A Clinical Pilot Study of Individual and Group Treatment for Adolescents with Chronic Pain and Their Parents: Effects of Acceptance and Commitment Therapy on Functioning
Abstract
:1. Introduction
2. Methods
2.1. Study Setting and Design
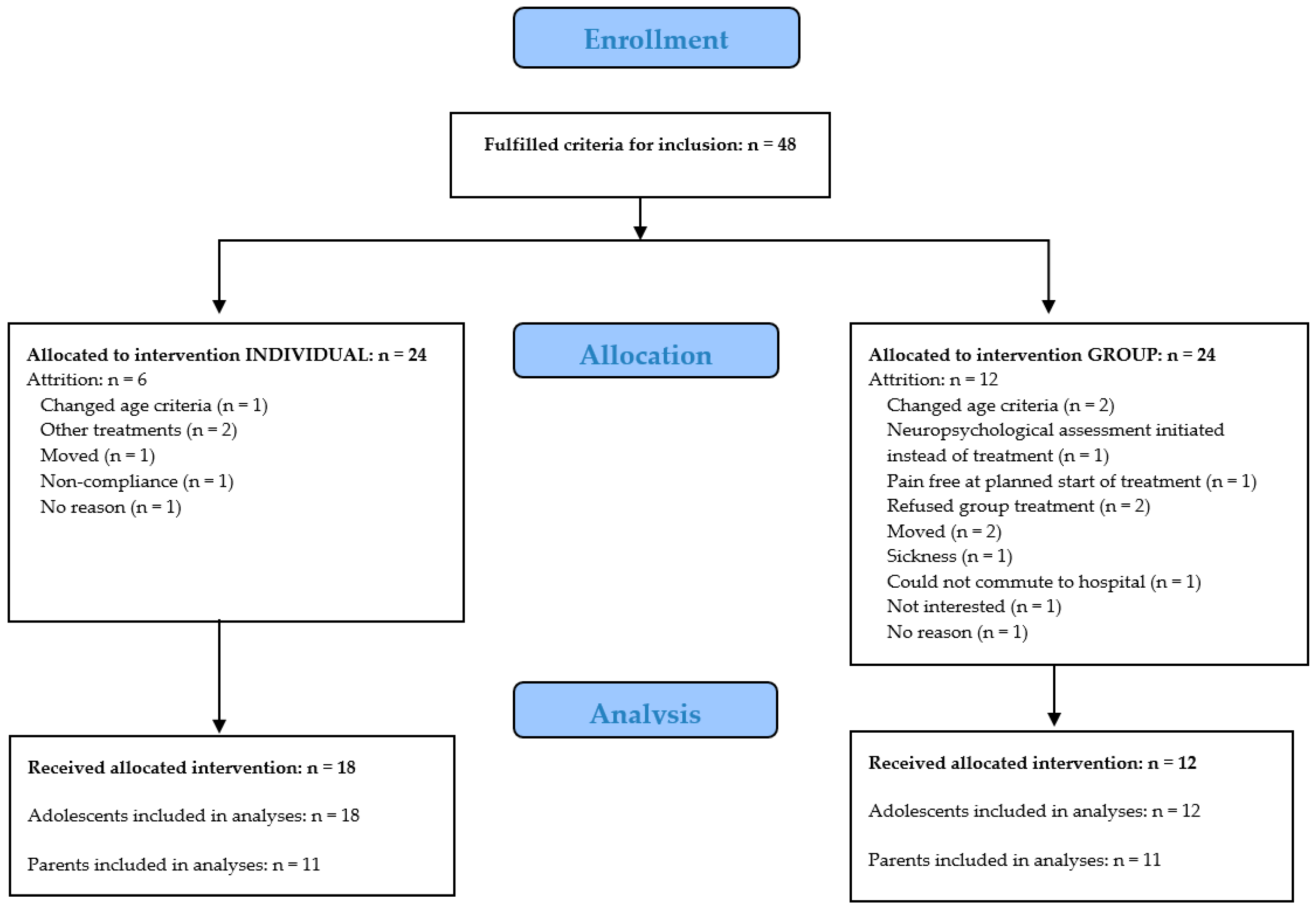
2.2. Participants
2.3. Intervention
2.4. Assessment
2.4.1. Adolescent Measures
2.4.1.1. Pain Intensity
2.4.1.2. Pain Interference Index (PII)
2.4.1.3. Pain Reactivity Scale (PRS)
2.4.1.4. Center for Epidemiological Studies Depression Scale Children (CES-DC)
2.4.1.5. Functional Disability Index (FDI)
2.4.1.6. Psychological Inflexibility in Pain Scale (PIPS)
2.4.2. Parental Measures
2.4.2.1. Hospital Anxiety and Depression Scale (HADS)
2.4.2.2. Pain Reactivity Scale Parent (PRS-P)
2.4.2.3. Parent Psychological Flexibility Questionnaire (PPFQ)
2.5. Data Management
2.6. Data Analysis
2.7. Ethical Considerations
3. Results
3.1. Initial Analyses
3.2. Descriptive Statistics
3.3. Initial Analyses: Comparison of Group and Individual Treatment
3.4. Effects of ACT-Treatment on Adolescent Functioning, Psychological Flexibility and Pain
3.5. Effects of ACT-Treatment on Parent Anxiety, Depression, Pain Reactivity and Psychological Flexibility
3.6. Analyses of Temporal Change Patterns
3.7. Clinically Significant Changes
3.7.1. Adolescents
3.7.2. Parents
3.8. Deterioration from Pre- to Post-Treatment Assessment
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hoftun, G.B.; Romundstad, P.R.; Zwart, J.-A.; Rygg, M. Chronic idiopathic pain in adolescence—High prevalence and disability: The young hunt study 2008. Pain 2011, 152, 2259–2266. [Google Scholar] [CrossRef] [PubMed]
