Auditory Cortex Maturation and Language Development in Children with Hearing Loss and Additional Disabilities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Biomarker of Auditory Development
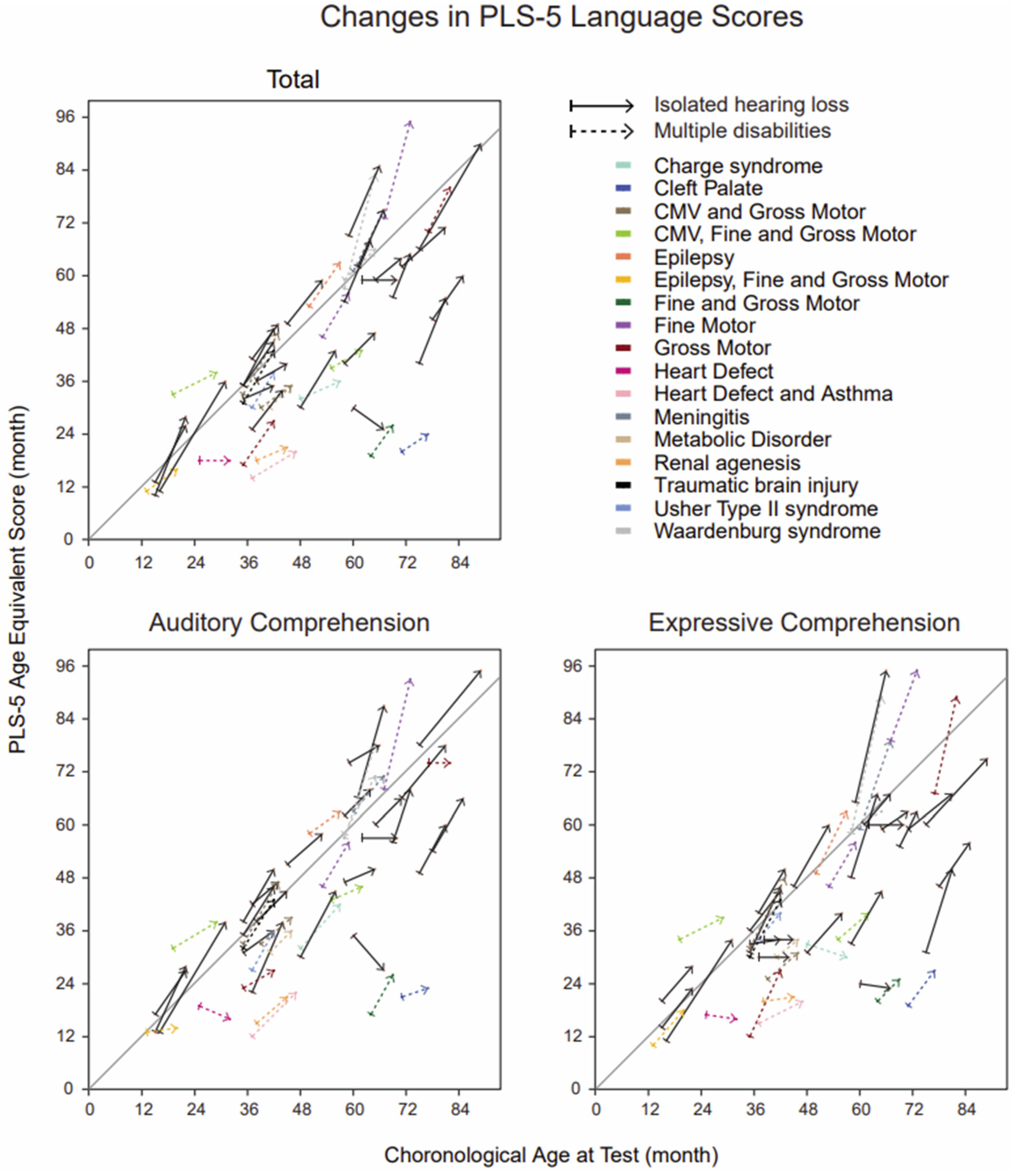
2.3. Language Assessment
2.4. Statistical Analysis
3. Results
3.1. Individual Latencies of P1 Cortical Auditory Evoked Responses
3.2. Group Differences in P1 Latencies
3.3. Group Differences in Language Outcomes
3.4. Regressions between P1 Latency and PLS-5
4. Discussion
Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tekin, M.; Arnos, K.S.; Pandya, A. Advances in hereditary deafness. Lancet 2001, 358, 1082–1090. [Google Scholar] [CrossRef]
- Finsterer, J.; Fellinger, J. Nuclear and mitochondrial genes mutated in nonsyndromic impaired hearing. Int. J. Pediatr. Otorhinolaryngol. 2005, 69, 621–647. [Google Scholar] [CrossRef] [PubMed]
- Home—OMIM. Available online: https://www.omim.org/ (accessed on 1 October 2023).
