Pharmacoepidemiological Analysis of Oral Contraceptive Use in Adolescents in a German Longitudinal Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Inclusion Criteria
2.3. Data Collection
2.4. Data Processing
2.5. Statistical Analysis
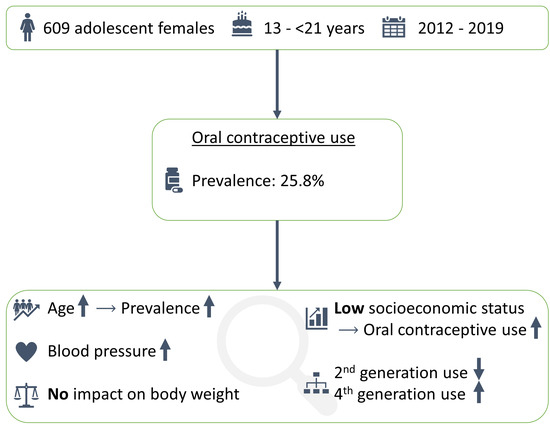
3. Results
3.1. Characteristics of the Study Population
3.2. Prevalence of OC Use
3.3. Median Age of Onset of OC Use
3.4. Changes in Active Ingredients of OC
3.5. Associations between OC Use and Blood Pressure
3.6. Body Weight and OC Use
4. Discussion
4.1. Prevalence of OC use
4.2. OC Use and SES
4.3. Changes in Generations of OC Used between 2013 and 2019
4.4. Associations between OC Use and Blood Pressure
4.5. Associations with Body Weight Gain
4.6. Limitations
4.7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- United Nations, Population Division. Contraceptive Use by Method 2019. Available online: https://www.un-ilibrary.org/content/books/9789210046527 (accessed on 10 February 2023).
- Ponce de Leon, R.G.; Ewerling, F.; Serruya, S.J.; Silveira, M.F.; Sanhueza, A.; Moazzam, A.; Becerra-Posada, F.; Coll, C.V.N.; Hellwig, F.; Victora, C.G.; et al. Contraceptive use in Latin America and the Caribbean with a focus on long-acting reversible contraceptives: Prevalence and inequalities in 23 countries. Lancet Glob. Health 2019, 7, e227–e235. [Google Scholar] [CrossRef] [Green Version]
- Alkema, L.; Kantorova, V.; Menozzi, C.; Biddlecom, A. National, regional, and global rates and trends in contraceptive prevalence and unmet need for family planning between 1990 and 2015: A systematic and comprehensive analysis. Lancet 2013, 381, 1642–1652. [Google Scholar] [CrossRef]
- Lindh, I.; Skjeldestad, F.E.; Gemzell-Danielsson, K.; Heikinheimo, O.; Hognert, H.; Milsom, I.; Lidegaard, Ø. Contraceptive use in the Nordic countries. Acta Obstet. Gynecol. Scand. 2017, 96, 19–28. [Google Scholar] [CrossRef] [Green Version]
- Du, Y.; Rosner, B.M.; Knopf, H.; Schwarz, S.; Dören, M.; Scheidt-Nave, C. Hormonal contraceptive use among adolescent girls in Germany in relation to health behavior and biological cardiovascular risk factors. J. Adolesc. Health 2011, 48, 331–337. [Google Scholar] [CrossRef] [Green Version]
- Machado, A.K.F.; Gräf, D.D.; Höfs, F.; Hellwig, F.; Barros, K.S.; Moreira, L.R.; Crespo, P.A.; Silveira, M.F. Prevalence and inequalities in contraceptive use among adolescents and young women: Data from a birth cohort in Brazil. Cad. Saude Publica 2021, 37, e00335720. [Google Scholar] [CrossRef]
- Poncet, L.C.; Huang, N.; Rei, W.; Lin, Y.-C.; Chen, C.-Y. Contraceptive use and method among immigrant women in France: Relationship with socioeconomic status. Eur. J. Contracept. Reprod. Health Care 2013, 18, 468–479. [Google Scholar] [CrossRef]
- Frost, J.J.; Darroch, J.E. Factors associated with contraceptive choice and inconsistent method use, United States, 2004. Perspect. Sex. Reprod. Health 2008, 40, 94–104. [Google Scholar] [CrossRef] [Green Version]
- Ziller, M.; Rashed, A.N.; Ziller, V.; Kostev, K. The prescribing of contraceptives for adolescents in German gynecologic practices in 2007 and 2011: A retrospective database analysis. J. Pediatr. Adolesc. Gynecol. 2013, 26, 261–264. [Google Scholar] [CrossRef]
- Arowojolu, A.O.; Gallo, M.F.; Lopez, L.M.; Grimes, D.A. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst. Rev. 2012, 7, CD004425. [Google Scholar] [CrossRef]
