Sonothrombolysis: State-of-the-Art and Potential Applications in Children
Abstract
:1. Introduction
2. Materials and Methods
3. Physics Principles
3.1. Thermal Effects
3.2. Mechanical Effects
3.3. Acoustic Cavitation
3.4. Microbubbles
4. Acoustic Parameters
5. Intravascular Sonothrombolysis
6. Preclinical In Vitro Studies
7. Preclinical Animal Studies
7.1. Intracranial Thrombosis
7.2. Extracranial Thrombosis
7.3. Vascular and Cardiovascular Conditions
8. Clinical Studies
8.1. Myocardial Infarction
8.2. Stroke
8.3. Pulmonary Embolism
8.4. Deep Venous Thrombosis and Peripheral Artery Occlusion
9. Safety Parameters
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mathias, W.; Arrieta, S.R.; Tavares, G.M.P.; Sbano, J.C.N.; Tsutsui, J.M.; Kutty, S.; Porter, T.R. Successful recanalization of thrombotic occlusion in pulmonary artery stent using sonothrombolysis. CASE 2019, 3, 14–17. [Google Scholar] [CrossRef]
- Tovey, C.; Wyatt, S. Diagnosis, investigation, and management of deep vein thrombosis. BMJ 2003, 326, 1180–1184. [Google Scholar] [CrossRef]
- Dixon, A.J.; Li, J.; Rickel, J.-M.R.; Klibanov, A.L.; Zuo, Z.; Hossack, J.A. Efficacy of Sonothrombolysis Using Microbubbles Produced by a Catheter-Based Microfluidic Device in a Rat Model of Ischemic Stroke. Ann. Biomed. Eng. 2019, 47, 1012–1022. [Google Scholar] [CrossRef]
- Anschuetz, R.; Bernard, H.R. Ultrasonic irradiation and atherosclerosis. Surgery 1965, 57, 549–553. [Google Scholar]
- Trübestein, G.; Engel, C.; Etzel, F.; Sobbe, A.; Cremer, H.; Stumpff, U. Thrombolysis by ultrasound. Clin. Sci. Mol. Med. Suppl. 1976, 3, 697s–698s. [Google Scholar] [CrossRef]
- Siegel, R.J.; Gaines, P.; Crew, J.R.; CumberlandM, D.C. Clinical trial of percutaneous peripheral ultrasound angiopalsty. J. Am. Coll. Cardiol. 1993, 22, 480–488. [Google Scholar] [CrossRef]
- Medel, R.; Crowley, R.W.; McKisic, M.S.; Dumont, A.S.; Kassell, N.F. Sonothrombolysis: An emerging modality for the management of stroke. Neurosurgery 2009, 65, 979–993; discussion 993. [Google Scholar] [CrossRef]
- Sehgal, C.M.; Leveen, R.F.; Shlansky-Goldberg, R.D. Ultrasound-assisted thrombolysis. Investig. Radiol. 1993, 28, 939–943. [Google Scholar] [CrossRef]
- Shlansky-Goldberg, R.D.; Cines, D.B.; Sehgal, C.M. Catheter-delivered ultrasound potentiates in vitro thrombolysis. J. Vasc. Interv. Radiol. 1996, 7, 313–320. [Google Scholar] [CrossRef]
- Hitchcock, K.E.; Holland, C.K. Ultrasound-assisted thrombolysis for stroke therapy: Better thrombus break-up with bubbles. Stroke 2010, 41, S50–S53. [Google Scholar] [CrossRef]
- Khan, I.R.; Reeves, J.G.; Riesenman, P.J.; Kasirajan, K. Simultaneous arterial and venous ultrasound-assisted thrombolysis for phlegmasia cerulea dolens. Ann. Vasc. Surg. 2011, 25, 696.e7–696.e10. [Google Scholar] [CrossRef]
- Mathias, W.; Tsutsui, J.M.; Tavares, B.G.; Fava, A.M.; Aguiar, M.O.D.; Borges, B.C.; Oliveira, M.T.; Soeiro, A.; Nicolau, J.C.; Ribeiro, H.B.; et al. MRUSMI Investigators Sonothrombolysis in ST-Segment Elevation Myocardial Infarction Treated With Primary Percutaneous Coronary Intervention. J. Am. Coll. Cardiol. 2019, 73, 2832–2842. [Google Scholar] [CrossRef]
- Tsivgoulis, G.; Katsanos, A.H.; Eggers, J.; Larrue, V.; Thomassen, L.; Grotta, J.C.; Seitidis, G.; Schellinger, P.D.; Mavridis, D.; Demchuk, A.; et al. Sonothrombolysis in Patients With Acute Ischemic Stroke With Large Vessel Occlusion: An Individual Patient Data Meta-Analysis. Stroke 2021, 52, 3786–3795. [Google Scholar] [CrossRef]
- Page, M.; McKenzie, J.; Bossuyt, P.; Boutron, I.; Hoffmann, T.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Jo, J.; Forrest, M.L.; Yang, X. Ultrasound-assisted laser thrombolysis with endovascular laser and high-intensity focused ultrasound. Med. Phys. 2021, 48, 579–586. [Google Scholar] [CrossRef]
- Petit, B.; Bohren, Y.; Gaud, E.; Bussat, P.; Arditi, M.; Yan, F.; Tranquart, F.; Allémann, E. Sonothrombolysis: The contribution of stable and inertial cavitation to clot lysis. Ultrasound Med. Biol. 2015, 41, 1402–1410. [Google Scholar] [CrossRef]
- Chen, X.; Wang, J.; Pacella, J.J.; Villanueva, F.S. Dynamic Behavior of Microbubbles during Long Ultrasound Tone-Burst Excitation: Mechanistic Insights into Ultrasound-Microbubble Mediated Therapeutics Using High-Speed Imaging and Cavitation Detection. Ultrasound Med. Biol. 2016, 42, 528–538. [Google Scholar] [CrossRef]
- Goyal, A.; Yu, F.T.H.; Tenwalde, M.G.; Chen, X.; Althouse, A.; Villanueva, F.S.; Pacella, J.J. Inertial Cavitation Ultrasound with Microbubbles Improves Reperfusion Efficacy When Combined with Tissue Plasminogen Activator in an In Vitro Model of Microvascular Obstruction. Ultrasound Med. Biol. 2017, 43, 1391–1400. [Google Scholar] [CrossRef]
- Auboire, L.; Fouan, D.; Grégoire, J.-M.; Ossant, F.; Plag, C.; Escoffre, J.-M.; Bouakaz, A. Acoustic and Elastic Properties of a Blood Clot during Microbubble-Enhanced Sonothrombolysis: Hardening of the Clot with Inertial Cavitation. Pharmaceutics 2021, 13, 1566. [Google Scholar] [CrossRef]
- Culp, W.C.; Flores, R.; Brown, A.T.; Lowery, J.D.; Roberson, P.K.; Hennings, L.J.; Woods, S.D.; Hatton, J.H.; Culp, B.C.; Skinner, R.D.; et al. Successful microbubble sonothrombolysis without tissue-type plasminogen activator in a rabbit model of acute ischemic stroke. Stroke 2011, 42, 2280–2285. [Google Scholar] [CrossRef] [PubMed]