- King, S.; Chambers, C.T.; Huguet, A.; MacNevin, R.C.; McGrath, P.J.; Parker, L.; MacDonald, A.J. The epidemiology of chronic pain in children and adolescents revisited: A systematic review. Pain 2011, 152, 2729–2738. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, A.S.; Palermo, T.M.; Stinson, J.; Handley, S.; Chambers, C.T. Systematic review of family functioning in families of children and adolescents with chronic pain. J. Pain 2010, 11, 1027–1038. [Google Scholar] [CrossRef] [PubMed]
- Groenewald, C.B.; Essner, B.S.; Wright, D.; Fesinmeyer, M.D.; Palermo, T.M. The economic costs of chronic pain among a cohort of treatment-seeking adolescents in the United States. J. Pain 2014, 15, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Eccleston, C.; Palermo, T.M.; Williams, A.C.; Lewandowski Holley, A.; Morley, S.; Fisher, E.; Law, E. Psychological therapies for the management of chronic and recurrent pain in children and adolescents. Cochrane Database Syst. Rev. 2014. [Google Scholar] [CrossRef]
- Hayes, S.C.; Luoma, J.B.; Bond, F.W.; Masuda, A.; Lillis, J. Acceptance and commitment therapy: Model, processes and outcomes. Behav. Res. Ther. 2006, 44, 1–25. [Google Scholar] [CrossRef] [PubMed]
- McGrath, P.J.; Walco, G.A.; Turk, D.C.; Dworkin, R.H.; Brown, M.T.; Davidson, K.; Eccleston, C.; Finley, G.A.; Goldschneider, K.; Haverkos, L.; et al. Core outcome domains and measures for pediatric acute and chronic/recurrent pain clinical trials: Pedimmpact recommendations. J. Pain 2008, 9, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Society of Clinical Psychology, American Psychological Association, division 12. Acceptance and Commitment Therapy for Chronic Pain. 2011. Available online: https://wwwdiv12org/psychological-treatments/disorders/chronic-or-persistent-pain/acceptance-and-commitment-therapy-for-chronic-pain/ (accessed on 1 September 2016).
