Enterocins Produced by Enterococci Isolated from Breast-Fed Infants: Antilisterial Potential
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Cultures and Media
2.2. L. monocytogenes Antibiotic Susceptibility Testing
2.3. Detection of Enterocin Genes
2.4. Bacteriocin Bioassay and Spectrum of Activity
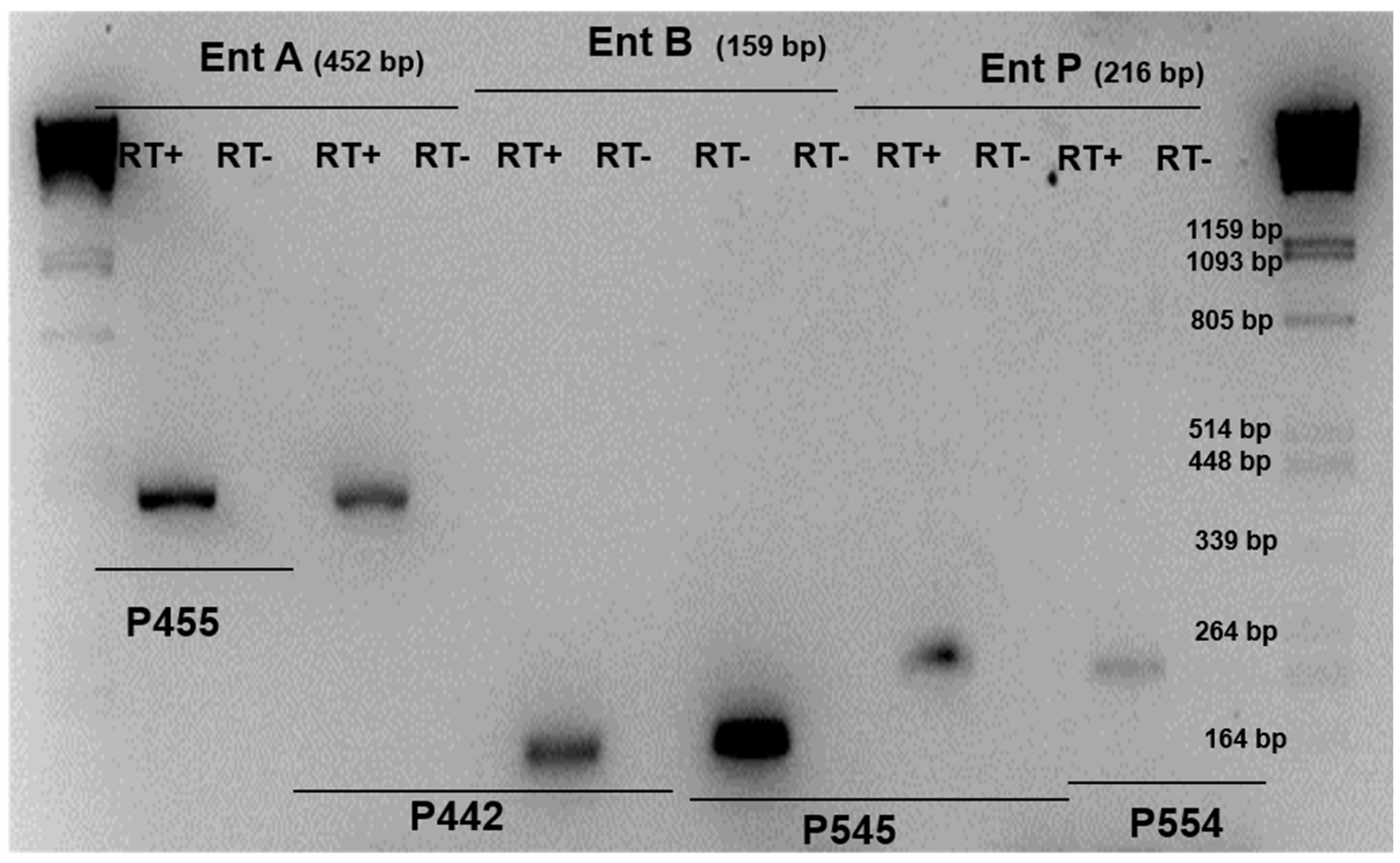
2.5. Total RNA Isolation and Reverse Transcription (RT)-PCR Analysis
2.6. Overlay Agar Spot Assay
3. Results
3.1. Antibiotic Susceptibility of L. monocytogenes
3.2. Detection of Enterocin Genes
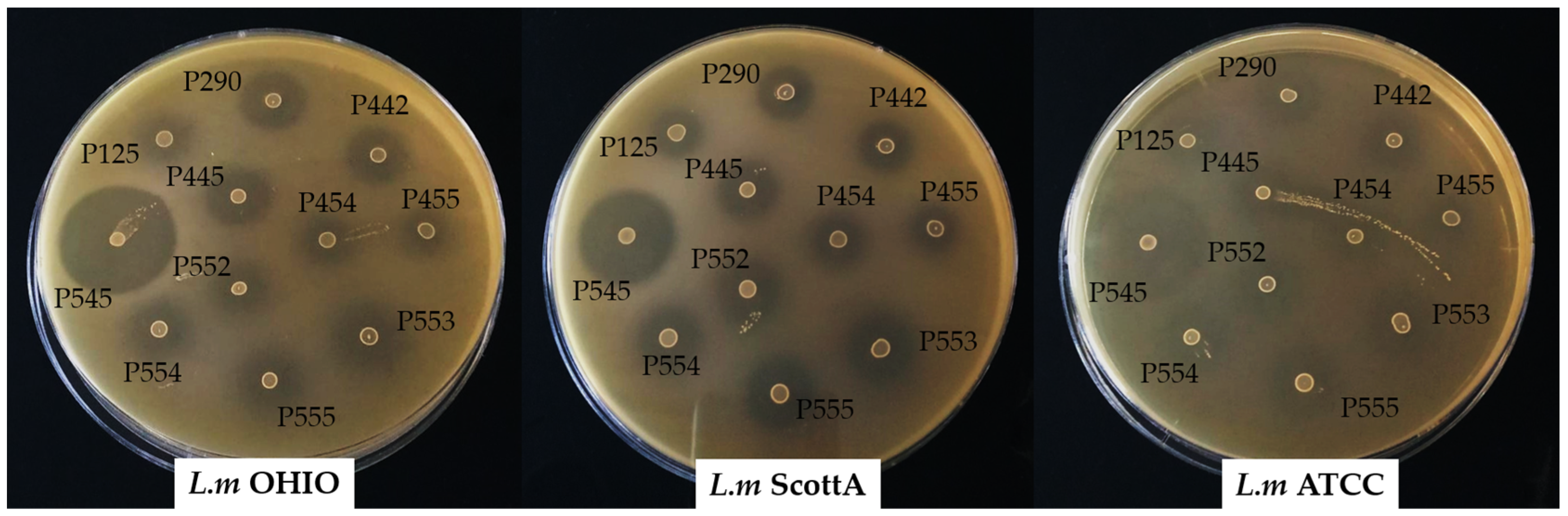
3.3. Antimicrobial Activity
3.4. Transcriptional Analysis of Enterocins
3.5. Overlay Agar Spot Assay
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cotter, P.; Ross, R.; Hill, C. Bacteriocins—A viable alternative to antibiotics? Nat. Rev. Microbiol. 2013, 11, 95–105. [Google Scholar] [CrossRef] [PubMed]
- McLauchlin, J.; Amar, C.F.L.; Grant, K.A. Neonatal cross-infection due to Listeria monocytogenes. Epidemiol. Infect. 2022, 150, e77. [Google Scholar] [CrossRef]
- Olaimat, A.N.; Al-Holy, M.A.; Shahbaz, H.M.; Al-Nabulsi, A.A.; Abu Ghoush, M.H.; Osaili, T.M.; Ayyash, M.M.; Holley, R.A. Emergence of antibiotic resistance in Listeria monocytogenes isolated from food products: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1277–1292. [Google Scholar] [CrossRef] [PubMed]
- Wilking, H.; Lachmann, R.; Holzer, A.; Halbedel, S.; Flieger, A.; Stark, K. Ongoing high incidence and case-fatality rates for invasive listeriosis, Germany, 2010–2019. Emerg. Infect. Dis. 2021, 27, 2485–2488. [Google Scholar] [CrossRef] [PubMed]