- Kleven, R.T.; Huang, S.; Ford, S.M.; Sakthivel, K.; Thomas, S.R.; Zuccarello, M.; Herr, A.B.; Holland, C.K. Effect of Recombinant Tissue Plasminogen Activator and 120-kHz Ultrasound on Porcine Intracranial Thrombus Density. Ultrasound Med. Biol. 2023, 49, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wang, J.; Huang, R.; Chen, G.; Zhong, L.; Shen, S.; Zhang, C.; Li, X.; Cao, S.; Liao, W.; et al. Microbubble-Mediated Sonothrombolysis Improves Outcome After Thrombotic Microembolism-Induced Acute Ischemic Stroke. Stroke 2016, 47, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, J.; Fukuda, T.; Abe, T.; Ogihara, M.; Kubota, J.; Sasaki, A.; Azuma, T.; Sasaki, K.; Shimizu, K.; Oishi, T.; et al. Ultrasound safety with midfrequency transcranial sonothrombolysis: Preliminary study on normal macaca monkey brain. Ultrasound Med. Biol. 2012, 38, 1040–1050. [Google Scholar] [CrossRef] [PubMed]
- Alexandrov, A.V.; Köhrmann, M.; Soinne, L.; Tsivgoulis, G.; Barreto, A.D.; Demchuk, A.M.; Sharma, V.K.; Mikulik, R.; Muir, K.W.; Brandt, G.; et al. Safety and efficacy of sonothrombolysis for acute ischaemic stroke: A multicentre, double-blind, phase 3, randomised controlled trial. Lancet Neurol. 2019, 18, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Barlinn, K.; Tsivgoulis, G.; Barreto, A.D.; Alleman, J.; Molina, C.A.; Mikulik, R.; Saqqur, M.; Demchuk, A.M.; Schellinger, P.D.; Howard, G.; et al. Outcomes following sonothrombolysis in severe acute ischemic stroke: Subgroup analysis of the CLOTBUST trial. Int. J. Stroke 2014, 9, 1006–1010. [Google Scholar] [CrossRef] [PubMed]
- Avgerinos, E.D.; Saadeddin, Z.; Abou Ali, A.N.; Fish, L.; Toma, C.; Chaer, M.; Rivera-Lebron, B.N.; Chaer, R.A. A meta-analysis of outcomes of catheter-directed thrombolysis for high- and intermediate-risk pulmonary embolism. J. Vasc. Surg. Venous Lymphat. Disord. 2018, 6, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Nacu, A.; Kvistad, C.E.; Naess, H.; Øygarden, H.; Logallo, N.; Assmus, J.; Waje-Andreassen, U.; Kurz, K.D.; Neckelmann, G.; Thomassen, L. NOR-SASS (Norwegian Sonothrombolysis in Acute Stroke Study): Randomized Controlled Contrast-Enhanced Sonothrombolysis in an Unselected Acute Ischemic Stroke Population. Stroke 2017, 48, 335–341. [Google Scholar] [CrossRef]
- Bader, K.B.; Bouchoux, G.; Holland, C.K. Sonothrombolysis. Adv. Exp. Med. Biol. 2016, 880, 339–362. [Google Scholar] [CrossRef]
- Shaw, G.J.; Dhamija, A.; Bavani, N.; Wagner, K.R.; Holland, C.K. Arrhenius temperature dependence of in vitro tissue plasminogen activator thrombolysis. Phys. Med. Biol. 2007, 52, 2953–2967. [Google Scholar] [CrossRef]
- Papadopoulos, N.; Kyriacou, P.A.; Damianou, C. Review of protocols used in ultrasound thrombolysis. J. Stroke Cerebrovasc. Dis. 2017, 26, 2447–2469. [Google Scholar] [CrossRef]
- Shaw, G.J.; Bavani, N.; Dhamija, A.; Lindsell, C.J. Effect of mild hypothermia on the thrombolytic efficacy of 120 kHz ultrasound enhanced thrombolysis in an in-vitro human clot model. Thromb. Res. 2006, 117, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Urban, M.W. Production of acoustic radiation force using ultrasound: Methods and applications. Expert Rev. Med. Devices 2018, 15, 819–834. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.Q.; Ooi, E.H.; Chiew, Y.S.; Foo, J.J.; Ng, E.Y.K.; Ooi, E.T. A computational framework for the multiphysics simulation of microbubble-mediated sonothrombolysis using a forward-viewing intravascular transducer. Ultrasonics 2023, 131, 106961. [Google Scholar] [CrossRef] [PubMed]
- Cerevast Transcranial Ultrasound Enhanced Thrombolysis. Available online: https://cerevast.com/science/sonothrombolysis/ (accessed on 20 October 2023).
- Qin, D.; Zou, Q.; Lei, S.; Wang, W.; Li, Z. Cavitation Dynamics and Inertial Cavitation Threshold of Lipid Coated Microbubbles in Viscoelastic Media with Bubble-Bubble Interactions. Micromachines 2021, 12, 1125. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.; Morgan, M. Mechanical Index. Available online: https://radiopaedia.org/articles/mechanical-index (accessed on 13 July 2023).
- Bell, D.; Morgan, M. Microbubbles. Available online: https://radiopaedia.org/articles/microbubbles (accessed on 6 June 2023).
- Rix, A.; Curaj, A.; Liehn, E.; Kiessling, F. Ultrasound microbubbles for diagnosis and treatment of cardiovascular diseases. Semin. Thromb. Hemost. 2020, 46, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Molina, C.A.; Ribo, M.; Rubiera, M.; Montaner, J.; Santamarina, E.; Delgado-Mederos, R.; Arenillas, J.F.; Huertas, R.; Purroy, F.; Delgado, P.; et al. Microbubble administration accelerates clot lysis during continuous 2-MHz ultrasound monitoring in stroke patients treated with intravenous tissue plasminogen activator. Stroke 2006, 37, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, P.S.; Cantisani, V.; Dietrich, C.F.; Gilja, O.H.; Saftoiu, A.; Bartels, E.; Bertolotto, M.; Calliada, F.; Clevert, D.-A.; Cosgrove, D.; et al. The EFSUMB Guidelines and Recommendations for the Clinical Practice of Contrast-Enhanced Ultrasound (CEUS) in Non-Hepatic Applications: Update 2017 (Long Version). Ultraschall Med. 2018, 39, e2–e44. [Google Scholar] [CrossRef]
- Microbubbles and Ultrasound: An Impactful Combination for Pediatric Imaging|Children’s Hospital of Philadelphia. Available online: https://www.chop.edu/news/microbubbles-and-ultrasound-impactful-combination-pediatric-imaging (accessed on 21 October 2023).