- Wicksell, R.K.; Melin, L.; Olsson, G.L. Exposure and acceptance in the rehabilitation of adolescents with idiopathic chronic pain—A pilot study. Eur. J. Pain 2007, 11, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Wicksell, R.K.; Melin, L.; Lekander, M.; Olsson, G.L. Evaluating the effectiveness of exposure and acceptance strategies to improve functioning and quality of life in longstanding pediatric pain—A randomized controlled trial. Pain 2009, 141, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Wicksell, R.K.; Dahl, J.; Magnusson, B.; Olsson, G.L. Using acceptance and commitment therapy in the rehabilitation of an adolescent female with chronic pain: A case example. Cogn. Behav. Pract. 2005, 12, 415–423. [Google Scholar] [CrossRef]
- Wicksell, R.K.; Olsson, G.L.; Hayes, S.C. Mediators of change in acceptance and commitment therapy for pediatric chronic pain. Pain 2011, 152, 2792–2801. [Google Scholar] [CrossRef] [PubMed]
- Gauntlett-Gilbert, J.; Connell, H.; Clinch, J.; McCracken, L.M. Acceptance and values-based treatment of adolescents with chronic pain: Outcomes and their relationship to acceptance. J. Pediatr. Psychol. 2013, 38, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Ghomian, S.; Shairi, M. The effectiveness of acceptance and commitment therapy for children with chronic pain on the function of 7 to 12 year-old. Int. J. Pediatr. 2014, 2, 47–55. [Google Scholar]
- Martin, S.; Wolters, P.L.; Toledo-Tamula, M.A.; Schmitt, S.N.; Baldwin, A.; Starosta, A.; Gillespie, A.; Widemann, B. Acceptance and commitment therapy in youth with neurofibromatosis type 1 (nf1) and chronic pain and their parents: A pilot study of feasibility and preliminary efficacy. Am. J. Med. Genet. Part A 2016, 170, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Eccleston, C.; Fisher, E.; Law, E.; Bartlett, J.; Palermo, T.M. Psychological interventions for parents of children and adolescents with chronic illness. Cochrane Database Syst. Rev. 2015. [Google Scholar] [CrossRef]
- McCracken, L.M.; Gauntlett-Gilbert, J. Role of psychological flexibility in parents of adolescents with chronic pain: Development of a measure and preliminary correlation analyses. Pain 2011, 152, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.P.; McCracken, L.M.; Weiss, K.E.; Harbeck-Weber, C. The role of parent psychological flexibility in relation to adolescent chronic pain: Further instrument development. J. Pain 2015, 16, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Kanstrup, M.; Holmstrom, L.; Ringstrom, R.; Wicksell, R.K. Insomnia in paediatric chronic pain and its impact on depression and functional disability. Eur. J. Pain 2014, 18, 1094–1102. [Google Scholar] [CrossRef] [PubMed]
- Holmstrom, L.; Kemani, M.K.; Kanstrup, M.; Wicksell, R.K. Evaluating the statistical properties of the pain interference index in children and adolescents with chronic pain. J. Dev. Behav. Pediatr. 2015, 36, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.; Nelson Schmitt, S.; Wolters, P.L.; Abel, B.; Toledo-Tamula, M.A.; Baldwin, A.; Wicksell, R.K.; Merchant, M.; Widemann, B. Development and validation of the english pain interference index and pain interference index-parent report. Pain Med 2015, 16, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Faulstich, M.E.; Carey, M.P.; Ruggiero, L.; Enyart, P.; Gresham, F. Assessment of depression in childhood and adolescence: An evaluation of the center for epidemiological studies depression scale for children (CES-DC). Am. J. Psychiatry 1986, 143, 1024–1027. [Google Scholar] [PubMed]
- Weissman, M.M.; Orvaschel, H.; Padian, N. Children’s symptom and social functioning self-report scales. Comparison of mothers’ and children’s reports. J. Nerv. Ment. Dis. 1980, 168, 736–740. [Google Scholar] [CrossRef] [PubMed]
