Prophylaxis of Patent Ductus Arteriosus with Paracetamol in Extremely Low Gestational Age Newborns (ELGANs): A Single-Institution Observational Study in Vietnam
Abstract
:1. Introduction
2. Methods
3. Comparison Cohort
4. Definitions
- (1)
- hsPDA was defined as a PDA diameter/birth weight (kg) of ≥ 1.4 (mm/kg) and one of the additional echocardiographic findings: LA/Ao > 1.4 and/or PDA diameter/LPA (left pulmonary artery) diameter > 0.5 [9,10]. Ductus that failed to meet these criteria were non-hsPDA. No PDA was defined as no flow through the ductus.
- (2)
- sPDA (symptomatic PDA) was defined as the presence of hsPDA and one of the following conditions attributable to the hsPDA, including the following [2,3]: (1) the need for vasopressor support; (2) a persistent or increasing need for ventilatory support and supplemental oxygen; (3) prerenal renal failure with metabolic acidosis; (4) intraventricular hemorrhage (IVH) Papile’s grade of ≥II [11] on ultrasound; (5) renal failure, defined as serum creatinine that is >1.5 mg/dL and/or serum creatinine rise of >0.3 mg/dL or serum creatinine rise of >1.5–1.9* the lowest previous serum creatinine value [12]; (6) necrotizing enterocolitis (NEC) ≥ Bell’s stage 2 or greater [13]; and (7) pulmonary hemorrhage. These were defined by meeting all three of following criteria: (1) fresh blood in the trachea or the endotracheal; (2) a rapid clinical deterioration need for intubation or increase FiO2 ≥ 10% for children on mechanical ventilation, decreased hematocrit > 10%; and (3) chest radiograph—patchy infiltrates or complete opacification).
5. Clinical Management
Statistical Analyses
6. Results
6.1. Patient Characteristics
6.2. Prophylactic Paracetamol Treatment
6.3. Outcomes of Prophylactic Paracetamol Treatment
6.4. Morbidities and Mortality
7. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Prevention and Management Protocol for Patent Ductus Arteriosus in Preterm Infants below 35 Weeks’ Gestational Age at Our Unit
- I.
- Overview
- II.
- Diagnosis
- 1.
- Clinical symptoms: often asymptomatic.
- Cardiac symptoms: Hyperdynamic precordial impulse, precordial systolic murmur, tachycardia, bounding pulses, widened pulse pressure, hypotension, cardiac failure, inability to discontinue mechanical ventilation without explained causes. Congestive heart failure usually appears after one week of age: cardiomegaly, pulmonary congestion, and hepatomegaly.
- Organ effects: Lungs (worsening respiratory status, increased pulmonary blood flow, pulmonary edema, pulmonary hemorrhage), gastrointestinal tract (poor feeding, necrotizing enterocolitis), kidneys (oliguria, increased blood creatinine), brain (intraventricular hemorrhage).
- PDA reopening: Worsening clinical status with increased respiratory support needs, significant apnea, inability to wean from mechanical ventilation, unexplained kidney failure, poor feeding or necrotizing enterocolitis, and new onset intraventricular hemorrhage.
- 2.
- Work-up:
- Echocardiography:
- -
- Screening: performed between 48 and 72 h for preterm infants.
- -
- Evaluation: whenever there are clinical symptoms.
- -
- Goals:
- ✓
- Exclude associated heart defects: PDA-dependent critical CHDs (especially coarctation), pulmonary stenosis.
- ✓
- Assess PDA diameter, ductal flow pattern, left atrium to aorta ratio (LA/Ao), and presence of reversed diastolic flow in the Superior Mesenteric Artery, descending aorta, or middle cranial artery.
- Chest X-ray: heart size, lung fields, associated injuries.
- Lab tests before pharmaceutical PDA closure:
- -
- For ibuprofen: blood test, renal function, stool examination, cranial ultrasonography, and abdominal ultrasound;
- -
- For Paracetamol: AST and ALT.
- Preoperative tests for preparing PDA ligation: ECG, complete blood count, renal function, electrolyte panel, and liver function.
- 3.
- Diagnosis: determined through echocardiography, and in case of surgical PDA ligation, results from at least two experienced cardiologists are necessary.
- ✓
- Hyperdynamic precordial impulse.
- ✓
- Tachycardia without explained causes.
- ✓
- Widened pulse pressure (Diastolic Blood Pressure (DBP) < ½ Systolic Blood Pressure (SBP)).
- ✓
- Left atrium diameter to aortic diameter ratio (LA/Ao) > 1.4.
- ✓
- Reversed diastolic flows in the superior mesenteric artery, descending aorta, or middle cerebral artery.
- ✓
- Increased respiratory support needs or inability to discontinue mechanical ventilation without explained causes.