- Mizushige, K.; Kondo, I.; Ohmori, K.; Hirao, K.; Matsuo, H. Enhancement of ultrasound-accelerated thrombolysis by echo contrast agents: Dependence on microbubble structure. Ultrasound Med. Biol. 1999, 25, 1431–1437. [Google Scholar] [CrossRef]
- Porter, T.R.; Xie, F. Ultrasound, microbubbles, and thrombolysis. Prog. Cardiovasc. Dis. 2001, 44, 101–110. [Google Scholar] [CrossRef]
- Cintas, P.; Nguyen, F.; Boneu, B.; Larrue, V. Enhancement of enzymatic fibrinolysis with 2-MHz ultrasound and microbubbles. J. Thromb. Haemost. 2004, 2, 1163–1166. [Google Scholar] [CrossRef]
- Xie, F.; Tsutsui, J.M.; Lof, J.; Unger, E.C.; Johanning, J.; Culp, W.C.; Matsunaga, T.; Porter, T.R. Effectiveness of lipid microbubbles and ultrasound in declotting thrombosis. Ultrasound Med. Biol. 2005, 31, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Ramaswami, R. Comparison of sonothrombolysis efficiencies of different ultrasound systems. J. Stroke Cerebrovasc. Dis. 2014, 23, 2730–2735. [Google Scholar] [CrossRef] [PubMed]
- Bor-Seng-Shu, E.; Nogueira, R.D.C.; Figueiredo, E.G.; Evaristo, E.F.; Conforto, A.B.; Teixeira, M.J. Sonothrombolysis for acute ischemic stroke: A systematic review of randomized controlled trials. Neurosurg. Focus 2012, 32, E5. [Google Scholar] [CrossRef] [PubMed]
- Arditi, M.; Gaud, E.; Petit, B.; Yan, F.; Frinking, P.; Allemann, E.; Tranquart, F. Effects of acoustic parameters on sonothrombolysis with contrast microbubbles. In Proceedings of the 2011 IEEE International Ultrasonics Symposium, Orlando, FL, USA, 18–21 October 2011; IEEE: Piscataway, NJ, USA; pp. 1786–1789. [Google Scholar]
- Goel, L.; Wu, H.; Zhang, B.; Kim, J.; Dayton, P.A.; Xu, Z.; Jiang, X. Safety Evaluation of a Forward-Viewing Intravascular Transducer for Sonothrombolysis: An In Vitro and Ex Vivo Study. Ultrasound Med. Biol. 2021, 47, 3231–3239. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhang, B.; Huang, C.-C.; Peng, C.; Zhou, Q.; Jiang, X. Ultrasound-Guided Intravascular Sonothrombolysis With a Dual Mode Ultrasound Catheter: In Vitro Study. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2022, 69, 1917–1925. [Google Scholar] [CrossRef] [PubMed]
- Hölscher, T.; Raman, R.; Fisher, D.J.; Ahadi, G.; Zadicario, E.; Voie, A. Effects of varying duty cycle and pulse width on high-intensity focused ultrasound (HIFU)-induced transcranial thrombolysis. J. Ther. Ultrasound 2013, 1, 18. [Google Scholar] [CrossRef] [PubMed]
- Ahadi, G.; Welch, C.S.; Grimm, M.J.; Fisher, D.J.; Zadicario, E.; Ernström, K.; Voie, A.H.; Hölscher, T. Transcranial sonothrombolysis using high-intensity focused ultrasound: Impact of increasing output power on clot fragmentation. J. Ther. Ultrasound 2013, 1, 22. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Yang, X. Laser enhanced high-intensity focused ultrasound thrombolysis: An in vitro study. J. Acoust. Soc. Am. 2013, 133, EL123–EL128. [Google Scholar] [CrossRef]
- Braun, T.; Sünner, L.; Hachenberger, M.; Müller, C.; Wietelmann, A.; Juenemann, M.; Pons-Kühnemann, J.; Kaps, M.; Gerriets, T.; Tschernatsch, M.; et al. Microbubble-mediated sonothrombolysis with BR38 of a venous full blood thrombus in a rat embolic stroke model. Ann. Transl. Med. 2021, 9, 1061. [Google Scholar] [CrossRef]
- Kleven, R.T.; Karani, K.B.; Hilvert, N.; Ford, S.M.; Mercado-Shekhar, K.P.; Racadio, J.M.; Rao, M.B.; Abruzzo, T.A.; Holland, C.K. Accelerated sonothrombolysis with Definity in a xenographic porcine cerebral thromboembolism model. Sci. Rep. 2021, 11, 3987. [Google Scholar] [CrossRef]
- Teng, Y.; Jin, H.; Nan, D.; Li, M.; Fan, C.; Liu, Y.; Lv, P.; Cui, W.; Sun, Y.; Hao, H.; et al. In vivo evaluation of urokinase-loaded hollow nanogels for sonothrombolysis on suture embolization-induced acute ischemic stroke rat model. Bioact. Mater. 2018, 3, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Zhu, Q.; Guo, M.; Gao, Y.; Dong, X.; Chen, Z.; Liu, Z.; Xie, F. Ultrasound and Intra-Clot Microbubbles Enhanced Catheter-Directed Thrombolysis in Vitro and in Vivo. Ultrasound Med. Biol. 2017, 43, 1671–1678. [Google Scholar] [CrossRef] [PubMed]
- Schleicher, N.; Tomkins, A.J.; Kampschulte, M.; Hyvelin, J.-M.; Botteron, C.; Juenemann, M.; Yeniguen, M.; Krombach, G.A.; Kaps, M.; Spratt, N.J.; et al. Sonothrombolysis with BR38 Microbubbles Improves Microvascular Patency in a Rat Model of Stroke. PLoS ONE 2016, 11, e0152898. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.T.; Flores, R.; Hamilton, E.; Roberson, P.K.; Borrelli, M.J.; Culp, W.C. Microbubbles improve sonothrombolysis in vitro and decrease hemorrhage in vivo in a rabbit stroke model. Investig. Radiol. 2011, 46, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Reuter, P.; Masomi, J.; Kuntze, H.; Fischer, I.; Helling, K.; Sommer, C.; Alessandri, B.; Heimann, A.; Gerriets, T.; Marx, J.; et al. Low-frequency therapeutic ultrasound with varied duty cycle: Effects on the ischemic brain and the inner ear. Ultrasound Med. Biol. 2010, 36, 1188–1195. [Google Scholar] [CrossRef] [PubMed]