- De Bastos, M.; Stegeman, B.H.; Rosendaal, F.R.; van Hylckama Vlieg, A.; Helmerhorst, F.M.; Stijnen, T.; Dekkers, O.M. Combined oral contraceptives: Venous thrombosis. Cochrane Database Syst. Rev. 2014, 3, CD010813. [Google Scholar] [CrossRef]
- Dragoman, M.V.; Tepper, N.K.; Fu, R.; Curtis, K.M.; Chou, R.; Gaffield, M.E. A systematic review and meta-analysis of venous thrombosis risk among users of combined oral contraception. Int. J. Gynaecol. Obstet. 2018, 141, 287–294. [Google Scholar] [CrossRef] [Green Version]
- O’Mahony, L.; Liddy, A.-M.; Barry, M.; Bennett, K. Hormonal contraceptive use in Ireland: Trends and co-prescribing practices. Br. J. Clin. Pharmacol. 2015, 80, 1315–1323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kharbanda, E.O.; Parker, E.D.; Sinaiko, A.R.; Daley, M.F.; Margolis, K.L.; Becker, M.; Sherwood, N.E.; Magid, D.J.; O’Connor, P.J. Initiation of oral contraceptives and changes in blood pressure and body mass index in healthy adolescents. J. Pediatr. 2014, 165, 1029–1033. [Google Scholar] [CrossRef] [Green Version]
- De Morais, T.L.; Giribela, C.; Nisenbaum, M.G.; Guerra, G.; Mello, N.; Baracat, E.; Consolim-Colombo, F.M. Effects of a contraceptive containing drospirenone and ethinylestradiol on blood pressure, metabolic profile and neurohumoral axis in hypertensive women at reproductive age. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 182, 113–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nisenbaum, M.G.; de Melo, N.R.; Giribela, C.R.G.; de Morais, T.L.; Guerra, G.M.; de Angelis, K.; Mostarda, C.; Baracat, E.C.; Consolim-Colombo, F.M. Effects of a contraceptive containing drospirenone and ethinyl estradiol on blood pressure and autonomic tone: A prospective controlled clinical trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 175, 62–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, Y.; Melchert, H.-U.; Schäfer-Korting, M. Use of oral contraceptives in Germany: Prevalence, determinants and use-associated health correlates. Results of National Health Surveys from 1984 to 1999. Eur. J. Obstet. Gynecol. Reprod. Biol. 2007, 134, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Nichols, M.; Robinson, G.; Bounds, W.; Newman, B.; Guillebaud, J. Effect of four combined oral contraceptives on blood pressure in the pill-free interval. Contraception 1993, 47, 367–376. [Google Scholar] [CrossRef]
- Le-Ha, C.; Beilin, L.J.; Burrows, S.; Huang, R.-C.; Oddy, W.H.; Hands, B.; Mori, T.A. Oral contraceptive use in girls and alcohol consumption in boys are associated with increased blood pressure in late adolescence. Eur. J. Prev. Cardiol. 2013, 20, 947–955. [Google Scholar] [CrossRef] [Green Version]
- Giribela, C.R.G.; Consolim-Colombo, F.M.; Nisenbaum, M.G.; de Moraes, T.L.; Giribela, A.H.G.; Baracat, E.C.; de Melo, N.R. Effects of a combined oral contraceptive containing 20 mcg of ethinylestradiol and 3 mg of drospirenone on the blood pressure, renin-angiotensin-aldosterone system, insulin resistance, and androgenic profile of healthy young women. Gynecol. Endocrinol. 2015, 31, 912–915. [Google Scholar] [CrossRef]
- Gallo, M.F.; Lopez, L.M.; Grimes, D.A.; Carayon, F.; Schulz, K.F.; Helmerhorst, F.M. Combination contraceptives: Effects on weight. Cochrane Database Syst. Rev. 2014, 1, CD003987. [Google Scholar] [CrossRef]