- Pohl, A.M.; Pouillot, R.; Bazaco, M.C.; Wolpert, B.J.; Healy, J.M.; Bruce, B.B.; Laughlin, M.E.; Hunter, J.C.; Dunn, J.R.; Hurd, S.; et al. Differences among incidence rates of invasive listeriosis in the U.S. FoodNet population by age, sex, race/ethnicity, and pregnancy status, 2008–2016. Foodborne Pathog. Dis. 2019, 16, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Herrador, Z.; Gherasim, A.; Lopez-Velez, R.; Benito, A. Listeriosis in Spain based on hospitalisation records, 1997 to 2015: Need for greater awareness. Eurosurveillance 2019, 24, 1800271. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Bai, L.; Ma, X.; Zhang, X.; Li, X.; Yang, X.; Huang, J.Y.; Fanning, S.; Guo, Y. Sentinel listeriosis surveillance in selected hospitals, China, 2013–2017. Emerg. Infect. Dis. 2019, 25, 2274–2277. [Google Scholar] [CrossRef]
- Zhang, X.; Niu, Y.; Liu, Y.; Lu, Z.; Wang, D.; Cui, X.; Chen, Q.; Ma, X. Isolation and characterization of clinical Listeria monocytogenes in Beijing, China, 2014–2016. Front. Microbiol. 2019, 10, 981. [Google Scholar] [CrossRef]
- Ke, Y.; Ye, L.; Zhu, P.; Sun, Y.; Zhu, Z. Listeriosis during pregnancy: A retrospective cohort study. BMC Pregnancy Childbirth 2022, 22, 261. [Google Scholar] [CrossRef]
- Wang, Z.; Tao, X.; Liu, S.; Zhao, Y.; Yang, X. An update review on Listeria infection in pregnancy. Infect. Drug Resist. 2021, 14, 1967–1978. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Santos, A.C.; Novais, C.; Peixe, L.; Freitas, A.R. Enterococcus spp. as a producer and target of bacteriocins: A double-edged sword in the antimicrobial resistance crisis context. Antibiotics 2021, 10, 1215. [Google Scholar] [CrossRef] [PubMed]
- Franz, C.M.; van Belkum, M.J.; Holzapfel, W.H.; Abriouel, H.; Gálvez, A. Diversity of enterococcal bacteriocins and their grouping in a new classification scheme. FEMS Microbiol. Rev. 2007, 31, 293–310. [Google Scholar] [CrossRef] [PubMed]
