Speech Sounds Production, Narrative Skills, and Verbal Memory of Children with 22q11.2 Microdeletion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
- (1)
- (2)
- (3)
- (4)
2.2. FISH and MLPA
2.3. Assessment of Articulatory Characteristics of Speech Sounds
2.4. Assessment of Spontaneous Language Abilities
2.5. Assessment of Immediate Verbal Memory
2.6. Statistics
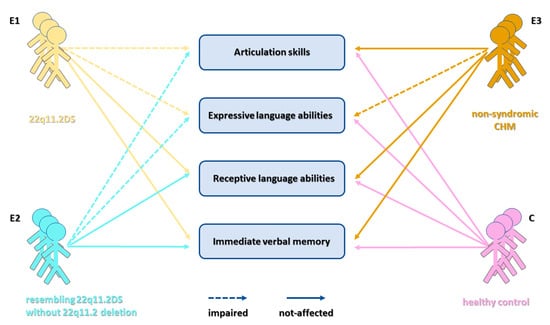
3. Results
3.1. Detection of 22q11.2 Microdeletion
3.2. Analysis of Articulatory Characteristics of Speech Sounds in Children with 22q11.2 Microdeletion
3.2.1. Pronunciation of Vowels
3.2.2. Pronunciation of Plosives
3.2.3. Pronunciation of Nasals
3.2.4. Pronunciation of Laterals
3.2.5. Pronunciation of Fricatives
3.2.6. Pronunciation of Affricates
3.2.7. Pronunciation of Semivowels
3.2.8. Pronunciation of Vibrant
3.3. Analysis of Spontaneous Language Abilities
3.4. Assessment of Immediate Verbal Memory
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blagojevic, C.; Heung, T.; Theriault, M.; Tomita-Mitchell, A.; Chakraborty, P.; Kernohan, K.; Bulman, D.E.; Bassett, A.S. Estimate of the contemporary live-birth prevalence of recurrent 22q11.2 deletions: A cross-sectional analysis from population-based newborn screening. CMAJ Open 2021, 9, E802–E809. [Google Scholar] [CrossRef]
- Kobrynski, L.J.; Sullivan, K.E. Velocardiofacial syndrome, DiGeorge syndrome: The chromosome 22q11.2 deletion syndromes. Lancet 2007, 370, 1443–1452. [Google Scholar] [CrossRef]
- Squarcione, C.; Torti, M.C.; Di Fabio, F.; Biondi, M. 22q11 deletion syndrome: A review of the neuropsychiatric features and their neurobiological basis. Neuropsychiatr. Dis. Treat. 2013, 9, 1873–1884. [Google Scholar] [CrossRef] [PubMed]
- Carelle-Calmels, N.; Saugier-Veber, P.; Girard-Lemaire, F.; Rudolf, G.; Doray, B.; Guerin, E.; Kuhn, P.; Arrive, M.; Gilch, C.; Schmitt, E.; et al. Genetic compensation in a human genomic disorder. N. Engl. J. Med. 2009, 360, 1211–1216. [Google Scholar] [CrossRef] [PubMed]
- McDonald-McGinn, D.M.; Fahiminiya, S.; Revil, T.; Nowakowska, B.A.; Suhl, J.; Bailey, A.; Mlynarski, E.; Lynch, D.R.; Yan, A.C.; Bilaniuk, L.T.; et al. Hemizygous mutations in SNAP29 unmask autosomal recessive conditions and contribute to atypical findings in patients with 22q11.2ds. J. Med. Genet. 2013, 50, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Cancrini, C.; Puliafito, P.; Digilio, M.C.; Soresina, A.; Martino, S.; Rondelli, R.; Consolini, R.; Ruga, E.M.; Cardinale, F.; Finocchi, A.; et al. Clinical features and follow-up in patients with 22q11.2 deletion syndrome. J. Pediatr. 2014, 164, 1475–1480.e1472. [Google Scholar] [CrossRef]
- Shprintzen, R.J. Velo-cardio-facial syndrome: 30 Years of study. Dev. Disabil. Res. Rev. 2008, 14, 3–10. [Google Scholar] [CrossRef]
- Firth, H.V.; Hurst, J.A. (Eds.) Oxford Desk Reference: Clinical Genetics; Oxford University Press: Oxford, UK, 2005. [Google Scholar]