- Nedelmann, M.; Ritschel, N.; Doenges, S.; Langheinrich, A.C.; Acker, T.; Reuter, P.; Yeniguen, M.; Pukropski, J.; Kaps, M.; Mueller, C.; et al. Combined contrast-enhanced ultrasound and rt-PA treatment is safe and improves impaired microcirculation after reperfusion of middle cerebral artery occlusion. J. Cereb. Blood Flow Metab. 2010, 30, 1712–1720. [Google Scholar] [CrossRef]
- Daffertshofer, M.; Huang, Z.; Fatar, M.; Popolo, M.; Schroeck, H.; Kuschinsky, W.; Moskowitz, M.A.; Hennerici, M.G. Efficacy of sonothrombolysis in a rat model of embolic ischemic stroke. Neurosci. Lett. 2004, 361, 115–119. [Google Scholar] [CrossRef]
- Doelare, S.A.N.; Nederhoed, J.H.; Evers, J.M.; Roos, S.T.; Kamp, O.; Musters, R.J.P.; Wisselink, W.; Jongkind, V.; Ebben, H.P.; Yeung, K.K. Feasibility of Microbubble-Accelerated Low-Dose Thrombolysis of Peripheral Arterial Occlusions Using an Ultrasound Catheter. J. Endovasc. Ther. 2022, 15266028221126938. [Google Scholar] [CrossRef]
- Choi, W.; Key, J.; Youn, I.; Lee, H.; Han, S. Cavitation-assisted sonothrombolysis by asymmetrical nanostars for accelerated thrombolysis. J. Control. Release 2022, 350, 870–885. [Google Scholar] [CrossRef]
- Wang, Z.; Pan, Y.; Huang, H.; Zhang, Y.; Li, Y.; Zou, C.; Huang, G.; Chen, Y.; Li, Y.; Li, J.; et al. Enhanced thrombolysis by endovascular low-frequency ultrasound with bifunctional microbubbles in venous thrombosis: In vitro and in vivo study. Front. Bioeng. Biotechnol. 2022, 10, 965769. [Google Scholar] [CrossRef]
- Zheng, X.; Pan, Y.; Zhang, Y.; Meng, K.; Zhou, J.; Wang, X.; Cui, Y.; Li, J.; Li, Y.; Chen, H. Interventional microbubble enhanced sonothrombolysis on left ventricular assist devices. Adv. Sci. 2022, 9, e2201291. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Jo, J.; Riegel, M.; Forrest, M.L.; Yang, X. The feasibility of ultrasound-assisted endovascular laser thrombolysis in an acute rabbit thrombosis model. Med. Phys. 2021, 48, 4128–4138. [Google Scholar] [CrossRef] [PubMed]
- Kutty, S.; Liu, N.; Zhou, J.; Xiao, Y.; Wu, J.; Danford, D.; Lof, J.; Xie, F.; Porter, T.R. Ultrasound induced microbubble cavitation for the treatment of catheterization induced vasospasm. JACC Basic Transl. Sci. 2017, 2, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.-H.; Yeh, J.-C.; Tsai, C.-H.; Yang, J.-T.; Lou, S.-L.; Seak, C.-J.; Wang, C.-Y.; Wei, K.-C.; Liu, H.-L. Improved thrombolytic effect with focused ultrasound and neuroprotective agent against acute carotid artery thrombosis in rat. Sci. Rep. 2017, 7, 1638. [Google Scholar] [CrossRef] [PubMed]
- Tomkins, A.J.; Hood, R.J.; Pepperall, D.; Null, C.L.; Levi, C.R.; Spratt, N.J. Thrombolytic recanalization of carotid arteries is highly dependent on degree of stenosis, despite sonothrombolysis. J. Am. Heart Assoc. 2016, 5, e002716. [Google Scholar] [CrossRef]
- Nederhoed, J.H.; Slikkerveer, J.; Meyer, K.W.; Wisselink, W.; Musters, R.J.P.; Yeung, K.K. Contrast-enhanced sonothrombolysis in a porcine model of acute peripheral arterial thrombosis and prevention of anaphylactic shock. Lab. Anim. 2014, 43, 91–94. [Google Scholar] [CrossRef]
- Kutty, S.; Wu, J.; Hammel, J.M.; Abraham, J.R.; Venkataraman, J.; Abdullah, I.; Danford, D.A.; Radio, S.J.; Lof, J.; Porter, T.R. Prevention of arteriovenous shunt occlusion using microbubble and ultrasound mediated thromboprophylaxis. J. Am. Heart Assoc. 2014, 3, e000689. [Google Scholar] [CrossRef]
- Damianou, C.; Hadjisavvas, V.; Mylonas, N.; Couppis, A.; Ioannides, K. MRI-guided sonothrombolysis of rabbit carotid artery. J. Stroke Cerebrovasc. Dis. 2014, 23, e113–e121. [Google Scholar] [CrossRef]
- Chen, R.; Paeng, D.-G.; Lam, K.H.; Zhou, Q.; Shung, K.K.; Matsuoka, N.; Humayun, M.S. In vivo Sonothrombolysis of Ear Marginal Vein of Rabbits Monitored with High-frequency Ultrasound Needle Transducer. J. Med. Biol. Eng. 2013, 33, 103–110. [Google Scholar] [CrossRef]
- Kutty, S.; Wu, J.; Hammel, J.M.; Xie, F.; Gao, S.; Drvol, L.K.; Lof, J.; Radio, S.J.; Therrien, S.L.; Danford, D.A.; et al. Microbubble mediated thrombus dissolution with diagnostic ultrasound for the treatment of chronic venous thrombi. PLoS ONE 2012, 7, e51453. [Google Scholar] [CrossRef]
- Borrelli, M.J.; O’Brien, W.D.; Hamilton, E.; Oelze, M.L.; Wu, J.; Bernock, L.J.; Tung, S.; Rokadia, H.; Culp, W.C. Influences of microbubble diameter and ultrasonic parameters on in vitro sonothrombolysis efficacy. J. Vasc. Interv. Radiol. 2012, 23, 1677–1684. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Yu, Q.; Liu, T.; Wang, H.; Zhuang, H.; Luo, Y.; Peng, Y. [Orthogonal analysis of physical parameters optimization of microbubble-enhanced sono-thrombolysis]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2012, 26, 1098–1101. [Google Scholar] [PubMed]