- Layton, B.A.; Walters, S.P.; Lam, L.H.; Boehm, A.B. Enterococcus species distribution among human and animal hosts using multiplex PCR. J. Appl. Microbiol. 2020, 109, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Hanchi, H.; Mottawea, W.; Sebei, K.; Hammami, R. The genus Enterococcus: Between probiotic potential and safety concerns—An update. Front. Microbiol. 2018, 9, 1791. [Google Scholar] [CrossRef] [PubMed]
- Bondi, M.; Laukova, A.; de Niederhausern, S.; Messi, P.; Papadopoulou, C.; Economou, V. Controversial Aspects Displayed by Enterococci: Probiotics or Pathogens? Biomed. Res. Int. 2020, 20, 9816185. [Google Scholar] [CrossRef] [PubMed]
- Flokas, M.E.; Karageorgos, S.A.; Detsis, M.; Alevizakos, M.; Mylonakis, E. Vancomycin-resistant enterococci colonisation, risk fac-tors and risk for infection among hospitalised paediatric patients: A systematic review and meta-analysis. Int. J. Antimicrob. Agents 2017, 49, 565–572. [Google Scholar] [CrossRef]
- Martín, R.; Langa, S.; Reviriego, C.; Jiménez, E.; Marín, M.L.; Xaus, J.; Fernández, L.; Rodríguez, J.M. Human milk is a source of lactic acid bacteria for the infant gut. J. Pediatr. 2003, 143, 754–758. [Google Scholar] [CrossRef]
- Landete, J.M.; Peirotén, A.; Medina, M.; Arqués, J.L.; Rodriguez, E. Virulence and antibiotic resistance of enterococci isolated from healthy breastfed infants. Microb. Drug Resist. 2018, 24, 63–69. [Google Scholar] [CrossRef]
- Francino, M.P. Antibiotics and the human gut microbiome: Dysbioses and accumulation of resistances. Front. Microbiol. 2016, 6, 1543. [Google Scholar] [CrossRef]
- Lebreton, F.; van Schaik, W.; McGuire, M.A.; Godfrey, P.; Griggs, A.; Mazumdar, V.; Corander, J.; Cheng, L.; Saif, S.; Young, S.; et al. Emergence of epidemic multidrug-resistant Enterococcus faecium from animal and commensal strains. mBio 2013, 4, e00534-13. [Google Scholar] [CrossRef]
- Neves, J.V. Editorial for Special Issue “Alternatives to Antibiotics: Bacteriocins and Antimicrobial Peptides”. Antibiotics 2022, 11, 860. [Google Scholar] [CrossRef]
