [Google Scholar] [CrossRef]
- Fang, S.B.; Lee, H.C.; Hu, J.J.; Hou, S.Y.; Liu, H.L.; Fang, H.W. Dose-dependent effect of Lactobacillus rhamnosus on quantitative reduction of faecal rotavirus shedding in children. J. Trop. Pediatr. 2009, 55, 297–301. [Google Scholar] [CrossRef]
- Larsen, C.N.; Nielsen, S.; Kaestel, P.; Brockmann, E.; Bennedsen, M.; Christensen, H.R.; Eskesen, D.C.; Jacobsen, B.L.; Michaelsen, K.F. Dose-response study of probiotic bacteria Bifidobacterium animalis subsp lactis BB-12 and Lactobacillus paracasei subsp paracasei CRL-341 in healthy young adults. Eur. J. Clin. Nutr. 2006, 60, 1284–1293. [Google Scholar] [CrossRef] [PubMed]
- Goodman, C.; Keating, G.; Georgousopoulou, E.; Hespe, C.; Levett, K. Probiotics for the prevention of antibiotic-associated diarrhoea: A systematic review and meta-analysis. BMJ Open 2021, 11, e043054. [Google Scholar] [CrossRef] [PubMed]
- Gianotti, L.; Morelli, L.; Galbiati, F.; Rocchetti, S.; Coppola, S.; Beneduce, A.; Gilardini, C.; Zonenschain, D.; Nespoli, A.; Braga, M. A randomized double-blind trial on perioperative administration of probiotics in colorectal cancer patients. World J Gastroenterol. 2010, 16, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Gu, Y.; Li, X.; Yang, W.; Jia, L.; Chen, C.; Han, X.; Huang, Y.; Zhao, L.; Li, P.; et al. Alterations of the Gut Microbiome in Hypertension. Front. Cell Infect. Microbiol. 2017, 7, 381. [Google Scholar] [CrossRef] [PubMed]
- Ejtahed, H.S.; Ardeshirlarijani, E.; Tabatabaei-Malazy, O.; Hoseini-Tavassol, Z.; Hasani-Ranjbar, S.; Soroush, A.R.; Larijani, B. Effect of probiotic foods and supplements on blood pressure: A systematic review of meta-analyses studies of controlled trials. J. Diabetes Metab. Disord. 2020, 19, 617–623. [Google Scholar] [CrossRef]
- Zhou, X.; Mao, B.; Tang, X.; Zhang, Q.; Zhao, J.; Zhang, H.; Cui, S. Exploring the Dose-Effect Relationship of Bifidobacterium longum in Relieving Loperamide Hydrochloride-Induced Constipation in Rats through Colon-Released Capsules. Int. J. Mol. Sci. 2023, 24, 6585. [Google Scholar] [CrossRef]
- Gross Margolis, K.; Vittorio, J.; Talavera, M.; Gluck, K.; Li, Z.; Iuga, A.; Stevanovic, K.; Saurman, V.; Israelyan, N.; Welch, M.G.; et al. Enteric serotonin and oxytocin: Endogenous regulation of severity in a murine model of necrotizing enterocolitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 313, G386–G398. [Google Scholar] [CrossRef]
- Alsharairi, N.A. Therapeutic Potential of Gut Microbiota and Its Metabolite Short-Chain Fatty Acids in Neonatal Necrotizing Enterocolitis. Life 2023, 13, 561. [Google Scholar] [CrossRef]
- Wen, K.; Liu, F.; Li, G.; Bai, M.; Kocher, J.; Yang, X.; Wang, H.; Clark-Deener, S.; Yuan, L. Lactobacillus rhamnosus GG Dosage Affects the Adjuvanticity and Protection Against Rotavirus Diarrhea in Gnotobiotic Pigs. J. Pediatr. Gastroenterol. Nutr. 2015, 60, 834–843. [Google Scholar] [CrossRef]
- Wen, K.; Li, G.; Bui, T.; Liu, F.; Li, Y.; Kocher, J.; Lin, L.; Yang, X.; Yuan, L. High dose and low dose Lactobacillus acidophilus exerted differential immune modulating effects on T cell immune responses induced by an oral human rotavirus vaccine in gnotobiotic pigs. Vaccine 2012, 30, 1198–1207. [Google Scholar] [CrossRef]
- Sun, Z.; Li, H.; Li, Y.; Qiao, J. Lactobacillus salivarius, a Potential Probiotic to Improve the Health of LPS-Challenged Piglet Intestine by Alleviating Inflammation as Well as Oxidative Stress in a Dose-Dependent Manner During Weaning Transition. Front. Vet. Sci. 2020, 7, 547425. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, Y.; Stanton, C.; Ross, R.P.; Zhao, J.; Chen, W.; Yang, B. Dose-Response Efficacy and Mechanisms of Orally Administered Bifidobacterium breve CCFM683 on IMQ-Induced Psoriasis in Mice. Nutrients 2023, 15, 1952. [Google Scholar] [CrossRef] [PubMed]