- Kim, J.S.; Leeman, J.E.; Kagemann, L.; Yu, F.T.H.; Chen, X.; Pacella, J.J.; Schuman, J.S.; Villanueva, F.S.; Kim, K. Volumetric quantification of in vitro sonothrombolysis with microbubbles using high-resolution optical coherence tomography. J. Biomed. Opt. 2012, 17, 070502. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.; Hynynen, K.; Goertz, D. In vitro and in vivo high-intensity focused ultrasound thrombolysis. Investig. Radiol. 2012, 47, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Flores, R.; Hennings, L.J.; Lowery, J.D.; Brown, A.T.; Culp, W.C. Microbubble-augmented ultrasound sonothrombolysis decreases intracranial hemorrhage in a rabbit model of acute ischemic stroke. Investig. Radiol. 2011, 46, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.; Dempfle, C.-E.; Della Martina, A.; Stroick, M.; Fatar, M.; Zohsel, K.; Allémann, E.; Hennerici, M.G.; Meairs, S. In vivo clot lysis of human thrombus with intravenous abciximab immunobubbles and ultrasound. Thromb. Res. 2009, 124, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Kawata, H.; Naya, N.; Takemoto, Y.; Uemura, S.; Nakajima, T.; Horii, M.; Takeda, Y.; Fujimoto, S.; Yamashita, A.; Asada, Y.; et al. Ultrasound accelerates thrombolysis of acutely induced platelet-rich thrombi similar to those in acute myocardial infarction. Circ. J. 2007, 71, 1643–1648. [Google Scholar] [CrossRef]
- Trübestein, G.; Engel, C.; Stumpff, U.; Wuttke, H.; Sobbe, A. [Embolism by ultrasound-thrombolysis]. Langenbecks Arch. Chir. 1976, 340, 199–205. [Google Scholar] [CrossRef]
- Mott, B.; Ammi, A.Y.; Le, D.E.; Davis, C.; Dykan, I.V.; Zhao, Y.; Nugent, M.; Minnier, J.; Gowda, M.; Alkayed, N.J.; et al. Therapeutic ultrasound increases myocardial blood flow in ischemic myocardium and cardiac endothelial cells: Results of in vivo and in vitro experiments. J. Am. Soc. Echocardiogr. 2019, 32, 1151–1160. [Google Scholar] [CrossRef]
- Li, H.; Lu, Y.; Sun, Y.; Chen, G.; Wang, J.; Wang, S.; Huang, C.; Zhong, L.; Si, X.; Liao, W.; et al. Diagnostic Ultrasound and Microbubbles Treatment Improves Outcomes of Coronary No-Reflow in Canine Models by Sonothrombolysis. Crit. Care Med. 2018, 46, e912–e920. [Google Scholar] [CrossRef]
- Looi, T.; Piorkowska, K.; Mougenot, C.; Waspe, A.; Hynynen, K.; Drake, J. An MR-based quantitative intraventricular hemorrhage porcine model for MR-guided focused ultrasound thrombolysis. Childs Nerv Syst 2018, 34, 1643–1650. [Google Scholar] [CrossRef]
- Tomkins, A.J.; Schleicher, N.; Murtha, L.; Kaps, M.; Levi, C.R.; Nedelmann, M.; Spratt, N.J. Platelet rich clots are resistant to lysis by thrombolytic therapy in a rat model of embolic stroke. Exp. Transl. Stroke Med. 2015, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Pacella, J.J.; Brands, J.; Schnatz, F.G.; Black, J.J.; Chen, X.; Villanueva, F.S. Treatment of microvascular micro-embolization using microbubbles and long-tone-burst ultrasound: An in vivo study. Ultrasound Med. Biol. 2015, 41, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Pajek, D.; Burgess, A.; Huang, Y.; Hynynen, K. High-intensity focused ultrasound sonothrombolysis: The use of perfluorocarbon droplets to achieve clot lysis at reduced acoustic power. Ultrasound Med. Biol. 2014, 40, 2151–2161. [Google Scholar] [CrossRef] [PubMed]
- Monteith, S.J.; Harnof, S.; Medel, R.; Popp, B.; Wintermark, M.; Lopes, M.B.S.; Kassell, N.F.; Elias, W.J.; Snell, J.; Eames, M.; et al. Minimally invasive treatment of intracerebral hemorrhage with magnetic resonance-guided focused ultrasound. J. Neurosurg. 2013, 118, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Ohtsuka, H.; Arimura, N.; Sonoda, S.; Kato, C.; Ushimaru, K.; Hara, N.; Tachibana, K.; Sakamoto, T. Sonothrombolysis for intraocular fibrin formation in an animal model. Ultrasound Med. Biol. 2009, 35, 1845–1853. [Google Scholar] [CrossRef] [PubMed]
- Ammi, A.Y.; Lindner, J.R.; Zhao, Y.; Porter, T.; Siegel, R.; Kaul, S. Efficacy and spatial distribution of ultrasound-mediated clot lysis in the absence of thrombolytics. Thromb. Haemost. 2015, 113, 1357–1369. [Google Scholar] [CrossRef] [PubMed]
- Auboire, L.; Sennoga, C.A.; Hyvelin, J.-M.; Ossant, F.; Escoffre, J.-M.; Tranquart, F.; Bouakaz, A. Microbubbles combined with ultrasound therapy in ischemic stroke: A systematic review of in-vivo preclinical studies. PLoS ONE 2018, 13, e0191788. [Google Scholar] [CrossRef]
- Chen, X.; Wu, W.; Wang, S.; Zhong, J.; Djama, N.M.; Wei, G.; Lai, Y.; Si, X.; Cao, S.; Liao, W.; et al. Magnetic Targeting Improves the Therapeutic Efficacy of Microbubble-Mediated Obstructive Thrombus Sonothrombolysis. Thromb. Haemost. 2019, 119, 1752–1766. [Google Scholar] [CrossRef]
- Gao, S.; Zhu, Q.; Dong, X.; Chen, Z.; Liu, Z.; Xie, F. Guided longer pulses from a diagnostic ultrasound and intraclot microbubble enhanced catheter-directed thrombolysis in vivo. J. Thromb. Thrombolysis 2017, 44, 48–56. [Google Scholar] [CrossRef]
- Veldman, A.; Nold, M.F.; Michel-Behnke, I. Thrombosis in the critically ill neonate: Incidence, diagnosis, and management. Vasc. Health Risk Manag. 2008, 4, 1337–1348. [Google Scholar] [PubMed]
- Tanke, R.B.; van Megen, R.; Daniëls, O. Thrombus detection on central venous catheters in the neonatal intensive care unit. Angiology 1994, 45, 477–480. [Google Scholar] [CrossRef] [PubMed]