- ✓
- Kidney failure with metabolic acidosis without explained causes.
- ✓
- Progressive necrotizing enterocolitis grade ≥ 2 and/or new onset of intraventricular hemorrhage grade ≥ 2.
- III.
- Treatment
- 1.
- Prevention of sPDA
- Indications: preterm birth ≤ 27 weeks; respiratory distress syndrome requiring surfactant therapy;
- Timing: within the first 24 h after birth;
- Dosage: IV Paracetamol, loading dose 20 mg/kg, followed by 7.5 mg/kg every 6 h for 4 days.
- 2
- Management of PDA
- a.
- Conservative management in the first week of life for all infants with respiratory distress:
- b.
- Medical intervention:
- c.
- Surgical intervention (PDA ligation):
- sPDA with contraindications to or failure of medical intervention;
- sPDA-related severe respiratory failure, progressive NEC grade II–III, or new onset of IVH grade III–IV.
- IV.
- Monitoring
- Monitor for PDA reopening (see clinical symptoms above).
| Recommendations | GRADE |
| Conservative management for all preterm infants with respiratory distress. If diuretics are needed, thiazides are preferable to loop diuretics. | 1B |
| Conservative management for all preterm infants with respiratory distress. If diuretics are needed, thiazides are preferable to loop diuretics. | 1B |
| Ibuprofen preferable over Indomethacin for PDA closure. | 1B |
| In preterm infants with respiratory distress and mechanical ventilation lasting > 1 week, drug closure is preferred over conservative management. | 2B |
| Surgical PDA ligation indicated in infants with symptomatic PDA, high ventilation settings, and pharmaceutical intervention failure. | 2B |
| Indomethacin not recommended for prophylaxis to reduce PDA in preterm infants. | 1B |
| Paracetamol recommended for prophylaxis in infants ≤ 32 weeks to reduce PDA occurrence. | 2C |
| Note: Strength of recommendation. 1: strong recommendation, 2: weak recommendation. Level of evidence—A: high quality, B: moderate quality, C: low quality. | |
Appendix B. Prevention and Management Flowchart for Patent Ductus Arteriosus in Preterm Infants with Gestational Age < 35 Weeks in Our Unit

References
- Sung, S.I.; Chang, Y.S.; Kim, J.; Choi, J.H.; Ahn, S.Y.; Park, W.S. Natural evolution of ductus arteriosus with noninterventional conservative management in extremely preterm infants born at 23–28 weeks of gestation. PLoS ONE 2019, 14, e0212256. [Google Scholar] [CrossRef] [PubMed]
- Jasani, B.; Mitra, S.; Shah, P.S. Paracetamol (acetaminophen) for patent ductus arteriosus in preterm or low birth weight infants. Cochrane Database Syst. Rev. 2022, 4, CD010061. [Google Scholar] [CrossRef]
- Mitra, S.; de Boode, W.P.; Weisz, D.E.; Shah, P.S. Interventions for patent ductus arteriosus (PDA) in preterm infants: An overview of Cochrane Systematic Reviews. Cochrane Database Syst. Rev. 2023, 4, CD013588. [Google Scholar] [PubMed]
- Sweet, D.G.; Carnielli, V.; Greisen, G.; Hallman, M.; Ozek, E.; Pas, A.T.; Plavka, R.; Roehr, C.C.; Saugstad, O.D.; Simeoni, U.; et al. European Consensus Guidelines on the Management of Respiratory Distress Syndrome–2019 Update. Neonatology 2019, 115, 432–450. [Google Scholar] [CrossRef] [PubMed]