- Olsson, G.; von Knorring, A.L. Depression among swedish adolescents measured by the self-rating scale center for epidemiology studies-depression child (CES-DC). Eur. Child Adolesc. Psychiatry 1997, 6, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Walker, L.S.; Greene, J.W. The functional disability inventory: Measuring a neglected dimension of child health status. J. Pediatr. Psychol. 1991, 16, 39–58. [Google Scholar] [CrossRef] [PubMed]
- Claar, R.L.; Walker, L.S. Functional assessment of pediatric pain patients: Psychometric properties of the functional disability inventory. Pain 2006, 121, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Kashikar-Zuck, S.; Flowers, S.R.; Claar, R.L.; Guite, J.W.; Logan, D.E.; Lynch-Jordan, A.M.; Palermo, T.M.; Wilson, A.C. Clinical utility and validity of the functional disability inventory among a multicenter sample of youth with chronic pain. Pain 2011, 152, 1600–1607. [Google Scholar] [CrossRef] [PubMed]
- Wicksell, R.K.; Renofalt, J.; Olsson, G.L.; Bond, F.W.; Melin, L. Avoidance and cognitive fusion—Central components in pain related disability? Development and preliminary validation of the psychological inflexibility in pain scale (pips). Eur. J. Pain 2008, 12, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Wicksell, R.K.; Olsson, G.L.; Hayes, S.C. Psychological flexibility as a mediator of improvement in acceptance and commitment therapy for patients with chronic pain following whiplash. Eur. J. Pain 2010, 14, 1059.e1–1059.e11. [Google Scholar] [CrossRef] [PubMed]
- Wicksell, R.K.; Lekander, M.; Sorjonen, K.; Olsson, G.L. The psychological inflexibility in pain scale (pips)—Statistical properties and model fit of an instrument to assess change processes in pain related disability. Eur. J. Pain 2010, 14, 771.e1–771.e14. [Google Scholar] [CrossRef] [PubMed]
- Snaith, R.P.; Zigmond, A.S. The hospital anxiety and depression scale. Br. Med. J. (Clin. Res. Ed.) 1986, 292, 344. [Google Scholar] [CrossRef]
- Bjelland, I.; Dahl, A.A.; Haug, T.T.; Neckelmann, D. The validity of the hospital anxiety and depression scale: An updated literature review. J. Psychosom. Res. 2002, 52, 69–77. [Google Scholar] [CrossRef]
- Lisspers, J.; Nygren, A.; Söderman, E. Hospital anxiety and depression scale (had): Some psychometric data for a swedish sample. Acta Psychiatr. Scand. 1997, 96, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Wiwe Lipsker, C.; Kanstrup, M.; Holmström, L.; Kemani, M.; Wicksell, R.K. The parent psychological flexibility questionnaire —Item reduction and validation in a clinical sample of Swedish parents of children with chronic pain. Children. accepted.
- Field, A. Discovering Statistics Using SPSS, 3rd ed.; SAGE Publications: London, England, 2009; p. 821. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; L. Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Jacobson, N.S.; Truax, P. Clinical significance: A statistical approach to defining meaningful change in psychotherapy research. J. Consult. Clin. Psychol. 1991, 59, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Wise, E.A. Methods for analyzing psychotherapy outcomes: A review of clinical significance, reliable change, and recommendations for future directions. J. Pers. Assess. 2004, 82, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Palermo, T.M. Enhancing daily functioning with exposure and acceptance strategies: An important stride in the development of psychological therapies for pediatric chronic pain. Pain 2009, 141, 189–190. [Google Scholar] [CrossRef] [PubMed]
- Zernikow, B.; Wager, J.; Hechler, T.; Hasan, C.; Rohr, U.; Dobe, M.; Meyer, A.; Hoebner-Moehler, B.; Wamsler, C.; Blankenburg, M. Characteristics of highly impaired children with severe chronic pain: A 5-year retrospective study on 2249 pediatric pain patients. BMC Pediatr. 2012, 12, 54. [Google Scholar] [CrossRef] [PubMed]