- Khilnani, P.; Goldstein, B.; Todres, I.D. Double lumen umbilical venous catheters in critically ill neonates: A randomized prospective study. Crit. Care Med. 1991, 19, 1348–1351. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.; Zipursky, A. Thrombotic disease in newborn infants. Clin. Perinatol. 1984, 11, 461–488. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Wang, Z.; Deng, Q.; Zhou, Y.; Cao, S.; Zhou, Q.; Chen, J.; Guo, R.; Hu, B. Low-intensity focused ultrasound guided dodecafluoropentane-loaded acoustic phase-change nanoparticles for treatment of porcine coronary microthromboembolism. Int. J. Cardiol. 2023, 371, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ghbeis, M.B.; Vander Pluym, C.J.; Thiagarajan, R.R. Hemostatic Challenges in Pediatric Critical Care Medicine-Hemostatic Balance in VAD. Front. Pediatr. 2021, 9, 625632. [Google Scholar] [CrossRef]
- White, H.D.; Chew, D.P. Acute myocardial infarction. Lancet 2008, 372, 570–584. [Google Scholar] [CrossRef]
- El Kadi, S.; Porter, T.R.; Zanstra, M.; Siegers, A.; van Loon, R.B.; Hopman, L.H.G.A.; van Rossum, A.C.; Kamp, O. Feasibility of sonothrombolysis in the ambulance for ST-elevation myocardial infarction. Int. J. Cardiovasc. Imaging 2021, 38, 1089–1098. [Google Scholar] [CrossRef]
- Bainey, K.R.; Abulhamayel, A.; Aziz, A.; Becher, H. Sonothrombolysis Augments Reperfusion in ST-Elevation Myocardial Infarction With Primary Percutaneous Coronary Intervention: Insights From the SONOSTEMI Study. CJC Open 2022, 4, 644–646. [Google Scholar] [CrossRef]
- Burns, J.C.; El-Said, H.; Tremoulet, A.H.; Friedman, K.; Gordon, J.B.; Newburger, J.W. Management of Myocardial Infarction in Children with Giant Coronary Artery Aneurysms after Kawasaki Disease. J. Pediatr. 2020, 221, 230–234. [Google Scholar] [CrossRef]
- McCrindle, B.W.; Rowley, A.H.; Newburger, J.W.; Burns, J.C.; Bolger, A.F.; Gewitz, M.; Baker, A.L.; Jackson, M.A.; Takahashi, M.; Shah, P.B.; et al. Diagnosis, Treatment, and Long-Term Management of Kawasaki Disease: A Scientific Statement for Health Professionals From the American Heart Association. Circulation 2017, 135, e927–e999. [Google Scholar] [CrossRef] [PubMed]
- Bilici, M.; Ture, M.; Balik, H. Myocardial infarction in children. In Myocardial Infarction; Pamukçu, B., Ed.; IntechOpen: London, UK, 2019; ISBN 978-1-78984-868-7. [Google Scholar]
- Donkor, E.S. Stroke in the 21st century: A snapshot of the burden, epidemiology, and quality of life. Stroke Res. Treat. 2018, 2018, 3238165. [Google Scholar] [CrossRef] [PubMed]
- Hollist, M.; Au, K.; Morgan, L.; Shetty, P.A.; Rane, R.; Hollist, A.; Amaniampong, A.; Kirmani, B.F. Pediatric stroke: Overview and recent updates. Aging Dis. 2021, 12, 1043–1055. [Google Scholar] [CrossRef] [PubMed]
- Ferriero, D.M.; Fullerton, H.J.; Bernard, T.J.; Billinghurst, L.; Daniels, S.R.; DeBaun, M.R.; deVeber, G.; Ichord, R.N.; Jordan, L.C.; Massicotte, P.; et al. Nursing. Management of stroke in neonates and children: A scientific statement from the american heart association/american stroke association. Stroke 2019, 50, e51–e96. [Google Scholar] [CrossRef] [PubMed]
- Moharir, M.D.; Shroff, M.; Stephens, D.; Pontigon, A.-M.; Chan, A.; MacGregor, D.; Mikulis, D.; Adams, M.; deVeber, G. Anticoagulants in pediatric cerebral sinovenous thrombosis: A safety and outcome study. Ann. Neurol. 2010, 67, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.A.; Molsberry, R.; Cuttica, M.J.; Desai, K.R.; Schimmel, D.R.; Khan, S.S. Time trends in pulmonary embolism mortality rates in the united states, 1999 to 2018. J. Am. Heart Assoc. 2020, 9, e016784. [Google Scholar] [CrossRef] [PubMed]
- Kaymaz, C.; Akbal, O.Y.; Tanboga, I.H.; Hakgor, A.; Yilmaz, F.; Ozturk, S.; Poci, N.; Turkday, S.; Ozdemir, N.; Konstantinides, S. Ultrasound-Assisted Catheter-Directed Thrombolysis in High-Risk and Intermediate-High-Risk Pulmonary Embolism: A Meta-Analysis. Curr. Vasc. Pharmacol. 2018, 16, 179–189. [Google Scholar] [CrossRef]
- Cushman, M. Epidemiology and risk factors for venous thrombosis. Semin. Hematol. 2007, 44, 62–69. [Google Scholar] [CrossRef]
- Song, P.; Rudan, D.; Zhu, Y.; Fowkes, F.J.I.; Rahimi, K.; Fowkes, F.G.R.; Rudan, I. Global, regional, and national prevalence and risk factors for peripheral artery disease in 2015: An updated systematic review and analysis. Lancet Glob. Health 2019, 7, e1020–e1030. [Google Scholar] [CrossRef]
- EKOSTM Endovascular System—Boston Scientific. Available online: https://www.bostonscientific.com/en-EU/products/thrombectomy-systems/ekosonic-endovascular-system.html (accessed on 21 October 2023).
- Shi, Y.; Shi, W.; Chen, L.; Gu, J. A systematic review of ultrasound-accelerated catheter-directed thrombolysis in the treatment of deep vein thrombosis. J. Thromb. Thrombolysis 2018, 45, 440–451. [Google Scholar] [CrossRef]
- Zhu, Q.; Dong, G.; Wang, Z.; Sun, L.; Gao, S.; Liu, Z. Intra-clot Microbubble-Enhanced Ultrasound Accelerates Catheter-Directed Thrombolysis for Deep Vein Thrombosis: A Clinical Study. Ultrasound Med. Biol. 2019, 45, 2427–2433. [Google Scholar] [CrossRef] [PubMed]