- Shearer, A.E.; Hildebrand, M.S.; Schaefer, A.M.; Smith, R.J. Genetic Hearing Loss Overview. In GeneReviews®; Adam, M.P., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. Available online: http://www.ncbi.nlm.nih.gov/books/NBK1434/ (accessed on 1 October 2023).
- Cupples, L.; Ching, T.Y.C.; Button, L.; Leigh, G.; Marnane, V.; Whitfield, J.; Gunnourie, M.; Martin, L. Language and speech outcomes of children with hearing loss and additional disabilities: Identifying the variables that influence performance at five years of age. Int. J. Audiol. 2018, 57 (Suppl. S2), S93–S104. [Google Scholar] [CrossRef]
- Picard, M. Children with permanent hearing loss and associated disabilities: Revisiting current epidemiological data and causes of deafness. Volta. Rev. 2004, 104, 221–236. [Google Scholar]
- Birman, C.S.; Elliott, E.J.; Gibson, W.P.R. Pediatric cochlear implants: Additional disabilities prevalence, risk factors, and effect on language outcomes. Otol. Neurotol. 2012, 33, 1347–1352. [Google Scholar] [CrossRef]
- Johnson, K.C.; Wiley, S. Cochlear Implantation in Children with Multiple Disabilties. In Clinical Management of Children with Cochlear Implants, 1st ed.; Plural Pub: San Diego, CA, USA, 2009; pp. 573–632. [Google Scholar]
- Guidelines for the Early Audiological Assessment and Management of Babies Referred from the Newborn Hearing Screening Programme. British Society of Audiology. Available online: https://www.thebsa.org.uk/resources/ (accessed on 10 November 2023).
- Clinical Guidance Document Assessment of Hearing in Infants and Young Children. American Academy of Audiology. Available online: https://www.audiology.org/wp-content/uploads/2021/05/Clin-Guid-Doc_Assess_Hear_Infants_Children_1.23.20.pdf (accessed on 10 November 2023).
- Gorga, M.P.; Johnson, T.A.; Kaminski, J.R.; Beauchaine, K.L.; Garner, C.A.; Neely, S.T. Using a combination of click- and tone burst–evoked auditory brain stem response measurements to estimate pure-tone thresholds. Ear Hear. 2006, 27, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Cohen, I.L.; Gardner, J.M.; Karmel, B.Z.; Phan, H.T.T.; Kittler, P.; Gomez, T.R.; Gonzalez, M.G.; Lennon, E.M.; Parab, S.; Barone, A. Neonatal brainstem function and 4-month arousal-modulated attention are jointly associated with autism. Autism Res. 2013, 6, 11–22. [Google Scholar] [CrossRef]
- Delgado, C.F.; Simpson, E.A.; Zeng, G.; Delgado, R.E.; Miron, O. Newborn auditory brainstem responses in children with developmental disabilities. J. Autism Dev. Disord. 2023, 53, 776–788. [Google Scholar] [CrossRef] [PubMed]
- Childhood Hearing Screening. American Speech-Language-Hearing Association. Available online: https://www.asha.org/practice-portal/professional-issues/childhood-hearing-screening (accessed on 11 November 2023).