- Kashikar-Zuck, S.; Goldschneider, K.R.; Powers, S.W.; Vaught, M.H.; Hershey, A.D. Depression and functional disability in chronic pediatric pain. Clin. J. Pain 2001, 17, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Hechler, T.; Kanstrup, M.; Holley, A.L.; Simons, L.E.; Wicksell, R.; Hirschfeld, G.; Zernikow, B. Systematic review on intensive interdisciplinary pain treatment of children with chronic pain. Pediatrics 2015, 136, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Hayes, S.C.; Duckworth, M.P. Acceptance and commitment therapy and traditional cognitive behavior therapy approaches to pain. Cogn. Behav. Pract. 2006, 13, 185–187. [Google Scholar] [CrossRef]
- Kemani, M.K.; Olsson, G.L.; Lekander, M.; Hesser, H.; Andersson, E.; Wicksell, R.K. Efficacy and cost-effectiveness of acceptance and commitment therapy and applied relaxation for longstanding pain: A randomized controlled trial. Clin. J. Pain 2015, 31, 1004–1016. [Google Scholar] [CrossRef] [PubMed]
- Palermo, T.M.; Eccleston, C. Parents of children and adolescents with chronic pain. Pain 2009, 146, 15–17. [Google Scholar] [CrossRef] [PubMed]
- Dunford, E.; Thompson, M.; Gauntlett-Gilbert, J. Parental behaviour in paediatric chronic pain: A qualitative observational study. Clin. Child Psychol. Psychiatry 2014, 19, 561–575. [Google Scholar] [CrossRef] [PubMed]
- Chow, E.T.; Otis, J.D.; Simons, L.E. The longitudinal impact of parent distress and behavior on functional outcomes among youth with chronic pain. J. Pain 2016, 17, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Palermo, T.M.; Law, E.F.; Fales, J.; Bromberg, M.H.; Jessen-Fiddick, T.; Tai, G. Internet-delivered cognitive-behavioral treatment for adolescents with chronic pain and their parents: A randomized controlled multicenter trial. Pain 2016, 157, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Wicksell, R.K.; Kanstrup, M.; Kemani, M.K.; Holmström, L.; Olsson, G.L. Acceptance and commitment therapy for children and adolescents with physical health concerns. Curr. Opin. Psychol. 2015, 2, 1–5. [Google Scholar] [CrossRef]
- Blackledge, J.T.; Hayes, S.C. Using acceptance and commitment training in the support of parents of children diagnosed with autism. Child Fam. Behav. Ther. 2006, 28, 1–18. [Google Scholar] [CrossRef]
- Brown, F.L.; Whittingham, K.; Boyd, R.N.; McKinlay, L.; Sofronoff, K. Improving child and parenting outcomes following paediatric acquired brain injury: A randomised controlled trial of stepping stones triple p plus acceptance and commitment therapy. J. Child Psychol. Psychiatry Allied Discipl. 2014, 55, 1172–1183. [Google Scholar] [CrossRef] [PubMed]
- Whittingham, K.; Sanders, M.; McKinlay, L.; Boyd, R.N. Interventions to reduce behavioral problems in children with cerebral palsy: An RCT. Pediatrics 2014, 133, e1249–e1257. [Google Scholar] [CrossRef] [PubMed]
- Burke, K.; Muscara, F.; McCarthy, M.; Dimovski, A.; Hearps, S.; Anderson, V.; Walser, R. Adapting acceptance and commitment therapy for parents of children with life-threatening illness: Pilot study. Fam. Syst. Health 2014, 32, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Eccleston, C.; Crombez, G.; Scotford, A.; Clinch, J.; Connell, H. Adolescent chronic pain: Patterns and predictors of emotional distress in adolescents with chronic pain and their parents. Pain 2004, 108, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Hoftun, G.B.; Romundstad, P.R.; Rygg, M. Association of parental chronic pain with chronic pain in the adolescent and young adult: Family linkage data from the hunt study. JAMA Pediatr. 2013, 167, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.C.; Fales, J.L. Parenting in the context of chronic pain: A controlled study of parents with chronic pain. Clin. J. Pain 2015, 31, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Holm, S.; Ljungman, G.; Asenlof, P.; Linton, S.J.; Soderlund, A. Treating youth in pain: Comparing tailored behavioural medicine treatment provided by physical therapists in primary care with physical exercises. Eur. J. Pain 2015. [Google Scholar] [CrossRef] [PubMed]