- Su, B.-H.; Lin, H.-Y.; Chiu, H.-Y.; Tsai, M.-L.; Chen, Y.-T.; Lu, I.-C. Therapeutic strategy of patent ductus arteriosus in extremely preterm infants. Pediatr. Neonatol. 2020, 61, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Silahli, M.; Gokmen, Z.; Tekin, M. Prophylactic intravenous paracetamol use in extremely premature infants for patent ductus arteriosus. J. Basic. Clin. Physiol. Pharmacol. 2020, 32, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Thu, N.Q.; Thu, T.T.H.; Tam, P.T.T. Characteristics of patent ductus arteriosus in preterms with respiratory distress syndrome at Children’s hospital 1. Ho Chi Minh City J. Med. 2018, 22, 197–203. [Google Scholar]
- Tam, P.T.T.; Tinh, N.T. Mortality and cost of treatment for extremely low gestational age neonates at NICU of Children’s hospital 1 in Ho Chi Minh City–Vietnam. Ho Chi Minh City J. Med. 2019, 23, 59–66. [Google Scholar]
- Hamrick, S.E.; Hansmann, G. Patent ductus arteriosus of the preterm infant. Pediatrics 2010, 125, 1020–1030. [Google Scholar] [CrossRef] [PubMed]
- Skinner, J. Diagnosis of patent ductus arteriosus. Semin. Neonatol. 2001, 6, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Atienza-Navarro, I.; Alves-Martinez, P.; Lubian-Lopez, S.; Garcia-Alloza, M. Germinal Matrix-Intraventricular Hemorrhage of the Preterm Newborn and Preclinical Models: Inflammatory Considerations. Int. J. Mol. Sci. 2020, 21, 8343. [Google Scholar] [CrossRef] [PubMed]
- Mian, A.N.; Askenazi, D.J.; Mhanna, M.J. Therapeutic Options for Neonatal Acute Kidney Injury (AKI). Curr. Treat. Options Pediatr. 2016, 2, 69–81. [Google Scholar] [CrossRef]
- Bell, M.J.; Ternberg, J.L.; Feigin, R.D.; Keating, J.P.; Marshall, R.; Barton, L.; Brotherton, T. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann. Surg. 1978, 187, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hundscheid, T.; Broek, M.v.D.; van der Lee, R.; de Boode, W.P. Understanding the pathobiology in patent ductus arteriosus in prematurity—Beyond prostaglandins and oxygen. Pediatr. Res. 2019, 86, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Canpolat, F.E.; Şimşek, G.K.; Webbe, J.; Büyüktiryaki, M.; Karaçağlar, N.B.; Elbayiyev, S.; Kutman, H.G.K. Late Administration of Surfactant May Increase the Risk of Patent Ductus Arteriosus. Front. Pediatr. 2020, 8, 130. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.O.O.; Ahmed, S.M.I.; Khidir, R.J.Y.; Shaheen, M.T.H.A.; Adam, M.H.M.; Ibrahim, B.A.Y.; Elmahdi, E.O.A.; Farah, A.S.M. Outcomes of neonatal hypothermia among very low birth weight infants: A Meta-analysis. Matern. Health Neonatol. Perinatol. 2021, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Roofthooft, D.W.E.; van Beynum, I.M.; de Klerk, J.C.A.; van Dijk, M.; Anker, J.N.v.D.; Reiss, I.K.M.; Tibboel, D.; Simons, S.H.P. Limited effects of intravenous paracetamol on patent ductus arteriosus in very low birth weight infants with contraindications for ibuprofen or after ibuprofen failure. Eur. J. Pediatr. 2015, 174, 1433–1440. [Google Scholar] [CrossRef] [PubMed]
- Juujärvi, S.; Saarela, T.; Hallman, M.; Aikio, O. Intravenous paracetamol was associated with closure of the ductus arteriosus in extremely premature infants. Acta Paediatr. 2018, 107, 605–610. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Prophylactic n = 58 | Historical * n = 47 | p-Value |
|---|---|---|---|
| Gender, male | 20 (34.5) | 22 (46.8) | 0.20 |
| Gestational age (weeks) (min–max) | 25.1 ± 1.1 (23+0/7–27+0/7) | 26 ± 0.9 (24+0/7–27+0/7) | 0.29 |
| Birth weight (g) (min–max) | 766.7 ± 183.5 (400–1300) | 871.2 ± 134.3 (500–1400) | 0.19 |
| Cesarian section | 5 (8.6) | 4 (8.5) | 0.98 |
| Multiple births | 28 (48.3) | 19 (40.4) | 0.42 |
| PROM ≥ 18 h | 7 (12.1) | 1 (2.1) | 0.06 |
| Antental steroids | 16 (27.6) | 12 (25.5) | 0.81 |