- Aldè, M.; Di Berardino, F.; Ambrosetti, U.; Barozzi, S.; Piatti, G.; Consonni, D.; Zanetti, D.; Pignataro, L.; Cantarella, G. Hearing outcomes in preterm infants with confirmed hearing loss. Int. J. Pediatr. Otorhinolaryngol. 2022, 161, 111262. [Google Scholar] [CrossRef]
- Corrales, C.E.; Oghalai, J.S. Cochlear implant considerations in children with additional disabilities. Curr. Otorhinolaryngol. Rep. 2013, 1, 61–68. [Google Scholar] [CrossRef]
- Sharma, A.; Glick, H.; Campbell, J.; Biever, A. Central auditory development in children with hearing loss: Clinical relevance of the P1 CAEP biomarker in hearing-impaired children with multiple disabilities. Hear. Balance Commun. 2013, 11, 1–17. [Google Scholar]
- Sharma, A.; Dorman, M.F.; Spahr, A.J. A sensitive period for the development of the central auditory system in children with cochlear implants: Implications for age of implantation. Ear Hear. 2002, 23, 532–539. [Google Scholar] [CrossRef]
- Dorman, M.F.; Sharma, A.; Gilley, P.; Martin, K.; Roland, P. Central auditory development: Evidence from CAEP measurements in children fit with cochlear implants. J. Commun. Disord. 2007, 40, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Dorman, M.; Spahr, A.; Todd, N.W. Early cochlear implantation in children allows normal development of central auditory pathways. Ann. Otol. Rhinol. Laryngol. Suppl. 2002, 189, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Gilley, P.M.; Dorman, M.F.; Baldwin, R. Deprivation-induced cortical reorganization in children with cochlear implants. Int. J. Audiol. 2007, 46, 494–499. [Google Scholar] [CrossRef]
- Broomfield, S.J.; Bruce, I.A.; Henderson, L.; Ramsden, R.T.; Green, K.M.J. Cochlear implantation in children with syndromic deafness. Int. J. Pediatr. Otorhinolaryngol. 2013, 77, 1312–1316. [Google Scholar] [CrossRef]
- Mesallam, T.A.; Yousef, M.; Almasaad, A. Auditory and language skills development after cochlear implantation in children with multiple disabilities. Eur. Arch. Oto-Rhino-Laryngol. 2019, 276, 49–55. [Google Scholar] [CrossRef]
- Ganesh, V.; Ram, B.; Nandhan, R.; Kameswaran, M. A retrospective clinical audit of outcomes of cochlear implantation in children with multiple disabilities in comparison with normal implantees: A south Indian experience. Indian J. Otolaryngol. Head. Neck Surg. 2021, 73, 140–146. [Google Scholar] [CrossRef]
- Rawes, C.; Ngaage, L.M.; Mackenzie, R.; Martin, J.; Cordingley, A.; Raine, C. A review of the outcomes of children with designated additional needs receiving cochlear implantation for severe to profound hearing loss. Cochlear Implants Int. 2021, 22, 338–344. [Google Scholar] [CrossRef]
- Eggermont, J.J. On the rate of maturation of sensory evoked potentials. Electroencephalogr. Clin. Neurophysiol. 1988, 70, 293–305. [Google Scholar] [CrossRef]
- Wunderlich, J.L.; Cone-Wesson, B.K.; Shepherd, R. Maturation of the cortical auditory evoked potential in infants and young children. Hear. Res. 2006, 212, 185–202. [Google Scholar] [CrossRef]