- Perez, R.H.; Zendo, T.; Sonomoto, K. Novel bacteriocins from lactic acid bacteria (LAB): Various structures and applications. Microb. Cell Fact. 2014, 13 (Suppl. 1), S3. [Google Scholar] [CrossRef]
- Cavera, V.L.; Arthur, T.D.; Kashtanov, D.; Chikindas, M.L. Bacteriocins and their position in the next wave of conventional antibiotics. Int. J. Antimicrob. Agents 2015, 46, 494–501. [Google Scholar] [CrossRef]
- Alvarez-Sieiro, P.; Montalbán-López, M.; Mu, D.; Kuipers, O.P. Bacteriocins of lactic acid bacteria: Extending the family. Appl. Microbiol. Biotechnol. 2016, 100, 2939–2951. [Google Scholar] [CrossRef]
- Cavicchioli, V.Q.; Camargo, A.C.; Todorov, S.D.; Nero, L.A. Novel bacteriocinogenic Enterococcus hirae and Pediococcus pentosaceus strains with antilisterial activity isolated from Brazilian artisanal cheese. J. Dairy Sci. 2017, 100, 2526–2535. [Google Scholar] [CrossRef]
- Moraes, P.M.; Perin, L.M.; Todorov, S.D.; Franco, S.D.; Silva, A.; Franco, B.D.G.M.; Nero, L.A. Bacteriocinogenic and virulence potential of Enterococcus isolates obtained from raw milk and cheese. J. Appl. Microbiol. 2012, 113, 318–328, Erratum in J. Appl. Microbiol. 2012, 113, 564. [Google Scholar] [CrossRef] [PubMed]
- Im, E.J.; Lee, H.H.; Kim, M.; Kim, M.K. Evaluation of enterococcal probiotic usage and review of potential health benefits, safety, and risk of antibiotic-resistant strain emergence. Antibiotics 2023, 12, 1327. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, L.; Langa, S.; Martin, V.; Maldonado, A.; Jimenez, E.; Martin, R.; Rodríguez, J.M. The human milk microbiota: Origin and potential roles in health and disease. Pharmacol. Res. 2013, 69, 1–10. [Google Scholar] [CrossRef] [PubMed]
- EUCAST Disk Diffusion Method for Antimicrobial Susceptibility Testing. Version 11.0. January 2023. Available online: www.eucast.org (accessed on 19 September 2023).