- Lopez, L.M.; Ramesh, S.; Chen, M.; Edelman, A.; Otterness, C.; Trussell, J.; Helmerhorst, F.M. Progestin-only contraceptives: Effects on weight. Cochrane Database Syst. Rev. 2016, 8, CD008815. [Google Scholar] [CrossRef]
- Slof-Op ’t Landt, M.C.T.; van Furth, E.F.; van Beijsterveldt, C.E.M.; Bartels, M.; Willemsen, G.; de Geus, E.J.; Ligthart, L.; Boomsma, D.I. Prevalence of dieting and fear of weight gain across ages: A community sample from adolescents to the elderly. Int. J. Public Health 2017, 62, 911–919. [Google Scholar] [CrossRef]
- Neininger, M.P.; Jeschke, S.; Kiesel, L.M.; Bertsche, T.; Bertsche, A. Physicians’ perspectives on adverse drug reactions in pediatric routine care: A survey. World J. Pediatr. 2022, 18, 50–58. [Google Scholar] [CrossRef]
- Quante, M.; Hesse, M.; Döhnert, M.; Fuchs, M.; Hirsch, C.; Sergeyev, E.; Casprzig, N.; Geserick, M.; Naumann, S.; Koch, C.; et al. The LIFE child study: A life course approach to disease and health. BMC Public Health 2012, 12, 1021. [Google Scholar] [CrossRef] [Green Version]
- Poulain, T.; Baber, R.; Vogel, M.; Pietzner, D.; Kirsten, T.; Jurkutat, A.; Hiemisch, A.; Hilbert, A.; Kratzsch, J.; Thiery, J.; et al. The LIFE Child study: A population-based perinatal and pediatric cohort in Germany. Eur. J. Epidemiol. 2017, 32, 145–158. [Google Scholar] [CrossRef]
- Hardin, A.P.; Hackell, J.M. Age Limit of Pediatrics. Pediatrics 2017, 140, e2017–e2151. [Google Scholar] [CrossRef] [Green Version]
- Herzig, M.; Bertsche, A.; Kiess, W.; Bertsche, T.; Neininger, M.P. Medicine and supplement use in infants, children, and adolescents depends on sex, age, and socioeconomic status: Results of a German longitudinal population-based cohort study (LIFE Child). Eur. J. Pediatr. 2022, 181, 2991–3003. [Google Scholar] [CrossRef]
- Lampert, T.; Müters, S.; Stolzenberg, H.; Kroll, L.E. Messung des sozioökonomischen Status in der KiGGS-Studie : Erste Folgebefragung (KiGGS Welle 1). Bundesgesundheitsblatt Gesundh. Gesundh. 2014, 57, 762–770. [Google Scholar] [CrossRef]
- Tricotel, A.; Raguideau, F.; Collin, C.; Zureik, M. Estimate of venous thromboembolism and related-deaths attributable to the use of combined oral contraceptives in France. PLoS ONE 2014, 9, e93792. [Google Scholar] [CrossRef] [Green Version]
- Schroeder, M.A. Diagnosing and dealing with multicollinearity. West. J. Nurs. Res. 1990, 12, 175–184; discussion 184-7. [Google Scholar] [CrossRef]
- Ribeiro, C.C.M.; Shimo, A.K.K.; Lopes, M.H.B.d.M.; Lamas, J.L.T. Effects of different hormonal contraceptives in women’s blood pressure values. Rev. Bras. Enferm. 2018, 71, 1453–1459. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: New York, NY, USA, 1988; ISBN 9781134742707. [Google Scholar]
- Bellizzi, S.; Mannava, P.; Nagai, M.; Sobel, H.L. Reasons for discontinuation of contraception among women with a current unintended pregnancy in 36 low and middle-income countries. Contraception 2020, 101, 26–33. [Google Scholar] [CrossRef]
- Woolley, N.O.; Macinko, J. Association between sociodemographic characteristics and sexual behaviors among a nationally representative sample of adolescent students in Brazil. Cad. Saude Publica 2019, 35, e00208517. [Google Scholar] [CrossRef]
- Gesetz zur Verbesserung der Information Über Einen Schwangerschaftsabbruch [Act on the Improvement of Information on Abortion]. Available online: http://www.bgbl.de/xaver/bgbl/start.xav?startbk=Bundesanzeiger_BGBl&jumpTo=bgbl119s0350.pdf (accessed on 21 November 2022).