- Mihatsch, W.A.; Vossbeck, S.; Eikmanns, B.; Hoegel, J.; Pohlandt, F. Effect of Bifidobacterium lactis on the incidence of nosocomial infections in very-low-birth-weight infants: A randomized controlled trial. Neonatology 2010, 98, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, T.; Majarikar, S.; Deshmukh, M.; Ananthan, A.; Balasubramanian, H.; Keil, A.; Patole, S. Probiotic sepsis in preterm neonates-a systematic review. Eur. J. Pediatr. 2022, 181, 2249–2262. [Google Scholar] [CrossRef]
- Preidis, G.A.; Weizman, A.V.; Kashyap, P.C.; Morgan, R.L. AGA Technical Review on the Role of Probiotics in the Management of Gastrointestinal Disorders. Gastroenterology 2020, 159, 708–738.e704. [Google Scholar] [CrossRef]
- van den Akker, C.H.P.; van Goudoever, J.B.; Shamir, R.; Domellöf, M.; Embleton, N.D.; Hojsak, I.; Lapillonne, A.; Mihatsch, W.A.; Berni Canani, R.; Bronsky, J.; et al. Probiotics and Preterm Infants: A Position Paper by the European Society for Paediatric Gastroenterology Hepatology and Nutrition Committee on Nutrition and the European Society for Paediatric Gastroenterology Hepatology and Nutrition Working Group for Probiotics and Prebiotics. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 664–680. [Google Scholar] [CrossRef]
- Patel, N.; Evans, K.; Berrington, J.; Szatkowski, L.; Costeloe, K.; Ojha, S.; Fleming, P.; Battersby, C. How frequent is routine use of probiotics in UK neonatal units? BMJ Paediatr. Open 2023, 7, e002012. [Google Scholar] [CrossRef]
- Hanna, M.; Ahmad, I.; Yanowitz, T.; Kim, J.; Hunter, C.; DiGeronimo, R.; Ahmad, K.A.; Sullivan, K.; Markel, T.A.; Hair, A.B.; et al. Current Patterns of Probiotic Use in U.S. Neonatal Intensive Care Units: A Multi-Institution Survey. Am. J. Perinatol. 2023; Online ahead of print. [Google Scholar] [CrossRef]
- Viswanathan, S.; Lau, C.; Akbari, H.; Hoyen, C.; Walsh, M.C. Survey and evidence based review of probiotics used in very low birth weight preterm infants within the United States. J. Perinatol. 2016, 36, 1106–1111. [Google Scholar] [CrossRef]
| Study ID | Selection | Comparability | Outcome | Total Score | |||||
|---|---|---|---|---|---|---|---|---|---|
| Representativeness of the exposed cohort | Selection of the non-exposed cohort | Ascertainment of exposure | Demonstration that outcome of interest was not present at start of study | Comparability of cohorts on the basis of the design or analysis | Assessment of outcome | Was follow-up long enough for outcomes to occur? | Adequacy of follow up of cohorts | ||
| Underwood_2013_USA | * | * | * | * | ** | * | * | * | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rath, C.; Athalye-Jape, G.; Rao, S.; Patole, S. Effect of Probiotic Dose Escalation on Gut Microbiota and Clinical Outcomes in Preterm Infants—A Systematic Review. Children 2023, 10, 1710. https://doi.org/10.3390/children10101710
Rath C, Athalye-Jape G, Rao S, Patole S. Effect of Probiotic Dose Escalation on Gut Microbiota and Clinical Outcomes in Preterm Infants—A Systematic Review. Children. 2023; 10(10):1710. https://doi.org/10.3390/children10101710
Chicago/Turabian StyleRath, Chandra, Gayatri Athalye-Jape, Shripada Rao, and Sanjay Patole. 2023. "Effect of Probiotic Dose Escalation on Gut Microbiota and Clinical Outcomes in Preterm Infants—A Systematic Review" Children 10, no. 10: 1710. https://doi.org/10.3390/children10101710






