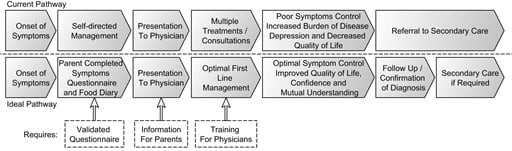
Cow’s Milk Protein Allergy from Diagnosis to Management: A Very Different Journey for General Practitioners and Parents
Abstract
:1. Introduction
2. Methods
Statistical Analysis
3. Results
3.1. Characteristics of GPs and Parents

| Number of Infants <1 Year Diagnosed as CMPA (12 Month Period) | n | % |
|---|---|---|
| 1–2 | 192 | 48% |
| 3–4 | 86 | 21% |
| 5–10 | 94 | 23% |
| 11+ | 31 | 8% |
| Number of Infants <1 Year Treated for CMPA (12 Month Period) | n | % |
| None | 40 | 10% |
| 1–2 | 98 | 24% |
| 3–4 | 102 | 25% |
| 5–10 | 113 | 28% |
| 11+ | 50 | 12% |
3.2. Symptom Presentation to Diagnosis: Parents vs. GPs

3.3. Burden of Disease on GPs and Families
3.4. Perception of Knowledge/Training by GPs and Parents
| Guideline | Not Aware | Aware but not Read | Somewhat Familiar | Very Familiar/Not Following | Very Familiar/Following |
|---|---|---|---|---|---|
| NICE | 18% | 24% | 45% | 6% | 7% |
| MAP | 55% | 23% | 17% | 3% | 2% |
| ESPGHAN | 81% | 13% | 4% | 1% | 0% |
- -
- “Go see a doctor, use your instincts and don’t doubt yourself!”
- -
- “Push push push until someone takes notice”
- -
- “Keep going back to see professionals and insist on referral to a paediatrician and dietitian.”
- -
- “Don’t take no for an answer and keep chasing appointments. Do not give up.”
4. Discussion

| Suggested Actions for GPs | Suggested Actions for Parents |
|---|---|
| Improve listening skills | Develop a symptoms diary that can be taken to the appointment |
| Allow more time for an allergy-focused history | Develop a simple food diary that can be taken to the appointment |
| More training on how to recognise and manage | Create awareness that there are guidelines for recognition and management of CMPA |
| Improve awareness of current guidelines | Create more awareness of CMPA through parent teaching/online training |
| Development of tools to aid diagnosis and management | Create leaflets with explanation of symptoms/diagnosis/treatment |
| More access to secondary and tertiary care |
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CMPA | Cow’s milk protein allergy |
| GP | general practitioner |
| UK | United Kingdom |
| NIAID | National Institute of Allergy and Infectious Disease |
| IgE | Immunoglobulin E |
| ESPGHAN | European Society of Paediatric Gastroenterology, Hepatology and Nutrition |
| EAACI | European Academy of Allergy and Clinical Immunology |
| DRACMA | Diagnosis and Rationale for Action against Cow’s Milk Allergy |
| NICE | National Institute for Clinical Excellence Guidelines |
| MAP | on food allergy diagnosis, Milk Allergy in Primary Care |
References
- Fiocchi, A.; Brozek, J.; Schunemann, H.; Bahna, S.L.; von Berg, A.; Beyer, K.; Bozzola, M.; Bradsher, J.; Compalati, E.; Ebisawa, M.; et al. World allergy organization (WAO) diagnosis and rationale for action against cow’s milk allergy (DRACMA) guidelines. World Allergy Organ. J. 2010, 3, 57–161. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Wegrzyn, A.; Katz, Y.; Mehr, S.S.; Koletzko, S. Non-IgE-mediated gastrointestinal food allergy. J. Allergy Clin. Immunol. 2015, 135, 1114–1124. [Google Scholar] [CrossRef] [PubMed]
- Skripak, J.M.; Matsui, E.C.; Mudd, K.; Wood, R.A. The natural history of IgE-mediated cow’s milk allergy. J. Allergy Clin. Immunol. 2007, 120, 1172–1177. [Google Scholar] [CrossRef] [PubMed]
- Boyce, J.A.; Assa’ad, A.; Burks, A.W.; Jones, S.M.; Sampson, H.A.; Wood, R.A.; Plaut, M.; Cooper, S.F.; Fenton, M.J.; Arshad, S.H.; et al. Guidelines for the diagnosis and management of food allergy in the united states: Summary of the niaid-sponsored expert panel report. J. Allergy Clin. Immunol. 2010, 126, 1105–1118. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.; O’Flynn, N. Diagnosis and assessment of food allergy in children and young people in primary care and community settings: Nice clinical guideline. Br. J. Gen. Pract. 2011, 61, 473–475. [Google Scholar] [CrossRef] [PubMed]