- Arie, S. European agency defends existing advice on later generation contraceptive pills. BMJ 2013, 346, f279. [Google Scholar] [CrossRef] [PubMed]
- Deutsche Gesellschaft für Gynäkologie und Geburtshilfe e.V. S3-Leitlinie Hormonelle Empfängnisverhütung. Available online: https://register.awmf.org/de/leitlinien/detail/015-015 (accessed on 12 December 2022).
- World Health Organization. Medical Eligibility Criteria for Contraceptive Use, 5th ed.; World Health Organization: Geneva, Switzerland, 2015; ISBN 9789241549158. [Google Scholar]
- Heinrich, J.; Brüske, I.; Schnappinger, M.; Standl, M.; Flexeder, C.; Thiering, E.; Tischer, C.; Tiesler, C.M.T.; Kohlböck, G.; Wenig, C.M.; et al. Die zwei deutschen Geburtskohorten GINIplus und LISAplus. Bundesgesundheitsblatt Gesundh. Gesundh. 2012, 55, 864–874. [Google Scholar] [CrossRef]
- Jacobsen, T.N.; Nohr, E.A.; Frydenberg, M. Selection by socioeconomic factors into the Danish National Birth Cohort. Eur. J. Epidemiol. 2010, 25, 349–355. [Google Scholar] [CrossRef] [Green Version]
- Jaddoe, V.W.V.; van Duijn, C.M.; van der Heijden, A.J.; Mackenbach, J.P.; Moll, H.A.; Steegers, E.A.P.; Tiemeier, H.; Uitterlinden, A.G.; Verhulst, F.C.; Hofman, A. The Generation R Study: Design and cohort update 2010. Eur. J. Epidemiol. 2010, 25, 823–841. [Google Scholar] [CrossRef]
- Boyd, A.; Golding, J.; Macleod, J.; Lawlor, D.A.; Fraser, A.; Henderson, J.; Molloy, L.; Ness, A.; Ring, S.; Davey Smith, G. Cohort Profile: The ‘children of the 90s’—The index offspring of the Avon Longitudinal Study of Parents and Children. Int. J. Epidemiol. 2013, 42, 111–127. [Google Scholar] [CrossRef] [Green Version]
- Gustavson, K.; von Soest, T.; Karevold, E.; Røysamb, E. Attrition and generalizability in longitudinal studies: Findings from a 15-year population-based study and a Monte Carlo simulation study. BMC Public Health 2012, 12, 918. [Google Scholar] [CrossRef] [Green Version]

| n | % | |
|---|---|---|
| Total | 609 | 100.0 |
| Age group | ||
| 13 | 93 | 15.3 |
| 14 | 87 | 14.3 |
| 15 | 128 | 21.0 |
| 16 | 98 | 16.1 |
| 17 | 97 | 15.9 |
| 18 | 63 | 10.3 |
| 19 | 25 | 4.1 |
| 20 | 18 | 3.0 |
| SES a | ||
| Low | 69 | 11.3 |
| Middle | 308 | 50.6 |
| High | 139 | 22.8 |
| Unknown | 93 | 15.3 |
| Monthly household equivalent income b | ||
| EUR ≤ 787 | 79 | 13.0 |
| EUR 788–999 | 58 | 9.5 |
| EUR 1000–1190 | 73 | 12.0 |
| EUR 1191–1400 | 62 | 10.2 |
| EUR 1401–1667 | 64 | 10.5 |
| EUR ≥ 1668 | 175 | 28.7 |
| Unknown | 98 | 16.1 |