- Ponton, C.W.; Eggermont, J.J.; Kwong, B.; Don, M. Maturation of human central auditory system activity: Evidence from multi-channel evoked potentials. Clin. Neurophysiol. 2000, 111, 220–236. [Google Scholar] [CrossRef]
- Pang, E.W.; Taylor, M.J. Tracking the development of the N1 from age 3 to adulthood: An examination of speech and non-speech stimuli. Clin. Neurophysiol. 2000, 111, 388–397. [Google Scholar] [CrossRef]
- Sharma, A.; Kraus, N.; McGee, T.J.; Nicol, T.G. Developmental changes in P1 and N1 central auditory responses elicited by consonant-vowel syllables. Electroencephalogr. Clin. Neurophysiol. 1997, 104, 540–545. [Google Scholar] [CrossRef]
- Eggermont, J.J.; Ponton, C.W. Auditory-evoked potential studies of cortical maturation in normal hearing and implanted children: Correlations with changes in structure and speech perception. Acta Otolaryngol. 2003, 123, 249–252. [Google Scholar] [CrossRef]
- 2012 Audiologic Guidelines for the Assessment of Hearing in Infants and Young Children. American Academy of Audiology. Available online: https://www.audiology.org/practice-guideline/2012-audiologic-guidelines-for-the-assessment-of-hearing-in-infants-and-young-children/ (accessed on 1 October 2023).
- Gilley, P.M.; Sharma, A.; Dorman, M.; Finley, C.C.; Panch, A.S.; Martin, K. Minimization of cochlear implant stimulus artifact in cortical auditory evoked potentials. Clin. Neurophysiol. 2006, 117, 1772–1782. [Google Scholar] [CrossRef]
- Sahli, A.S.; Belgin, E. Adaptation, validity, and reliability of the Preschool Language Scale-Fifth Edition (PLS-5) in the Turkish context: The Turkish Preschool Language Scale-5 (TPLS-5). Int. J. Pediatr. Otorhinolaryngol. 2017, 98, 143–149. [Google Scholar] [CrossRef]
- Zimmerman, I.L.; Steiner, V.G.; Pond, R.E. Preschool Language Scale, Fifth Edition. 2012. Available online: http://doi.apa.org/getdoi.cfm?doi=10.1037/t15141-000 (accessed on 27 August 2023).
- R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. 2022. Available online: https://www.R-project.org/ (accessed on 1 August 2020).
- R Studio Team. RStudio: Integrated Development Environment for R. Boston, MA: RStudio, PBC. 2022. Available online: http://www.rstudio.com/ (accessed on 1 August 2020).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67. Available online: http://www.jstatsoft.org/v67/i01/ (accessed on 27 August 2023). [CrossRef]
- Schielzeth, H.; Dingemanse, N.J.; Nakagawa, S.; Westneat, D.F.; Allegue, H.; Teplitsky, C.; Réale, D.; Dochtermann, N.A.; Garamszegi, L.Z.; Araya-Ajoy, Y.G. Robustness of linear mixed-effects models to violations of distributional assumptions. Methods Ecol. Evol. 2020, 11, 1141–1152. [Google Scholar] [CrossRef]
- le Cessie, S.; Goeman, J.J.; Dekkers, O.M. Who is afraid of non-normal data? Choosing between parametric and non-parametric tests. Eur. J. Endocrinol. 2020, 182, E1–E3. [Google Scholar] [CrossRef] [PubMed]
- Skovlund, E.; Fenstad, G.U. Should we always choose a nonparametric test when comparing two apparently nonnormal distributions? J. Clin. Epidemiol. 2001, 54, 86–92. [Google Scholar] [CrossRef]