- Gul, R.B.; Ali, P.A. Clinical trials: The challenge of recruitment and retention of participants. J. Clin. Nurs. 2010, 19, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Kazdin, A.E. Mediators and mechanisms of change in psychotherapy research. Annu. Rev. Clin. Psychol. 2007, 3, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Vlaeyen, J.W.; Morley, S. Cognitive-behavioral treatments for chronic pain: What works for whom? Clin. J. Pain 2005, 21, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Palermo, T.M.; Law, E.F.; Zhou, C.; Holley, A.L.; Logan, D.; Tai, G. Trajectories of change during a randomized controlled trial of internet-delivered psychological treatment for adolescent chronic pain: How does change in pain and function relate? Pain 2015, 156, 626–634. [Google Scholar] [CrossRef] [PubMed]
| Prior to treatment: Assessment of pain and pain-related disability through semi-structured screening interviews with psychologist, pain physician and physiotherapist (3 × 1 h) followed by team discussion regarding suitability for treatment; feedback to the patient and a joint decision regarding the initiation of treatment. | ||
| Pre-treatment assessments | ||
| Adolescent session | Parent session | |
| Sessions 1–3: Preparing for behavior change. | (1) Introduction to behavior analysis of difficult pain-related situations, such as ABC-analysis * with antecedent (2) Pain education with physician directed towards adolescents (e.g., information about the pain system and differences between adaptive avoidance reactions to acute pain and potentially dysfunctional avoidance reactions to long-term pain). | (3) Pain education with physician directed towards parents. |
| Sessions 4–6: Shifting perspective. | (4) Individual life values: What is important in life? How have previous strategies to avoid pain and distress led away from a valued life? (5) Introduction to the concept of increased functioning also in the presence of persisting pain. | (6) Introduction to ABC-analysis of difficult pain-related parent-child situations. Pain reduction as opposed to valued living. Clarification of parental values. Being an effective coach to your child. Home assignment: practice ABC-analyses on parent-child interactions. |
| Mid-treatment assessments | ||
| Sessions 7–8: Acceptance and cognitive defusion. | (7) Evaluation of previous strategies and creative hopelessness (i.e., how have previous attempts at symptom reduction prevented a valued living). (8) Acceptance and cognitive defusion. | |
| Sessions 9–17: Values-oriented behavioral activation. | (9) Goal setting, gradual behavior activation and exposure to previously-avoided situations, in line with life values. (10) Physiotherapist: Goal setting focused on physical activities in line with chosen values. (12) Physician: Joint session. Continued pain education and discussion about symptoms in relation to behavior change. (13) Exposure, continued. (14) Physiotherapist: Evaluation and gradual increase of values oriented physical activities. (15) Exposure, continued. (16) Recruiting family and friends for support. (17) Formulating individual plan for relapse prevention and summary of treatment. | (11) Practice of acceptance and defusion in order to facilitate behaviors in line with long-term goals and values also in the presence of own worry and distress. Follow up on parent-as-coach. (12) Physician: Joint session. |