| Highest educational status of parents | ||
| No school certificate | 3 | 0.5 |
| Lower secondary education | 43 | 7.1 |
| Middle secondary education | 165 | 27.1 |
| Higher secondary education | 285 | 46.8 |
| Other | 11 | 1.8 |
| Unknown | 102 | 16.7 |
| Highest occupational status of parents | ||
| Unemployed | 19 | 3.1 |
| Worker, employed, civil service | 280 | 46.0 |
| Self-employed | 85 | 14.0 |
| Other | 21 | 3.4 |
| Unknown | 204 | 33.5 |
| n | Prevalence in % (95% CI) | aOR (95% CI) | p-Value | |
|---|---|---|---|---|
| Total | 157/609 | 25.8 (22.5, 29.1) | - | - |
| Age | ||||
| 13 | 1/93 | 1.1 (0.0, 3.2) | 0.09 (0.01, 0.71) | 0.022 |
| 14 | 11/87 | 12.6 (6.9, 18.4) | Reference | |
| 15 | 26/128 | 20.3 (14.1, 26.6) | 1.98 (0.89, 4.40) | 0.096 |
| 16 | 28/98 | 28.6 (20.4, 36.7) | 3.20 (1.42, 7.18) | 0.005 |
| 17 | 42/97 | 43.3 (35.1, 51.5) | 6.85 (3.06, 15.31) | <0.001 |
| 18 | 29/63 | 46.0 (34.9, 57.1) | 6.05 (2.44, 15.04) | <0.001 |
| 19 | 13/25 | 52.0 (36.0, 68.0) | 7.25 (1.98, 26.52) | 0.003 |
| 20 | 7/18 | 38.9 (22.2, 55.6) | 6.78 (1.29, 35.63) | 0.024 |
| SES | ||||
| Low | 25/69 | 36.2 (26.1, 46.4) | Reference | |
| Middle | 79/308 | 25.6 (21.1, 30.2) | 0.47 (0.26, 0.86) | 0.014 |
| High | 24/139 | 17.3 (12.2, 22.3) | 0.30 (0.15, 0.62) | 0.001 |
| ATC-Classification | 2012 [n/nyear a (%)] | 2013 [n/nyear a (%)] | 2014 [n/nyear a (%)] | 2015 [n/nyear a (%)] | 2016 [n/nyear a (%)] | 2017 [n/nyear a (%)] | 2018 [n/nyear a (%)] | 2019 [n/nyear a (%)] | Frequency [n/ntotal (%)] | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Progestin | Estrogen | ||||||||||
| Dienogest | Ethinylestradiol | G03AA16 | 9/28 (32.1) | 13/39 (33.3) | 18/50 (36.0) | 18/48 (37.5) | 16/44 (36.4) | 13/30 (43.3) | 10/21 (47.6) | 11/33 (33.3) | 108/293 (36.9) |
| Levonorgestrel | Ethinylestradiol | G03AA07 | 4/28 (14.3) | 7/39 (17.9) | 5/50 (10.0) | 7/48 (14.6) | 10/44 (22.7) | 7/30 (23.3) | 5/21 (23.8) | 16/33 (48.5) | 61/293 (20.8) |
| Chlormadinone | Ethinylestradiol | G03AA15/G03AB07 | 2/28 (7.1) | 9/39 (23.1) | 16/50 (32.0) | 13/48 (27.1) | 11/44 (25.0) | 5/30 (16.7) | 3/21 (14.3) | 2/33 (6.1) | 61/293 (20.8) |
| Drospirenone | Ethinylestradiol | G03AA12 | 5/28 (17.9) | 3/39 (7.7) | 4/50 (8.0) | 3/48 (6.3) | - | 1/30 (3.3) | 1/21 (4.8) | 1/33 (3.0) | 18/293 (6.1) |
| Desogestrel | G03AC09 | - | 1/39 (2.6) | - | 3/48 (6.3) | 4/44 (9.1) | 2/30 (6.7) | 1/21 (4.8) | 1/33 (3.0) | 12/293 (4.1) | |