- Dori, N.; Green, T.; Weizman, A.; Gothelf, D. The Effectiveness and Safety of Antipsychotic and Antidepressant Medications in Individuals with 22q11.2 Deletion Syndrome. J. Child Adolesc. Psychopharmacol. 2017, 27, 83–90. [Google Scholar] [CrossRef]
- Solot, C.B.; Sell, D.; Mayne, A.; Baylis, A.L.; Persson, C.; Jackson, O.; McDonald-McGinn, D.M. Speech-Language Disorders in 22q11.2 Deletion Syndrome: Best Practices for Diagnosis and Management. Am. J. Speech-Lang. Pathol. 2019, 28, 984–999. [Google Scholar] [CrossRef]
- Solot, C.B.; Gerdes, M.; Kirschner, R.E.; McDonald-McGinn, D.M.; Moss, E.; Woodin, M.; Aleman, D.; Zackai, E.H.; Wang, P.P. Communication issues in 22q11.2 deletion syndrome: Children at risk. Genet. Med. 2001, 3, 67–71. [Google Scholar] [CrossRef]
- Widdershoven, J.C.; Beemer, F.A.; Kon, M.; Dejonckere, P.H.; Mink van der Molen, A.B. Possible mechanisms and gene involvement in speech problems in the 22q11.2 deletion syndrome. J. Plast. Reconstr. Aesthetic Surg. 2008, 61, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Persson, C.; Lohmander, A.; Jonsson, R.; Oskarsdottir, S.; Soderpalm, E. A prospective cross-sectional study of speech in patients with the 22q11 deletion syndrome. J. Commun. Disord. 2003, 36, 13–47. [Google Scholar] [CrossRef] [PubMed]
- Solot, C.B.; Knightly, C.; Handler, S.D.; Gerdes, M.; McDonald-McGinn, D.M.; Moss, E.; Wang, P.; Cohen, M.; Randall, P.; Larossa, D.; et al. Communication disorders in the 22Q11.2 microdeletion syndrome. J. Commun. Disord. 2000, 33, 187–203; quiz 203–204. [Google Scholar] [CrossRef] [PubMed]
- Solot, C.B.; Moore, T.M.; Crowley, T.B.; Gerdes, M.; Moss, E.; McGinn, D.E.; Emanuel, B.S.; Zackai, E.H.; Gallagher, S.; Calkins, M.E.; et al. Early language measures associated with later psychosis features in 22q11.2 deletion syndrome. Am. J. Med. Genetics. Part B Neuropsychiatr. Genet. 2020, 183, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Gaynor, J.W.; Stopp, C.; Wypij, D.; Andropoulos, D.B.; Atallah, J.; Atz, A.M.; Beca, J.; Donofrio, M.T.; Duncan, K.; Ghanayem, N.S.; et al. Neurodevelopmental outcomes after cardiac surgery in infancy. Pediatrics 2015, 135, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Cuturilo, G.; Drakulic, D.; Krstic, A.; Gradinac, M.; Ilisic, T.; Parezanovic, V.; Milivojevic, M.; Stevanovic, M.; Jovanovic, I. The role of modern imaging techniques in the diagnosis of malposition of the branch pulmonary arteries and possible association with microdeletion 22q11.2. Cardiol. Young 2013, 23, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Rakonjac, M.; Cuturilo, G.; Stevanovic, M.; Jovanovic, I.; Jelicic Dobrijevic, L.; Mijovic, M.; Drakulic, D. Speech and language abilities of children with the familial form of 22q11.2 deletion syndrome. Genetika 2016, 48, 57–72. [Google Scholar] [CrossRef]
- Kostic, D.; Vladisavljevic, S. Testovi za Ispitivanje Govora i Jezika [Tests for Speech and Language]; Zavod za Udzbenike i Nastavna Sredstva: Belgrade, Serbia, 1983. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org (accessed on 24 January 2024).