| 5 min APGAR score ≤ 6 | 38 (65.6) | 29 (61.7) | 0.69 |
| Delivery room resuscitation: | |||
| 7 (12.1) | 5 (10.6) | 0.84 |
| 32 (55.2) | 24 (51.1) | |
| 19 (32,7) | 18 (38.3) | |
| Surfactant administration in the delivery room | 0 (0.0) | 0 (0.0) | - |
| Respiratory support during transportation: | |||
| 6 (10.3) | 2 (4.3) | 0.50 |
| 27 (46.6) | 23 (48.9) | |
| 25 (43.1) | 22 (46.8) | |
| Temperature at admission (°C) | 33.6 ± 1.8 | 34.2 ± 1.2 | 0.43 |
| MV within the first 24 h | 51 (87.9) | 38 (80.9) | 0.32 |
| Surfactant administration: | |||
| 29 (50) | 20 (42.6) | 0.45 |
| 29 (50) | 17 (36.2) | 0.16 |
| Age at start surfactant (h) | 6 (4–8) | 6 (4–9) | 0.75 |
| Age at start IV paracetamol (h) | 9 (6–20) | 10 (4–21) | 0.58 |
| Parameters | sPDA | p-Value | |
|---|---|---|---|
| No n = 24 | Yes n = 34 | ||
| Gender, male | 9 (37.5) | 11 (32.4) | 0.68 |
| GA (weeks) | 25.1 ± 1.1 | 25.1 ± 1.1 | 0.71 |
| BW (g) | 768.1 ± 185.1 | 765.7 ± 185.1 | 0.78 |
| SGA | 1 (4.2) | 2 (5.9) | 0.63 |
| Cesarian delivery | 2 (8.3) | 3 (8.8) | 0.66 |
| Multiple pregnancies | 15 (62.5) | 13 (38.2) | 0.07 |
| PROM ≥ 18 h | 2 (8.3) | 5 (14.7) | 0.72 |
| Chorioamnionitis | 1 (4.2) | 2 (5.9) | 0.96 |
| No antenatal steroids | 16 (66.7) | 20 (58.8) | 0.54 |
| 5 min APGAR score ≤ 6 | 15 (68.2) | 23 (79.3) | 0.36 |
| Resuscitation via ETT | 7 (29.2) | 12 (35.3) | 0.62 |
| Temperature at admission | 33.7 ± 1.7 | 33.5 ± 1.8 | 0.57 |
| MV within the first 24 h | 20 (83.3) | 31 (91.2) | 0.43 |
| Age at start surfactant (h) | 6 (4–7) | 6 (4–8) | 0.79 |
| Age at start IV paracetamol (h) | 8 (6–18) | 10 (5–20) | 0.69 |
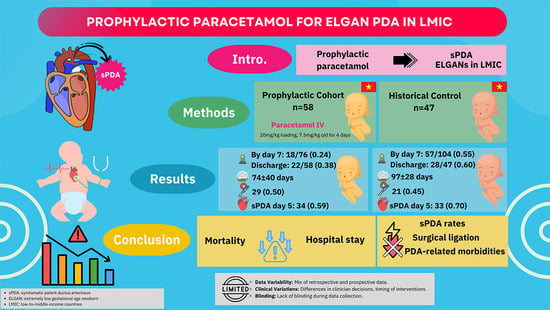
| Outcome | Prophylactic n = 58 | Historical * n = 47 | p-Value |
|---|---|---|---|
| sPDA on 5 days of life | 34 (58.6) | 33 (70.2) | 0.22 |
| Hypotension requiring vasopressor support within the first 3 days of life | 24 (41.4) | 19 (40.4) | 0.92 |
| Pulmonary hemorrhage | 18 (31.0) | 17 (36.2) | 0.58 |
| Peri/intraventricular hemorrhage | 28 (48.3) | 24 (51.0) | 0.78 |
| Peri/intraventricular hemorrhage, Grade ≥ 2 | 10 (17.2) | 16 (34.0) | 0.05 |
| Necrotizing enterocolitis, Grade ≥ 2 | 6 (10.3) | 9 (19.1) | 0.20 |
| Renal failure | 9 (15.5) | Not available | - |
| BPD (grade moderate to severe) | 20 (34.5) | 24 (51.1) | 0.09 |
| ROP was treated with laser or Avastin | 12/44 (27.3) | 7/36 (19.4) | 0.41 |
| Respiratory support duration (days) | 62 ± 38 | 64 ± 36 | 0.71 |
| Invasive mechanical ventilation duration (days) | 36 ± 30 | 21 ± 25 | 0.20 |
| Hospital stay (day) | 74 ± 40 | 97 ± 28 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.T.; Nguyen, D.T.N.; Pham, T.T.T.; Oei, J.-L. Prophylaxis of Patent Ductus Arteriosus with Paracetamol in Extremely Low Gestational Age Newborns (ELGANs): A Single-Institution Observational Study in Vietnam. Children 2023, 10, 1934. https://doi.org/10.3390/children10121934
Nguyen TT, Nguyen DTN, Pham TTT, Oei J-L. Prophylaxis of Patent Ductus Arteriosus with Paracetamol in Extremely Low Gestational Age Newborns (ELGANs): A Single-Institution Observational Study in Vietnam. Children. 2023; 10(12):1934. https://doi.org/10.3390/children10121934
Chicago/Turabian StyleNguyen, Tinh Thu, Dung Thi Ngoc Nguyen, Tam Thi Thanh Pham, and Ju-Lee Oei. 2023. "Prophylaxis of Patent Ductus Arteriosus with Paracetamol in Extremely Low Gestational Age Newborns (ELGANs): A Single-Institution Observational Study in Vietnam" Children 10, no. 12: 1934. https://doi.org/10.3390/children10121934