| Nomegestrol | Estradiol | G03AA14 | - | 1/39 (2.6) | 3/50 (6.0) | 2/48 (4.2) | 1/44 (2.3) | 1/30 (3.3) | 1/21 (4.8) | - | 9/293 (3.1) |
| Cyproterone | Estrogen | G03HB01 | 1/28 (3.6) | 2/39 (5.1) | 2/50 (4.0) | 1/48 (2.1) | 1/44 (2.3) | - | - | 1/33 (3.0) | 8/293 (2.7) |
| Desogestrel | Ethinylestradiol | G03AA09 | - | 1/39 (2.6) | - | - | 1/44 (2.3) | 1/30 (3.3) | - | - | 3/293 (1.0) |
| Unknown active ingredient | G03xxxx | 7/28 (25.0) | 2/39 (5.1) | 2/50 (4.0) | 1/48 (2.1) | - | - | - | 1/33 (3.0) | 13/293 (4.4) | |
| Second generation b | - | 4/28 (14.3) | 7/39 (17.9) | 5/50 (10.0) | 7/48 (14.6) | 10/44 (22.7) | 7/30 (23.3) | 5/21 (23.8) | 16/33 (48.5) | 61/293 (20.8) | |
| Third generation c | - | - | 2/39 (5.1) | - | 3/48 (6.3) | 5/44 (11.4) | 3/30 (10.0) | 1/21 (4.8) | 1/33 (3.0) | 15/293 (5.1) | |
| Fourth generation d | - | 17/28 (60.7) | 28/39 (71.8) | 43/50 (86.0) | 37/48 (77.1) | 29/44 (65.9) | 20/30 (66.7) | 15/21 (71.4) | 15/33 (45.5) | 204/293 (69.6) | |
| ANCOVA a | ANOVA b | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Non-Users of OC | Users of OC | Result ANCOVA | Non-Users of OC | Users of OC | Result ANOVA | |||||
| Mean | SD c | Mean | SD c | Mean | SD c | Mean | SD c | |||
| Systolic blood pressure | 108.60 mmHg | 7.17 mmHg | 111.74 mmHg | 8.03 mmHg | F(1, 580) = 16.306 p < 0.001 ηp2 = 0.027 | 109.23 mmHg | 7.41 mmHg | 111.55 mmHg | 8.31 mmHg | F(1, 220) = 4.837 p = 0.029 ηp2 = 0.022 |
| Diastolic blood pressure | 67.24 mmHg | 5.42 mmHg | 69.15 mmHg | 5.66 mmHg | F(1, 580) = 8.274 p = 0.004 ηp2 = 0.014 | 67.52 mmHg | 5.52 mmHg | 69.17 mmHg | 5.84 mmHg | F(1, 220) = 4.676 p = 0.032 ηp2 = 0.021 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herzig, M.; Bertsche, A.; Hilbert, C.; Kiess, W.; Bertsche, T.; Neininger, M.P. Pharmacoepidemiological Analysis of Oral Contraceptive Use in Adolescents in a German Longitudinal Cohort Study. Children 2023, 10, 393. https://doi.org/10.3390/children10020393
Herzig M, Bertsche A, Hilbert C, Kiess W, Bertsche T, Neininger MP. Pharmacoepidemiological Analysis of Oral Contraceptive Use in Adolescents in a German Longitudinal Cohort Study. Children. 2023; 10(2):393. https://doi.org/10.3390/children10020393
Chicago/Turabian StyleHerzig, Markus, Astrid Bertsche, Cornelia Hilbert, Wieland Kiess, Thilo Bertsche, and Martina Patrizia Neininger. 2023. "Pharmacoepidemiological Analysis of Oral Contraceptive Use in Adolescents in a German Longitudinal Cohort Study" Children 10, no. 2: 393. https://doi.org/10.3390/children10020393