- Sánchez-Hidalgo, M.; Maqueda, M.; Gálvez, A.; Abriouel, H.; Valdivia, E.; Martínez-Bueno, M. The genes coding for enterocin EJ97 production by Enterococcus faecalis EJ97 are located on a conjugative plasmid. Appl. Environ. Microbiol. 2003, 69, 1633–1641. [Google Scholar] [CrossRef] [PubMed]
- Aymerich, T.; Holo, H.; Havarstein, L.S.; Hugas, M.; Garriga, M.; Nes, I.F. Biochemical and genetic characterization of enterocin A from Enterococcus faecium, a new antilisterial bacteriocin in the pediocin family of bacteriocins. Appl. Environ. Microbiol. 1996, 62, 1676–1682. [Google Scholar] [CrossRef] [PubMed]
- Du Toit, M.; Franz, C.; Dicks, L.M.T.; Holzapfel, W.H. Preliminary characterization of bacteriocins produced by Enterococcus faecium and Enterococcus faecalis isolated from pig faeces. J. Appl. Microbiol. 2000, 88, 482–494. [Google Scholar] [CrossRef]
- Gutiérrez, J.; Criado, R.; Citti, R.; Martín, M.; Herranz, C.; Nes, I.F.; Cintas, L.M.; Hernández, P.E. Cloning, production and functional expression of enterocin P, a sec-dependent bacteriocin produced by Enterococcus faecium P13, in Escherichia coli. Int. J. Food Microbiol. 2005, 103, 239–250. [Google Scholar] [CrossRef]
- Ruiz-Rodríguez, M.; Martínez-Bueno, M.; Martín-Vivaldi, M.; Valdivia, E.; Soler, J.J. Bacteriocins with a broader antimicrobial spectrum prevail in enterococcal symbionts isolated from the hoopoe’s uropygial gland. FEMS Microbiol. Ecol. 2013, 85, 495–502. [Google Scholar] [CrossRef]
- Yousif, N.M.K.; Dawyndt, P.; Abriouel, H.; Wijaya, A.; Schillinger, U.; Vancanneyt, M.; Swings, J.; Dirar, H.A. Molecular characterization, technological properties and safety aspects of enterococci from ‘Hussuwa’, an African fermented sorghum product. J. Appl. Microbiol. 2005, 98, 216–228. [Google Scholar] [CrossRef]
- Martínez-Bueno, M.; Valdivia, E.; Gálvez, A.; Coyette, J.; Maqueda, M. Analysis of the gene cluster involved in production and immunity of the peptide antibiotic AS-48 in Enterococcus faecalis. Mol. Microbiol. 1998, 27, 347–358. [Google Scholar] [CrossRef]
- Holo, H.; Nilssen, O.; Nes, I.F. Lactococcin A, a new bacteriocin from Lactococcus lactis subsp. cremoris: Isolation and charac-terization of the protein and its gene. J. Bacteriol. 1991, 173, 3879–3887. [Google Scholar] [CrossRef]
- Baquero, F.; Lanza, V.F.; Duval, M.; Coque, T.M. Ecogenetics of antibiotic resistance in Listeria monocytogenes. Mol. Microbiol. 2020, 113, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Charpentier, E.; Courvalin, P. Antibiotic resistance in Listeria spp. Antimicrob. Agents Chemother. 1999, 43, 2103–2210. [Google Scholar] [CrossRef] [PubMed]
- Poyart-Salmeron, C.; Carlier, C.; Trieu-Cuot, P.; Courtier, A.L.; Courvalin, P. Transferable plasmid-mediated antibiotic resistance in Listeria monocytogenes. Lancet 1990, 335, 1419–1426. [Google Scholar]