| Post-treatment assessments: Concluding team session together with both adolescent and parent. | ||
| Total Sample | Group Condition | Individual Condition | |
|---|---|---|---|
| Children | 30 | 12 | 18 |
| Age m (SD) | 16.0 (1.6) | 16.3 (1.5) | 15.8 (1.6) |
| Gender | |||
| Girls n (%) | 24 (80.0) | 11 (91.7) | 13 (72.2) |
| Boys n (%) | 6 (20.0) | 1 (8.3) | 5 (27.8) |
| Pain characteristics | |||
| Head n (%) | 27 (90.0) | 10 (83.0) | 17 (94.0) |
| Abdominal n (%) | 12 (40.0) | 4 (33.0) | 8 (44.0) |
| Back n (%) | 13 (43.0) | 7 (58.0) | 6 (33.0) |
| Joint n (%) | 5 (17.0) | 4 (33.0) | 1 (5.5) |
| Other (e.g., parts of limbs) n (%) | 18 (60.0) | 9 (75.0) | 9 (50.0) |
| CRPS a n(%) | 1 (3.3) | - | 1 (5.5) |
| Widespread n (%) | 6 (20.0) | 4 (33.0) | 2 (11.0) |
| Pain locations > 3 n (%) | 16 (53.0) | 8 (67.0) | 8 (44.0) |
| Pain duration in months m (SD) | 57.87 (49.5) | 43.18 (36.3) | 67.35 (55.4) |
| Pain duration ≥36 months m (SD) | 17 (60.7) | 6 (54.5) | 11 (64.7) |
| Current pain intensity (0–6) m (SD) | 3.31 (1.4) | 3.75 (1.0) | 3.00 (1.7) |
| Continuous pain n (%) | 22 (73.3) | 10 (83.3) | 12 (66.7) |
| Pain every day n (%) | 5 (16.7) | 2 (16.7) | 3 (16.7) |
| Pain every week n (%) | 3 (10.0) | - | 3 (16.7) |
| Current pain medication n (%) | 15 (50.0) | 6 (50.0) | 9 (50.0) |
| School absence n (%) | |||
| None | 4 (13.3) | 3 (25.0) | 1 (5.6) |
| Moderate | 16 (53.3) | 4 (33.3) | 12 (66.7) |
| Extensive (>1 day/week) | 4 (13.3) | 3 (25.0) | 1 (5.6) |
| Total absence | 4 (13.3) | 1 (8.3) | 3 (16.7) |
| N/A | 1 (3.3) | 1 (8.3) | 1 (5.6) |
| Parents | 28 | 12 | 16 |
| Mothers n (%) | 24 (86.0) | 10 (83.3) | 14 (87.5) |
| Age m (SD) | 47.3 (4.8) | 48.42 (4.5) | 46.5 (5.0) |
| Parent pain duration ≥1 year n (%) | 16 (57.1) | 7 (58.3) | 9 (56.2) |
| Marital status n (%) | |||
| Married | 14 (50.0) | 6 (83.0) | 8 (50.0) |
| Co-habiting | 6 (21.4) | 2 (16.7) | 4 (25.0) |
| In a relationship | 2 (7.1) | 1 (8.3) | 1 (6.3) |
| Single | 6 (21.4) | 3 (25.0) | 3 (18.8) |
| Educational status n (%) | |||
| Basic/high school | 16 (57.1) | 5 (41.7) | 11 (68.8) |
| University studies | 12 (42.9) | 7 (58.3) | 5 (31.3) |
| Occupational status n (%) | |||
| Full time work/study | 20 (71.4) | 9 (75.0) | 11 (68.8) |
| Part time work/study | 5 (17.9) | - | 5 (31.3) |
| Not working/studying | 3 (10.7) | 3 (25.0) | - |
| Outcome Variable | Pre-Md (Min–Max) | Mid-Md (Min–Max) | Post-Md (Min–Max) | |
|---|---|---|---|---|
| Children | ||||
| PII (0–36) | Total | 24.5 (5–35) | 20.0 (4–35) | 12.5 (1–35) |
| Group | 24.5 (11–35) | 22.5 (9–35) | 13.5 (6–35) | |
| Individual | 22.5 (5–34) | 18.0 (4–30) | 11.0 (1–32) | |
| PRS (0–30) | Total | 21.5 (13–29) | 21.0 (10–30) | 13.0 (0–29) |
| Group | 20.0 (14–27) | 21.5 (11–30) | 15.0 (8–29) | |
| Individual | 23.0 (13–29) | 16.0 (10–29) | 10.0 (0–28) | |
| CES-DC (0–60) | Total | 28.0 (10–47) | 27.0 (15–52) | 20.0 (6–47) |
| Group | 26.0 (10–47) | 30.5 (15–52) | 22.0 (9–47) | |
| Individual | 28.5 (12–45) | 26.0 (16–46) | 17.0 (6–46) | |
| FDI-P (0–60) | Total | 15.5 (3–57) | 10.5 (0–37) | 6.0 (0–39) |
| Group | 19.0 (9–39) | 9.5 (6–36) | 6.5 (0–34) | |
| Individual | 15.0 (3–57) | 11.0 (0–37) | 6.0 (0–39) | |
| PIPS (12–84) | Total | 54.0 (27–81) | 49.5 (33–72) | 37.0 (17–75) |
| Group | 54.0 (27–81) | 51.0 (33–72) | 40.5 (17–45) | |
| Individual | 55.5 (38–76) | 48.0 (35.50–71) | 32.5 (22–65) | |
| Pain intensity (0–6) | Total | 4.0 (0–6) | 4.0 (1–6) | 3.0 (0–6) |
| Group | 4.0 (2–6) | 4.0 (3–6) | 4.0 (1–5) | |
| Individual | 3.0 (0–6) | 3.0 (1–6) | 2.0 (0–6) | |
| Parents | ||||
| HADS (0–42) | Total | 15.0 (0–31) | 17.0 (0–30) | 13.5 (0–32) |