- Lomer, M.C.; Parkes, G.C.; Sanderson, J.D. Review article: Lactose intolerance in clinical practice--myths and realities. Aliment. Pharmacol. Ther. 2008, 27, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Guarino, A.; Albano, F.; Ashkenazi, S.; Gendrel, D.; Hoekstra, J.H.; Shamir, R.; Szajewska, H.; ESPGHAN/ESPID Evidence-Based Guidelines for the Management of Acute Gastroenteritis in Children in Europe Expert Working Group. European society for paediatric gastroenterology, hepatology, and nutrition/european society for paediatric infectious diseases evidence-based guidelines for the management of acute gastroenteritis in children in europe: Executive summary. J. Pediatr. Gastroenterol. Nutr. 2008, 46, 619–621. [Google Scholar] [PubMed]
- Hill, D.J.; Hosking, C.S. The cow milk allergy complex: Overlapping disease profiles in infancy. Eur. J. Clin. Nutr. 1995, 49, S1–S12. [Google Scholar] [PubMed]
- Muraro, A.; Werfel, T.; Hoffmann-Sommergruber, K.; Roberts, G.; Beyer, K.; Bindslev-Jensen, C.; Cardona, V.; Dubois, A.; duToit, G.; Eigenmann, P.; et al. EAACIfood allergy and anaphylaxis guidelines: Diagnosis and management of food allergy. Allergy 2014, 69, 1008–1025. [Google Scholar] [CrossRef] [PubMed]
- Skypala, I.J.; Venter, C.; Meyer, R.; deJong, N.W.; Fox, A.T.; Groetch, M.; Oude Elberink, J.N.; Sprikkelman, A.; Diamandi, L.; Vlieg-Boerstra, B.J.; et al. The development of a standardised diet history tool to support the diagnosis of food allergy. Clin. Transl. Allergy 2015, 5, 7. [Google Scholar] [CrossRef] [PubMed]
- Heine, R.G. Pathophysiology, diagnosis and treatment of food protein-induced gastrointestinal diseases. Curr. Opin. Allergy Clin. Immunol. 2004, 4, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, S.; Niggemann, B.; Arato, A.; Dias, J.A.; Heuschkel, R.; Husby, S.; Mearin, M.L.; Papadopoulou, A.; Ruemmele, F.M.; Staiano, A.; et al. Diagnostic approach and management of cow’s-milk protein allergy in infants and children: ESPGHAN GI committee practical guidelines. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Venter, C.; Brown, T.; Shah, N.; Walsh, J.; Fox, A.T. Diagnosis and management of non-ige-mediated cow’s milk allergy in infancy—A UK primary care practical guide. Clin. Transl. Allergy 2013, 3, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luyt, D.; Ball, H.; Makwana, N.; Green, M.R.; Bravin, K.; Nasser, S.M.; Clark, A.T.; Standards of Care Committee (SOCC) of the British Society for Allergy and Clinical Immunology (BSACI). BSACI guideline for the diagnosis and management of cow’s milk allergy. Clin. Exp. Allergy 2014, 44, 642–672. [Google Scholar] [CrossRef] [PubMed]
- Sladkevicius, E.; Nagy, E.; Lack, G.; Guest, J.F. Resource implications and budget impact of managing cow milk allergy in the UK. J. Med. Econ. 2010, 13, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Du Toit, G.; Meyer, R.; Shah, N.; Heine, R.G.; Thomson, M.A.; Lack, G.; Fox, A.T. Identifying and managing cow’s milk protein allergy. Arch. Dis. Child Educ. Pract. Ed. 2010, 95, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Heine, R.G. Gastroesophageal reflux disease, colic and constipation in infants with food allergy. Curr. Opin. Allergy Clin. Immunol. 2006, 6, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K. Update on infantile colic and management options. Curr. Opin. Investig. Drugs 2007, 8, 921–926. [Google Scholar] [PubMed]