- Caruso, M.; Fraccalvieri, R.; Pasquali, F.; Santagada, G.; Latorre, L.M.; Difato, L.M.; Miccolupo, A.; Normanno, G.; Parisi, A. Antimicrobial susceptibility and multilocus sequence typing of Listeria monocytogenes isolated over 11 years from food, humans, and the environment in Italy. Foodborne Pathog. Dis. 2020, 17, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Vicente, M.F.; Perez-Díaz, J.C.; Baquero, F.; de Pedro, M.A.; Berenguer, J. Penicillin-binding protein 3 of Listeria monocytogenes as the primary lethal target for beta-lactams. Antimicrob. Agents Chemother. 1990, 34, 539–542. [Google Scholar] [CrossRef]
- Mata, M.T.; Baquero, F.; Perez-Diaz, J.C. A multidrug efflux transporter in Listeria monocytogenes. FEMS Microbiol. Lett. 2000, 187, 185–188. [Google Scholar] [CrossRef]
- De Vuyst, L.; Moreno, M.F.; Revets, H. Screening for enterocins and detection of hemolysin and vancomycin resistance in enterococci of different origins. Int. J. Food Microbiol. 2003, 84, 299–318. [Google Scholar] [CrossRef]
- Pangallo, D.; Harichová, J.; Karelová, E.; Drahovská, H.; Chovanová, K.; Ferianc, P.; Timko, J. Molecular investigation of enterococci isolated from different environmental sources. Biologia 2004, 59, 829–837. [Google Scholar]
- Özdemir, G.B.; Oryasm, E.; Biyik, H.H.; Özteber, M.; Bozdoğan, B. Phenotypic and genotypic characterization of bacteriocins in enterococcal isolates of different sources. Ind. J. Microbiol. 2011, 51, 182–187. [Google Scholar] [CrossRef]
- Poeta, P.; Costa, D.; Rojo-Bezares, B.; Zarazaga, M.; Klibi, N.; Rodrigues, J.; Torres, C. Detection of antimicrobial activities and bacteriocin structural genes in faecal enterococci of wild animals. Microbiol. Res. 2007, 162, 257–263. [Google Scholar] [CrossRef]
- Casaus, F.; Nilsen, T.; Cintas, L.M.; Nes, L.F.; Hernández, P.E.; Holo, H. Enterocin B, a new bacteriocin from Enterococcus faecium TI36 which can act synergistically with enterocin A. Microbiology 1997, 143, 2287–2294. [Google Scholar] [CrossRef]
- Franz, C.M.A.P.; Worobo, R.W.W.; Quadri, L.E.N.; Schillinger, U.; Holzapfel, W.H.; Vederas, J.C.; Stiles, M.E. Atypical genetic locus associated with constitutive production of enterocin B by Enterococcus faecium BFE 900. Appl. Environ. Microbiol. 1999, 65, 2170–2178. [Google Scholar] [CrossRef] [PubMed]
- Foulquié Moreno, M.R.; Callewaert, R.; Devreese, B.; Van Beeumen, J.; De Vuyst, L. Isolation and biochemical characterisation of enterocins produced by enterococci from different sources. J. Appl. Microbiol. 2003, 94, 214–229. [Google Scholar] [CrossRef] [PubMed]
- Arqués, J.L.; Rodríguez, E.; Langa, S.; Landete, J.M.; Medina, M. Antimicrobial activity of lactic acid bacteria in dairy products and gut: Effect on pathogens. Biomed. Res. Int. 2015, 2015, 584183. [Google Scholar] [CrossRef] [PubMed]
- Ogaki, M.B.; Rocha, K.R.; Terra, M.R.; Furlaneto, M.C.; Furlaneto-Maia, L. Screening of the enterocin-encoding genes and antimicrobial activity in Enterococcus species. J. Microbiol. Biotechnol. 2016, 26, 1026–1034. [Google Scholar] [CrossRef] [PubMed]