| Group | 13.5 (4–19) | 12.5 (0–28) | 17.0 (0–23) | |
| Individual | 17.0 (0–31) | 20.0 (6–30) | 10.5 (0–32) | |
| HADS-A (0–21) | Total | 9.0 (0–17) | 9.5 (0–18) | 7.5 (0–16) |
| Group | 7.5 (4–13) | 6.0 (0–17) | 6.5 (0–15) | |
| Individual | 11.0 (0–17) | 10.5 (3–18) | 7.5 (0–16) | |
| HADS-D (0–21) | Total | 5.5 (0–14) | 8.5 (0–15) | 3.5 (0–16) |
| Group | 4.5 (0–12) | 6.0 (0–11) | 7.0 (0–12) | |
| Individual | 5.5 (0–14) | 9.0 (0–15) | 3.0 (0–16) | |
| PRS-P (0–30) | Total | 22.5 (5–30) | 20.0 (4–30) | 15.0 (1–28) |
| Group | 22.0 (13–30) | 15.0 (5–23) | 14.0 (5–28) | |
| Individual | 22.5 (5–30) | 24.0 (4–30) | 15.0 (1–28) | |
| PPFQ (0–60) | Total | 32.0 (9–51) | 38.0 (6–48) | 42.0 (15–55) |
| Group | 34.5 (19–51) | 40.0 (31–47) | 42.0 (23–55) | |
| Individual | 24.5 (9–46) | 25.5 (6–48) | 41.5 (15–54) | |
| Outcome Variable | Wilcoxon Signed Rank Test Pre–Mid Change | Wilcoxon Signed Rank Test Mid–Post Changes | Wilcoxon Signed Rank Test Pre–Post Changes | Effect Size (r) a Pre–Post | Clinically Significant Change b Pre–Post | Deterioration c Pre–Post |
|---|---|---|---|---|---|---|
| Children | ||||||
| PII | z = −2.203, p = 0.026 | z = −2.962, p = 0.002 * | z = −3.949, p < 0.001 * | r = −0.51 | 14 of 30 | - |
| PRS | z = −0.930, p = 0.362 | z = −3.651, p < 0.001 * | z = −3.765, p < 0.001 * | r = −0.49 | 14 of 29 | 2 of 29 |
| CES-DC | z = −1.264, p = 0.213 | z = −3.597, p < 0.001 * | z = −2.788, p = 0.004 * | r = −0.37 | 11 of 28 | 2 of 28 |
| FDI-P | z = −2.584, p = 0.008 * | z = −0.142, p = 0.901 | z = −2.134, p = 0.032 | r = −0.35 | 4 of 19 | 2 of 19 |
| PIPS | z = −2.199, p = 0.027 | z = −4.314, p < 0.001 * | z = −4.607, p < 0.001 * | r = −0.59 | 19 of 30 | - |
| Pain intensity | z = −0.525, p = 0.697 | z = −1.206, p = 0.255 | z = −0.980, p = 0.346 | r = − 0.13 | 4 of 26 | 1 of 26 |
| Parents | ||||||
| HADS | z = −0.299, p = 0.777 | z = −0.142, p = 0.899 | z = −0.222, p = 0.838 | - | - | - |
| HADS-A | z = −0.916, p = 0.373 | z = −0.234, p = 0.836 | z = −0.843, p = 0.413 | - | - | - |
| HADS-D | z = −0.643, p = 0.537 | z = −0.114, p = 0.933 | z = −0.694, p = 0.508 | - | - | - |
| PRS-P | z = −2.138, p = 0.031 | z = −2.987, p = 0.001 * | z = −3.672, p < 0.001 * | r = −0.57 | 16 of 21 | - |
| PPFQ | z = −2.315, p = 0.019 | z = −3.144, p = 0.001 * | z = −4.117, p < 0.001 * | r = −0.62 | 12 of 22 | - |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanstrup, M.; Wicksell, R.K.; Kemani, M.; Wiwe Lipsker, C.; Lekander, M.; Holmström, L. A Clinical Pilot Study of Individual and Group Treatment for Adolescents with Chronic Pain and Their Parents: Effects of Acceptance and Commitment Therapy on Functioning. Children 2016, 3, 30. https://doi.org/10.3390/children3040030
Kanstrup M, Wicksell RK, Kemani M, Wiwe Lipsker C, Lekander M, Holmström L. A Clinical Pilot Study of Individual and Group Treatment for Adolescents with Chronic Pain and Their Parents: Effects of Acceptance and Commitment Therapy on Functioning. Children. 2016; 3(4):30. https://doi.org/10.3390/children3040030
Chicago/Turabian StyleKanstrup, Marie, Rikard K. Wicksell, Mike Kemani, Camilla Wiwe Lipsker, Mats Lekander, and Linda Holmström. 2016. "A Clinical Pilot Study of Individual and Group Treatment for Adolescents with Chronic Pain and Their Parents: Effects of Acceptance and Commitment Therapy on Functioning" Children 3, no. 4: 30. https://doi.org/10.3390/children3040030
APA StyleKanstrup, M., Wicksell, R. K., Kemani, M., Wiwe Lipsker, C., Lekander, M., & Holmström, L. (2016). A Clinical Pilot Study of Individual and Group Treatment for Adolescents with Chronic Pain and Their Parents: Effects of Acceptance and Commitment Therapy on Functioning. Children, 3(4), 30. https://doi.org/10.3390/children3040030