- Meyer, R.; Fleming, C.; Dominguez-Ortega, G.; Lindley, K.; Michaelis, L.; Thapar, N.; Elawad, M.; Chakravarti, V.; Fox, A.T.; Shah, N. Manifestations of food protein induced gastrointestinal allergies presenting to a single tertiary paediatric gastroenterology unit. World Allergy Organ. J. 2013, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Sladkevicius, E.; Guest, J.F. Budget impact of managing cow milk allergy in the netherlands. J. Med. Econ. 2010, 13, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Valentine, A.Z.; Knibb, R.C. Exploring quality of life in families of children living with and without a severe food allergy. Appetite 2011, 57, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Ortega, G.; Borrelli, O.; Meyer, R.; Dziubak, R.; de Koker, C.; Godwin, H.; Fleming, C.; Thapar, N.; Elawad, M.; Kiparissi, F.; et al. Extraintestinal manifestations in children with gastrointestinal food allergy. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Klinnert, M.D.; Silveira, L.; Harris, R.; Moore, W.; Atkins, D.; Fleischer, D.M.; Menard-Katcher, C.; Aceves, S.; Spergel, J.M.; Franciosi, J.P.; et al. Health-related quality of life over time in children with eosinophilic esophagitis and their families. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Meyer, R.; Rommel, N.; van Oudenhove, L.; Fleming, C.; Dziubak, R.; Shah, N. Feeding difficulties in children with food protein-induced gastrointestinal allergies. J. Gastroenterol. Hepatol. 2014, 29, 1764–1769. [Google Scholar] [CrossRef] [PubMed]
- Staiano, A. Food refusal in toddlers with chronic diseases. J. Pediatr. Gastroenterol. Nutr. 2003, 37, 225–227. [Google Scholar] [CrossRef] [PubMed]
- Saps, M.; Lu, P.; Bonilla, S. Cow’s-milk allergy is a risk factor for the development of FGIDs in children. J. Pediatr. Gastroenterol. Nutr. 2011, 52, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Quitadamo, P.; Papadopoulou, A.; Wenzl, T.; Urbonas, V.; Kneepkens, C.M.; Roman, E.; Orel, R.; Pavkov, D.J.; Dias, J.A.; Vandenplas, Y.; et al. European pediatricians’ approach to children with GER symptoms: Survey of the implementation of 2009 NASPGHAN-ESPGHAN guidelines. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Maslin, K.; Meyer, R.; Reeves, L.; Mackenzie, H.; Swain, A.; Stuart-Smith, W.; Loblay, R.; Groetch, M.; Venter, C. Food allergy competencies of dietitians in the united kingdom, australia and united states of america. Clin. Transl. Allergy 2014, 4, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandenplas, Y.; Dupont, C.; Eigenmann, P.; Host, A.; Kuitunen, M.; Ribes-Koninckx, C.; Shah, N.; Shamir, R.; Staiano, A.; Szajewska, H.; et al. A workshop report on the development of the cow’s milk-related symptom score awareness tool for young children. Acta Paediatr. 2015, 104, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Fagnano, M.; Berkman, E.; Wiesenthal, E.; Butz, A.; Halterman, J.S. Depression among caregivers of children with asthma and its impact on communication with health care providers. Public Health 2012, 126, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lozinsky, A.C.; Meyer, R.; Anagnostou, K.; Dziubak, R.; Reeve, K.; Godwin, H.; Fox, A.T.; Shah, N. Cow’s Milk Protein Allergy from Diagnosis to Management: A Very Different Journey for General Practitioners and Parents. Children 2015, 2, 317-329. https://doi.org/10.3390/children2030317
Lozinsky AC, Meyer R, Anagnostou K, Dziubak R, Reeve K, Godwin H, Fox AT, Shah N. Cow’s Milk Protein Allergy from Diagnosis to Management: A Very Different Journey for General Practitioners and Parents. Children. 2015; 2(3):317-329. https://doi.org/10.3390/children2030317
Chicago/Turabian StyleLozinsky, Adriana C., Rosan Meyer, Katherine Anagnostou, Robert Dziubak, Kate Reeve, Heather Godwin, Adam T. Fox, and Neil Shah. 2015. "Cow’s Milk Protein Allergy from Diagnosis to Management: A Very Different Journey for General Practitioners and Parents" Children 2, no. 3: 317-329. https://doi.org/10.3390/children2030317
APA StyleLozinsky, A. C., Meyer, R., Anagnostou, K., Dziubak, R., Reeve, K., Godwin, H., Fox, A. T., & Shah, N. (2015). Cow’s Milk Protein Allergy from Diagnosis to Management: A Very Different Journey for General Practitioners and Parents. Children, 2(3), 317-329. https://doi.org/10.3390/children2030317