- Darbandi, A.; Asadi, A.; Ari, M.M.; Ohadi, E.; Talebi, M.; Zadeh, M.H.; Emamie, A.D.; Ghanavati, R.; Kakanj, M. Bacteriocins: Properties and potential use as antimicrobials. J. Clin. Lab. Anal. 2022, 36, 24093. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Pang, X.; Wu, Y.; Liu, X.; Zhang, X. Enterocins: Classification, Synthesis, Antibacterial Mechanisms and Food Applications. Molecules 2022, 27, 2258. [Google Scholar] [CrossRef]
- Zdolec, N.; Kiš, M. Antimicrobial properties of food enterococci. In Lactic Acid Bacteria in Food Biotechnology; Innovations and Functional Aspects; Ray, R.C., Paramithiotis, P., Azevedo, V.A.C., Montet, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; Volume 11, pp. 195–203. [Google Scholar] [CrossRef]
- Umu, Ö.C.O.; Bäuerl, C.; Oostindjer, M.; Pope, P.B.; Hernández, P.E.; Pérez-Martínez, G.; Diep, D.B. The potential of class II bacteriocins to modify gut microbiota to improve host health. PLoS ONE 2016, 11, e0164036. [Google Scholar] [CrossRef]
- Dobson, A.; Cotter, P.D.; Ross, R.P.; Hill, C. Bacteriocin production: A probiotic trait? Appl. Environ. Microbiol. 2012, 78, 1–6. [Google Scholar] [CrossRef]
- Ramirez, J.; Guarner, F.; Bustos Fernandez, L.; Maruy, A.; Sdepanian, V.L.; Cohen, H. Antibiotics as major disruptors of gut microbiota. Front. Cell. Infect. Microbiol. 2020, 10, 572912. [Google Scholar] [CrossRef]
- McDonnell, L.; Gilkes, A.; Ashworth, M.; Rowland, V.; Harries, T.H.; Armstrong, D.; White, P. Association between antibiotics and gut microbiome dysbiosis in children: Systematic review and meta-analysis. Gut Microbes 2021, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Franz, C.M.A.P.; Huch, M.; Abriouel, H.; Holzapfel, W.; Gálvez, A. Enterococci as probiotics and their implications in food safety. Int. J. Food Microbiol. 2011, 151, 125–140. [Google Scholar] [CrossRef]
- Giraffa, G. Functionality of enterococci in dairy products. Int. J. Food Microbiol. 2003, 88, 215–222. [Google Scholar] [CrossRef]
- Dapkevicius, M.d.L.E.; Sgardioli, B.; Câmara, S.P.A.; Poeta, P.; Malcata, F.X. Current Trends of Enterococci in Dairy Products: A Comprehensive Review of Their Multiple Roles. Foods 2021, 10, 821. [Google Scholar] [CrossRef]
- Eijsink, V.G.; Axelsson, L.D.; Diep, B.; Håvarstein, L.S.; Holo, H.; Nes, I.F. Production of class II bacteriocins by lactic acid bacteria; an example of biological warfare and communication. Antonie Leeuwenhoek 2002, 81, 639–654. [Google Scholar] [CrossRef] [PubMed]
- Gensollen, T.; Iyer, S.S.; Kasper, D.L.; Blumberg, R.S. How colonization by microbiota in early life shapes the immune system. Science 2016, 352, 539–544. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yang, Q.; Tian, L.; Zhang, Z.; Qiu, L.; Tao, X.; Wei, H. Protection of surface layer protein from Enterococcus faecium WEFA23 against Listeria monocytogenes CMCC54007 infection by modulating intestinal permeability and immunity. Appl. Microbiol. Biotechnol. 2021, 105, 4269–4284. [Google Scholar] [CrossRef] [PubMed]
- Popović, N.; Djokić, J.; Brdarić, E.; Dinić, M.; Terzić-Vidojević, A.; Golić, N.; Veljović, K. The influence of heat-killed Enterococcus faecium BGPAS1-3 on the tight junction protein expression and immune function in differentiated Caco-2 cells infected with Listeria monocytogenes ATCC 19111. Front. Microbiol. 2019, 10, 412. [Google Scholar] [CrossRef] [PubMed]