- Rakonjac, M.; Cuturilo, G.; Stevanovic, M.; Jelicic, L.; Subotic, M.; Jovanovic, I.; Drakulic, D. Differences in speech and language abilities between children with 22q11.2 deletion syndrome and children with phenotypic features of 22q11.2 deletion syndrome but without microdeletion. Res. Dev. Disabil. 2016, 55, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Cuturilo, G.; Drakulic, D.; Jovanovic, I.; Krstic, A.; Djukic, M.; Skoric, D.; Mijovic, M.; Stefanovic, I.; Milivojevic, M.; Stevanovic, M. Improving the Diagnosis of Children with 22q11.2 Deletion Syndrome: A Single-center Experience from Serbia. Indian Pediatr. 2016, 53, 786–789. [Google Scholar] [CrossRef]
- D’Antonio, L.L.; Scherer, N.J.; Miller, L.L.; Kalbfleisch, J.H.; Bartley, J.A. Analysis of speech characteristics in children with velocardiofacial syndrome (VCFS) and children with phenotypic overlap without VCFS. Cleft Palate-Craniofacial J. 2001, 38, 455–467. [Google Scholar] [CrossRef]
- Wernovsky, G. Current insights regarding neurological and developmental abnormalities in children and young adults with complex congenital cardiac disease. Cardiol. Young 2006, 16 (Suppl. S1), 92–104. [Google Scholar] [CrossRef]
- Goodship, J.; Cross, I.; Scambler, P.; Burn, J. Monozygotic twins with chromosome 22q11 deletion and discordant phenotype. J. Med. Genet. 1995, 32, 746–748. [Google Scholar] [CrossRef] [PubMed]
- Sebastian-Lazaro, D.; Brun-Gasca, C.; Fornieles, A. Voice and speech of children with 22q11 deletion syndrome. Rev. Neurol. 2019, 68, 99–106. [Google Scholar] [PubMed]
- Scherer, N.J.; D’Antonio, L.L.; Kalbfleisch, J.H. Early speech and language development in children with velocardiofacial syndrome. Am. J. Med. Genet. 1999, 88, 714–723. [Google Scholar] [CrossRef]
- Roizen, N.J.; Antshel, K.M.; Fremont, W.; AbdulSabur, N.; Higgins, A.M.; Shprintzen, R.J.; Kates, W.R. 22q11.2DS deletion syndrome: Developmental milestones in infants and toddlers. J. Dev. Behav. Pediatr. 2007, 28, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Everaert, E.; Selten, I.; Boerma, T.; Houben, M.; Vorstman, J.; de Wilde, H.; Derksen, D.; Haverkamp, S.; Wijnen, F.; Gerrits, E. The Language Profile of Preschool Children With 22q11.2 Deletion Syndrome and the Relationship With Speech Intelligibility. Am. J. Speech-Lang. Pathol. 2023, 32, 128–144. [Google Scholar] [CrossRef] [PubMed]
- Swillen, A.; Vogels, A.; Devriendt, K.; Fryns, J.P. Chromosome 22q11 deletion syndrome: Update and review of the clinical features, cognitive-behavioral spectrum, and psychiatric complications. Am. J. Med. Genet. 2000, 97, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Persson, C.; Niklasson, L.; Oskarsdottir, S.; Johansson, S.; Jonsson, R.; Soderpalm, E. Language skills in 5-8-year-old children with 22q11 deletion syndrome. Int. J. Lang. Commun. Disord. 2006, 41, 313–333. [Google Scholar] [CrossRef] [PubMed]
- Boerma, T.; Everaert, E.; Vlieger, D.; Steggink, M.; Selten, I.; Houben, M.; Vorstman, J.; Gerrits, E.; Wijnen, F. Grammatical skills of Dutch children with 22q11.2 Deletion Syndrome in comparison with children with Developmental Language Disorder: Evidence from spontaneous language and standardized assessment. Front. Commun. 2023, 8, 1111584. [Google Scholar] [CrossRef]
- Van Den Heuvel, E.; ReuterskioLd, C.; Solot, C.; Manders, E.; Swillen, A.; Zink, I. Referential communication abilities in children with 22q11.2 deletion syndrome. Int. J. Speech-Lang. Pathol. 2017, 19, 490–502. [Google Scholar] [CrossRef]
- McCabe, K.L.; Marlin, S.; Cooper, G.; Morris, R.; Schall, U.; Murphy, D.G.; Murphy, K.C.; Campbell, L.E. Visual perception and processing in children with 22q11.2 deletion syndrome: Associations with social cognition measures of face identity and emotion recognition. J. Neurodev. Disord. 2016, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- Hemphill, L.; Uccelli, P.; Winner, K.; Chang, C.J.; Bellinger, D. Narrative discourse in young children with histories of early corrective heart surgery. J. Speech Lang. Hear. Res. 2002, 45, 318–331. [Google Scholar] [CrossRef] [PubMed]
- Selten, I.; Boerma, T.; Everaert, E.; Gerrits, E.; Houben, M.; Wijnen, F.; Vorstman, J. Behaviors related to autism spectrum disorder in children with developmental language disorder and children with 22q11.2 deletion syndrome. Autism Dev. Lang. Impair. 2023, 8, 23969415231179844. [Google Scholar] [CrossRef] [PubMed]