- Fagerland, M.W. T-tests, non-parametric tests, and large studies--a paradox of statistical practice? BMC Med. Res. Methodol. 2012, 12, 78. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Wunderlich, J.L.; Cone-Wesson, B.K. Maturation of CAEP in infants and children: A review. Hear. Res. 2006, 212, 212–223. [Google Scholar] [CrossRef]
- Moore, J.K.; Linthicum, F.H. The human auditory system: A timeline of development. Int. J. Audiol. 2007, 46, 460–478. [Google Scholar] [CrossRef]
- Tooley, U.A.; Bassett, D.S.; Mackey, A.P. Environmental influences on the pace of brain development. Nat. Rev. Neurosci. 2021, 22, 372–384. [Google Scholar] [CrossRef]
- Sharma, A.; Dorman, M.F.; Kral, A. The influence of a sensitive period on central auditory development in children with unilateral and bilateral cochlear implants. Hear. Res. 2005, 203, 134–143. [Google Scholar] [CrossRef]
- Sharma, A.; Martin, K.; Roland, P.; Bauer, P.; Sweeney, M.H.; Gilley, P.; Dorman, M. P1 latency as a biomarker for central auditory development in children with hearing impairment. J. Am. Acad. Audiol. 2005, 16, 564–573. [Google Scholar] [CrossRef]
- Clarós, P.; Remjasz, A.; Clarós-Pujol, A.; Pujol, C.; Clarós, A.; Wiatrow, A. Long-term outcomes in down syndrome children after cochlear implantation: Particular issues and considerations. Otol. Neurotol. 2019, 40, 1278–1286. [Google Scholar] [CrossRef]
- Eshraghi, A.A.; Nazarian, R.; Telischi, F.F.; Martinez, D.; Hodges, A.; Velandia, S.; Cejas-Cruz, I.; Balkany, T.J.; Lo, K.; Lang, D. Cochlear implantation in children with autism spectrum disorder. Otol. Neurotol. 2015, 36, e121–e128. [Google Scholar] [CrossRef]
- Ferro, F.; Tozzi, A.E.; Erba, I.; Dall’Oglio, I.; Campana, A.; Cecchetti, C.; Geremia, C.; Rega, M.L.; Tontini, G.; Tiozzo, E.; et al. Impact of telemedicine on health outcomes in children with medical complexity: An integrative review. Eur. J. Pediatr. 2021, 180, 2389–2400. [Google Scholar] [CrossRef] [PubMed]
- Cardon, G.; Sharma, A. Central auditory maturation and behavioral outcome in children with auditory neuropathy spectrum disorder who use cochlear implants. Int. J. Audiol. 2013, 52, 577–586. [Google Scholar] [CrossRef]
- Lee, S.Y.; Han, J.H.; Song, H.K.; Kim, N.J.; Yi, N.; Kyong, J.S.; Choi, B.Y. Central auditory maturation and behavioral outcomes after cochlear implantation in prelingual auditory neuropathy spectrum disorder related to OTOF variants (DFNB9): Lessons from pilot study. PLoS ONE 2021, 16, e0252717. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.A.F.; Couto, M.I.V.; Magliaro, F.C.L.; Tsuji, R.K.; Bento, R.F.; de Carvalho, A.C.M.; Matas, C.G. Cortical maturation in children with cochlear implants: Correlation between electrophysiological and behavioral measurement. PLoS ONE 2017, 12, e0171177. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, M.I.; Silva, L.A.F.; Goffi Gomez, M.V.S.; Koji, T.R.; Bento, R.F.; Martinho de Carvalho, A.C.; Gentile, M.C. Central auditory nervous system stimulation through the cochlear implant use and its behavioral impacts: A longitudinal study of case series. Case Rep. Otolaryngol. 2021, 2021, 8888450. [Google Scholar] [CrossRef]