- Rajput, K.; Dubey, R.C.; Kumar, A. Probiotic potential and immunomodulatory properties in Enterococcus faecium GMB24 and Enterococcus hirae SMB16 isolated from goat and sheep milk. Arch. Microbiol. 2022, 204, 619. [Google Scholar] [CrossRef]
- Salvucci, E.; Saavedra, L.; Hebert, E.M.; Haro, C.; Sesma, F. Enterocin CRL35 inhibits Listeria monocytogenes in a murine model. Foodborne Pathog. Dis. 2012, 9, 68–74. [Google Scholar] [CrossRef]
- Song, M.W.; Kim, K.-T.; Paik, H.-D. Probiotics as a functional health supplement in infant formulas for the improvement of intestinal microflora and immunity. Food Rev. Int. 2021, 39, 858–874. [Google Scholar] [CrossRef]

| Enterocin | Primers | Sequence (5’ to 3’) | Product Size (pb) | References |
|---|---|---|---|---|
| EJ97 | E21-4 | GCAGCTAAGCTAACGACT | 279 | [30] |
| E21-9 | AGGGGAATTTGAACAGA | |||
| A | P9 | GAGATTTATCTCCATAATCT | 452 | [31] |
| P10 | GTACCACTCATAGTGGAA | |||
| B | EntB(f) | GAAAATGATCACAGAATGCCTA | 159 | [32] |
| EntB(r) | GTTGCATTTAGAGTATACATTTG | |||
| P | EntP1 | ATGAGAAAAAAATTATTTAGTTT | 216 | [33] |
| EntP2 | TTAATGTCCCATACCTGCCAAACC | |||
| MR10A | LICIJ1 | ATGGGAGCAATCGCAAAA | 135 | [34] |
| LICJ2A | TTAAATATGTTTTTTAATCCA | |||
| L50 | L50F | ATGGGAGCAATCGCAAAATTAG | 98 | [35] |
| L50R | ATTGCCCATCCTTCTCCAAT | |||
| AS-48 | As48-1 | AATAAACTACATGGGT | 377 | [36] |
| As-48-5 | CCAAGCAATAACTGCTCTTT |
| Strain | Enterocin | Gene 1 | Inhibition EU/mL 2 | ||
|---|---|---|---|---|---|
| Expression | L.m OHIO | L.m ScottA | L.m ATCC | ||
| E. faecium INIA P125 | A | A (-) | - | - | - |
| E. faecalis INIA P290 | AS-48 | AS-48 (++) | 2560 | 1280 | 2560 |
| E. faecium INIA P442 | A, B | A (++), B (+++) | 5120 | 2560 | 5120 |
| E. faecium INIA P445 | A, B | A (+), B (++) | 2560 | 1280 | 2560 |
| E. faecium INIA P454 | A, B | A (+), B (-) | - | - | - |
| E. faecium INIA P455 | A | A (++) | - | - | - |
| E. faecium INIA P545 | B, P | B (++++), P (+) | 81,920 | 10,240 | 81,920 |
| E. faecium INIA P552 | A | A (-) | - | - | - |
| E. faecium INIA P553 | P | P (++) | - | - | - |
| E. faecium INIA P554 | P | P (+) | - | - | - |
| E. faecium INIA P555 | P | P (+) | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Landete, J.M.; Montiel, R.; Rodríguez-Mínguez, E.; Arqués, J.L. Enterocins Produced by Enterococci Isolated from Breast-Fed Infants: Antilisterial Potential. Children 2024, 11, 261. https://doi.org/10.3390/children11020261
Landete JM, Montiel R, Rodríguez-Mínguez E, Arqués JL. Enterocins Produced by Enterococci Isolated from Breast-Fed Infants: Antilisterial Potential. Children. 2024; 11(2):261. https://doi.org/10.3390/children11020261
Chicago/Turabian StyleLandete, José María, Raquel Montiel, Eva Rodríguez-Mínguez, and Juan L. Arqués. 2024. "Enterocins Produced by Enterococci Isolated from Breast-Fed Infants: Antilisterial Potential" Children 11, no. 2: 261. https://doi.org/10.3390/children11020261