- Doelare, S.A.N.; Jean Pierre, D.M.; Nederhoed, J.H.; Smorenburg, S.P.M.; Lely, R.J.; Jongkind, V.; Hoksbergen, A.W.J.; Ebben, H.P.; Yeung, K.K. MUST collaborators Microbubbles and ultrasound accelerated thrombolysis for peripheral arterial occlusions: The outcomes of a single arm phase II trial. Eur. J. Vasc. Endovasc. Surg. 2021, 62, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Jaffray, J.; Bauman, M.; Massicotte, P. The impact of central venous catheters on pediatric venous thromboembolism. Front. Pediatr. 2017, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Higgerson, R.A.; Lawson, K.A.; Christie, L.M.; Brown, A.-M.; McArthur, J.A.; Totapally, B.R.; Hanson, S.J. National Association of Children’s Hospitals and Related Institution’s Pediatric Intensive Care Unit FOCUS group Incidence and risk factors associated with venous thrombotic events in pediatric intensive care unit patients. Pediatr. Crit. Care Med. 2011, 12, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Mandel-Shorer, N.; Tzvi-Behr, S.; Harvey, E.; Revel-Vilk, S. Central venous catheter-related venous thrombosis in children with end-stage renal disease undergoing hemodialysis. Thromb. Res. 2018, 172, 150–157. [Google Scholar] [CrossRef]
- Witmer, C.; Raffini, L. Treatment of venous thromboembolism in pediatric patients. Blood 2020, 135, 335–343. [Google Scholar] [CrossRef]
- Dinia, L.; Rubiera, M.; Ribo, M.; Maisterra, O.; Ortega, G.; del Sette, M.; Alvarez-Sabin, J.; Molina, C.A. Reperfusion after stroke sonothrombolysis with microbubbles may predict intracranial bleeding. Neurology 2009, 73, 775–780. [Google Scholar] [CrossRef]
- Brouwer, A.J.; Groenendaal, F.; Koopman, C.; Nievelstein, R.-J.A.; Han, S.K.; de Vries, L.S. Intracranial hemorrhage in full-term newborns: A hospital-based cohort study. Neuroradiology 2010, 52, 567–576. [Google Scholar] [CrossRef]
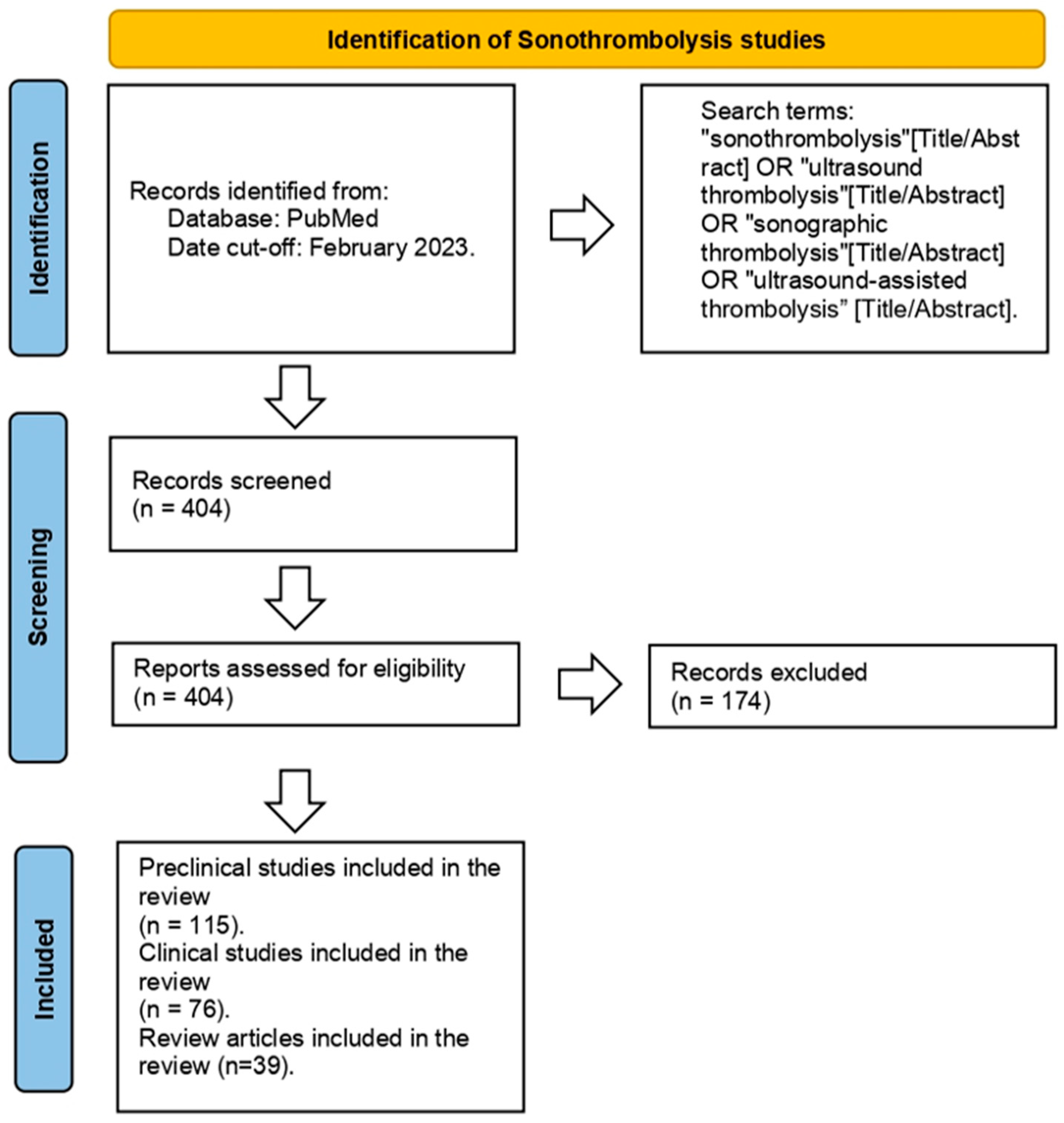

| Study Type | Title Author PMID | Ultrasound Parameters | Type of Sonothrombolysis | Comments |
|---|---|---|---|---|
| Preclinical In Vitro | ||||
| Ultrasound-assisted laser thrombolysis with endovascular laser and high-intensity focused ultrasound. Jo et al. [15] PMID: 33280145 |
Frequency: 0.5 MHz Laser fluence levels: 0 mJ/cm2, 2 mJ/cm2, and 4 mJ/cm2 | Ultrasound only | Endovascular laser combined with US at low power and pressure levels can achieve effective thrombolysis. | |
| Sonothrombolysis: the contribution of stable and inertial cavitation to clot lysis. Petit et al. [16] PMID: 25601463 | Frequency: 1 MHz Acoustic pressures: 200, 350, and 1300 kPA | Combinations of tPA + US + MB | The combination of tPA + US + MB demonstrated that inertial cavitation (1300 kPA) enhanced fibrin degradation compared with tPA alone. | |
| Dynamic behavior of microbubbles during long ultrasound tone-burst excitation: mechanistic insights into ultrasound/microbubble-mediated therapeutics using high-speed imaging and cavitation detection. Chen et al. [17] PMID: 26603628 | Frequency: 1 MHz Focal length: 42.5 mm Various peak negative pressures: 0.25, 0.5, 1.0, and 1.5 MPa | US + MB | Ongoing cavitation activity during long tone bursts may lead to additional therapeutic benefits. | |
| Inertial cavitation ultrasound with microbubbles improves reperfusion efficacy when combined with tissue plasminogen activator in an in vitro model of microvascular obstruction. Goyal et al. [18] PMID: 28395964 | Pulsed US: 1 MHz IC: 1.0 MPa, 1000 cycles, 0.33 Hz Stable cavitation: 0.23 MPa, 20% duty cycle, 0.33 Hz | US + tPA + MB | These findings suggest an IC regime can be used with tPA to achieve a high degree of fibrinolysis for both venous and arterial type microthrombi. | |
| Acoustic and elastic properties of a blood clot during microbubble-enhanced sonothrombolysis: hardening of the clot with inertial cavitation. Auboire et al. [19] PMID: 34683859 | 1 MHz sinusoidal ultrasound waves 0.8 Hz pulse repetition frequency | tPA alone (3 μg/mL) US + MB tPA + US + MB | The combination of tPA and sonothrombolysis with microbubbles augments sound speed and clot hardening, which in turn decreases the penetration of thrombolytic drugs and their efficacy. | |
| Preclinical animal | ||||
| Efficacy of sonothrombolysis using microbubbles produced by a catheter-based microfluidic device in a rat model of ischemic stroke. Dixon et al. [3] PMID: 30689066 |
Frequency: 1 MHz Ultrasound energy was applied at a 10% duty factor in bursts of 100 cycles with a peak negative pressure of 500 kPa. | Group A: Control; Group B: 0.09 mg/kg tPA; Group C: 0.9 mg/kg tPA; Group D: 0.09 mg/kg tPA + US + MB. | Sonothrombolysis + US reduced cerebral infarct volumes by approximately 50% compared with no therapy, in addition to significantly improving functional neurological outcomes at 24 h and permitting tPA dose reduction by 3.3-fold compared with tPA alone. | |
| Successful microbubble sonothrombolysis without tissue-type plasminogen activator in a rabbit model of acute ischemic stroke. Culp et al. [20] PMID: 21700942 |
Frequency: 1 MHz Intensity: 0.8 W/cm2 | Embolized without treatment (control) tPA only tPA + US perflutren lipid MB (0.16 mg/kg) + US albumin 3 μm MB (5 × 10⁹ MB) + US Tagged albumin 3 μm MB + US |
Infarct volume percent was lower for rabbits treated with lipid MB + US, 3 μm MB + US, and tagged 3 μm MB + US compared with controls. Microbubble groups had lower infarct volumes than controls. | |
| Effect of recombinant tissue plasminogen activator and 120 kHz ultrasound on porcine intracranial thrombus density. Kleven et al. [21] PMID: 36336551 | Frequency: 120 kHZ | tPA alone Ultrasound with tPA | No enhancement of tPA-mediated thrombolysis was noted with the addition of sonothrombolysis. | |