- Benasich, A.A.; Choudhury, N.; Friedman, J.T.; Realpe-Bonilla, T.; Chojnowska, C.; Gou, Z. The infant as a prelinguistic model for language learning impairments: Predicting from event-related potentials to behavior. Neuropsychologia 2006, 44, 396–411. [Google Scholar] [CrossRef]
- Choudhury, N.; Benasich, A.A. Maturation of auditory evoked potentials from 6 to 48 months: Prediction to 3 and 4 year language and cognitive abilities. Clin. Neurophysiol. 2011, 122, 320–338. [Google Scholar] [CrossRef]
- Choudhury, N.; Leppanen, P.H.T.; Leevers, H.J.; Benasich, A.A. Infant information processing and family history of specific language impairment: Converging evidence for RAP deficits from two paradigms. Dev. Sci. 2007, 10, 213–236. [Google Scholar] [CrossRef]



| All Children 1 | Complete Dataset | ||||
|---|---|---|---|---|---|
| Multiple Disabilities | Isolated Hearing Loss | Multiple Disabilities | Isolated Hearing Loss | ||
| Number of the patients | 36 | 26 | 22 | 23 | |
| Demographics | Age (mos; mean ± SD) | 41.3 ± 17.6 | 48 ± 20.0 | 46.1 ± 16.8 | 49.9 ± 19.6 |
| Gender (% of females) | 56.4 | 53.8 | 50 | 47.8 | |
| ASL (% of ASL Users) | 16.7 | 30.8 | 22.7 | 34.8 | |
| Hearing rehabilitation | Hearing aid (%) | 33.3 | 46.2 | 45.5 | 47.8 |
| Cochlear implant (%) 2 | 66.7 | 53.8 | 54.5 | 52.2 | |
| Age of Amplification (mos; mean ± SD) 3 | 15.1 ± 17.1 | 9.35 ± 13.21 | 19.18 ± 18.75 | 10.30 ± 13.78 | |
| Age of implantation (mos; mean ± SD) 4 | 24.3 ± 17.5 | 18.4 ± 13.3 | 32.2 ± 20.0 | 19.4 ± 14.2 | |
| Duration of Hearing Rehabilitation | 26.28 ± 19.1 | 38.69 ± 21.07 | 19.18 ± 20.32 | 10.30 ± 21.41 | |
| Speech therapy | In person | 52.8 | 50.0 | 54.5 | 52.2 |
| Telehealth | 38.9 | 42.3 | 40.9 | 39.1 | |
| Length (mos; mean ± SD) | 6.9 ± 1.4 | 7.6 ± 2.5 | 6.9 ± 1.4 | 7.6 ± 2.6 | |
| P1 Latency | ||||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Follow-Up | |||||||
| All Children | Abnormal P1 | Normal P1 | All Children | Abnormal P1 | Normal P1 | |||
| Baseline | PLS-5 Total | −0.25 | −0.34 | SUP | N/A | |||
| PLS-5 AC | −0.24 | −0.33 | SUP | |||||
| Language | PLS-5 EC | −0.28 | −0.34 | SUP | ||||
| score | Follow-up | PLS-5 Total | −0.33 * | −0.43 * | SUP | −0.43 *** | SUP | −0.64 *** |
| PLS-5 AC | −0.25 | −0.41 ~ | SUP | −0.38 * | SUP | −0.62 *** | ||
| PLS-5 EC | −0.29 | −0.37 | SUP | −0.39 ** | SUP | −0.61 *** | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamminmäki, S.; Cormier, K.; Davidson, H.; Grigsby, J.; Sharma, A. Auditory Cortex Maturation and Language Development in Children with Hearing Loss and Additional Disabilities. Children 2023, 10, 1813. https://doi.org/10.3390/children10111813
Lamminmäki S, Cormier K, Davidson H, Grigsby J, Sharma A. Auditory Cortex Maturation and Language Development in Children with Hearing Loss and Additional Disabilities. Children. 2023; 10(11):1813. https://doi.org/10.3390/children10111813
Chicago/Turabian StyleLamminmäki, Satu, Kayla Cormier, Hanna Davidson, Jim Grigsby, and Anu Sharma. 2023. "Auditory Cortex Maturation and Language Development in Children with Hearing Loss and Additional Disabilities" Children 10, no. 11: 1813. https://doi.org/10.3390/children10111813