- Glaser, B.; Mumme, D.L.; Blasey, C.; Morris, M.A.; Dahoun, S.P.; Antonarakis, S.E.; Reiss, A.L.; Eliez, S. Language skills in children with velocardiofacial syndrome (deletion 22q11.2). J. Pediatr. 2002, 140, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Gerdes, M.; Solot, C.; Wang, P.P.; McDonald-McGinn, D.M.; Zackai, E.H. Taking advantage of early diagnosis: Preschool children with the 22q11.2 deletion. Genet. Med. 2001, 3, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Kambanaros, M.; Grohmann, K.K. Linguistic and Nonverbal Abilities over Time in a Child Case of 22q11 Deletion Syndrome. Biolinguistics 2017, 11, 57–81. [Google Scholar] [CrossRef]
- Hovels-Gurich, H.H.; Konrad, K.; Skorzenski, D.; Nacken, C.; Minkenberg, R.; Messmer, B.J.; Seghaye, M.C. Long-term neurodevelopmental outcome and exercise capacity after corrective surgery for tetralogy of Fallot or ventricular septal defect in infancy. Ann. Thorac. Surg. 2006, 81, 958–966. [Google Scholar] [CrossRef] [PubMed]
- Campbell, L.E.; Azuma, R.; Ambery, F.; Stevens, A.; Smith, A.; Morris, R.G.; Murphy, D.G.; Murphy, K.C. Executive functions and memory abilities in children with 22q11.2 deletion syndrome. Aust. New Zealand J. Psychiatry 2010, 44, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Van Den Heuvel, E.; Manders, E.; Swillen, A.; Zink, I. Atypical language characteristics and trajectories in children with 22q11.2 deletion syndrome. J. Commun. Disord. 2018, 75, 37–56. [Google Scholar] [CrossRef]
- Lajiness-O’Neill, R.R.; Beaulieu, I.; Titus, J.B.; Asamoah, A.; Bigler, E.D.; Bawle, E.V.; Pollack, R. Memory and learning in children with 22q11.2 deletion syndrome: Evidence for ventral and dorsal stream disruption? Child Neuropsychol. 2005, 11, 55–71. [Google Scholar] [CrossRef]
- Majerus, S.; Van der Linden, M.; Braissand, V.; Eliez, S. Verbal short-term memory in individuals with chromosome 22q11.2 deletion: Specific deficit in serial order retention capacities? Am. J. Ment. Retard. 2007, 112, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Baker, K.; Baldeweg, T.; Sivagnanasundaram, S.; Scambler, P.; Skuse, D. COMT Val108/158 Met modifies mismatch negativity and cognitive function in 22q11 deletion syndrome. Biol. Psychiatry 2005, 58, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Latreche, C.; Maeder, J.; Mancini, V.; Bortolin, K.; Schneider, M.; Eliez, S. Altered developmental trajectories of verbal learning skills in 22q11.2DS: Associations with hippocampal development and psychosis. Psychol. Med. 2023, 53, 4923–4932. [Google Scholar] [CrossRef] [PubMed]
- Maeder, J.; Sandini, C.; Zoller, D.; Schneider, M.; Bostelmann, M.; Pouillard, V.; Caroni, P.; Kliegel, M.; Eliez, S. [Formula: See text] Long-term verbal memory deficit and associated hippocampal alterations in 22q11.2 deletion syndrome. Child Neuropsychol. 2020, 26, 289–311. [Google Scholar] [CrossRef]




| Group | No. of Patients | 22q11.2 Deletion | 22q11.2DS Phenotype | Non-Syndromic CHD |
|---|---|---|---|---|
| E1 | 15 | yes | yes | no |
| E2 | 14 | no | yes | no |
| E3 | 14 | no | no | yes |
| C | 14 | no | no | no |
| Group | Understood the Content | Did Not Understand the Content | Total |
|---|---|---|---|
| E1 | 11 | 4 | 15 |
| E2 | 12 | 2 | 14 |
| E3 | 14 | 0 | 14 |
| C | 14 | 0 | 14 |
| Total | 51 | 6 | 57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rakonjac, M.; Cuturilo, G.; Kovacevic-Grujicic, N.; Simeunovic, I.; Kostic, J.; Stevanovic, M.; Drakulic, D. Speech Sounds Production, Narrative Skills, and Verbal Memory of Children with 22q11.2 Microdeletion. Children 2024, 11, 489. https://doi.org/10.3390/children11040489
Rakonjac M, Cuturilo G, Kovacevic-Grujicic N, Simeunovic I, Kostic J, Stevanovic M, Drakulic D. Speech Sounds Production, Narrative Skills, and Verbal Memory of Children with 22q11.2 Microdeletion. Children. 2024; 11(4):489. https://doi.org/10.3390/children11040489
Chicago/Turabian StyleRakonjac, Marijana, Goran Cuturilo, Natasa Kovacevic-Grujicic, Ivana Simeunovic, Jovana Kostic, Milena Stevanovic, and Danijela Drakulic. 2024. "Speech Sounds Production, Narrative Skills, and Verbal Memory of Children with 22q11.2 Microdeletion" Children 11, no. 4: 489. https://doi.org/10.3390/children11040489
APA StyleRakonjac, M., Cuturilo, G., Kovacevic-Grujicic, N., Simeunovic, I., Kostic, J., Stevanovic, M., & Drakulic, D. (2024). Speech Sounds Production, Narrative Skills, and Verbal Memory of Children with 22q11.2 Microdeletion. Children, 11(4), 489. https://doi.org/10.3390/children11040489