| Microbubble-mediated sonothrombolysis improves outcome after thrombotic micro-embolism-induced acute ischemic stroke. Lu et al. [22] PMID: 27048701 | Frequency: 14 MHz MI = 0.17 | tPA only group (10 mg/kg) Microbubble only (0.008 mL/min) Microbubble-enhanced (0.008 mL/min) + half-dose tPA group (5 mg/kg) | Microbubble-mediated sonothrombolysis significantly reduced cerebral infarction and improved neurological deficit (US + MB group). | |
| Ultrasound safety with midfrequency transcranial sonothrombolysis: preliminary study on normal Macaca monkey brain. Shimizu et al. [23] PMID: 22475695 |
Frequency = 490 kHz Intensity = 0.72 W/cm2 | Two elder rhesus monkeys received 0.9 mg/kg of alteplase. | None of the monkeys showed neurologic deficits after sonothrombolysis. | |
| Clinical studies | ||||
| Sonothrombolysis in ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention. Mathias, Jr. et al. [12] PMID: 30894317 | Control group: low MI (<0.2) Therapeutic group: 1.8 MHz 1.2 to 1.3 MI <5-μs pulse | Microbubble-enhanced | In the high MI sonothrombolysis group, there was sustained improvement in systolic function and reduced need for defibrillators at 6-month follow up. | |
| Safety and efficacy of sonothrombolysis for acute ischemic stroke: a multicenter, double-blind, phase 3, randomized controlled trial. Alexandrov et al. [24] PMID: 30878103 | A 2 MHz pulsed-wave transcranial ultrasound for 120 min (total average power 32 mW; maximum spatial peak temporal average intensity 207 mW/cm2; pulse repetition frequency 8·3 kHz; pulse duration 5 μs) | Ultrasound in conjunction with intravenous alteplase (0.9 mg/kg) | No clinical benefit after 90 days was found. | |
| Outcomes following sonothrombolysis in severe acute ischemic stroke: subgroup analysis of the CLOTBUST trial. Barlinn et al. [25] PMID: 25079049 | Frequency: 2 MHz | Ultrasound in conjunction with intravenous alteplase (0.9 mg/kg) | Researchers observed an absolute increase in 3-month functional independence rate with sonothrombolysis compared with the IV tPA only group. | |
| A meta-analysis of outcomes of catheter-directed thrombolysis for high- and intermediate-risk pulmonary embolism. Avgerinos et al. [26] PMID ID: 29909859 | Variable | Ultrasound in conjunction with tPA (variable dosages) | Sonothrombolysis may be more effective than standard catheter-directed thrombolysis in the higher risk population. | |
| Successful recanalization of thrombotic occlusion in pulmonary artery stent using sonothrombolysis. Mathias, Jr. et al. [1] PMID: 30828677 |
Frequency: 1.7 MHz MI: 1.3 Pulse duration: 20 μs | Microbubble-enhanced (6 mL in total) Bolus of 0.05 mg/kg of alteplase followed by 24 h infusion at 5 mg/kg/hr (day prior to sonothrombolysis) | Sonothrombolysis with microbubbles resulted in recanalization of pulmonary artery obstruction in a child with congenital heart disease. | |
| NOR-SASS (Norwegian sonothrombolysis in acute stroke study): randomized controlled contrast-enhanced sonothrombolysis in an unselected acute ischemic stroke population. Nacu et al. [27] PMID: 27980128 | Frequency: 2 MHz MI: <1.0. | Microbubble-enhanced (Sonovue, 10 mL) Tenecteplase 0.4 mg/kg or alteplase 0.9 mg/kg | No significant clinical effect of sonothrombolysis was demonstrated. The trial stopped prematurely, secondary to lack of funding. | |
| Condition | Zone | Number of Patients Evaluated |
|---|---|---|
| Pulmonary Embolism | Asia | 42 |
| Europe | 217 | |
| Middle East | 441 | |
| North America | 1538 | |
| South America a | 1 | |
| Total | 2239 | |
| Acute Ischemic Stroke | Asia | 84 |
| Central America | 1 | |
| Europe | 1184 | |
| Middle East | 42 | |
| Multicentric b | 733 | |
| North America | 55 | |
| South America | 61 | |
| Total | 2160 | |
| Myocardial Infarction | Europe | 23 |
| Middle East | 141 | |
| North America | 196 | |
| South America | 400 | |
| Total | 760 | |
| Brain Hemorrhage | Europe | 300 |
| North America | 33 | |
| Total | 333 | |
| Deep Venous Thrombosis | Asia | 50 |
| Europe | 23 | |
| Total | 73 | |
| Peripheral Artery Occlusion | Asia | 1 |
| Europe | 44 | |
| Total | 45 | |
| Venous Graft Thrombosis | Middle East | 20 |
| North America | 1 | |
| Total | 21 | |
| Occluded Transjugularportosystemic Shunt | Europe | 1 |
| Total | 1 | |
| Grand Total | 5632 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ward, R.E.; Martinez-Correa, S.; Tierradentro-García, L.O.; Hwang, M.; Sehgal, C.M. Sonothrombolysis: State-of-the-Art and Potential Applications in Children. Children 2024, 11, 57. https://doi.org/10.3390/children11010057
Ward RE, Martinez-Correa S, Tierradentro-García LO, Hwang M, Sehgal CM. Sonothrombolysis: State-of-the-Art and Potential Applications in Children. Children. 2024; 11(1):57. https://doi.org/10.3390/children11010057
Chicago/Turabian StyleWard, Rebecca E., Santiago Martinez-Correa, Luis Octavio Tierradentro-García, Misun Hwang, and Chandra M. Sehgal. 2024. "Sonothrombolysis: State-of-the-Art and Potential Applications in Children" Children 11, no. 1: 57. https://doi.org/10.3390/children11010057






