Effects of Gestational Age and Early Parenting on Children’s Social Inhibition at 6 Years
Abstract
1. Introduction
2. Materials and method
2.1. Participants
2.2. Measures
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chronis-Tuscano, A.; Degnan, K.A.; Pine, D.S.; Perez-Edgar, K.; Henderson, H.A.; Diaz, Y.; Raggi, V.L.; Fox, N.A. Stable early maternal report of behavioral inhibition predicts lifetime social anxiety disorder in adolescence. J. Am. Acad. Child Adoles. Psychiatry 2009, 48, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Kagan, J.; Reznick, J.S.; Clarke, C.; Snidman, N.; Garcia-Coll, C. Behavioral inhibition to the unfamiliar. Child Dev. 1984, 55, 2212–2225. [Google Scholar] [CrossRef]
- Fox, N.A.; Henderson, H.A.; Rubin, K.H.; Calkins, S.D.; Schmidt, L.A. Continuity and discontinuity of behavioral inhibition and exuberance: Psychophysiological and behavioral influences across the first four years of life. Child Dev. 2001, 72, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Hirshfeld, D.R.; Rosenbaum, J.F.; Biederman, J.; Bolduc, E.A.; Faraone, S.V.; Snidman, N.; Reznick, J.S.; Kagan, J. Stable behavioral inhibition and its association with anxiety disorder. J. Am. Acad. Child Adoles. Psychiatry 1992, 31, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Biederman, J.; Hirshfeld-Becker, D.R.; Rosenbaum, J.F.; Hérot, C.; Friedman, D.; Snidman, N.; Kagan, J.; Faraone, S.V. Further evidence of association between behavioral inhibition and social anxiety in children. Am. J. Psychiatry 2001, 158, 1673–1679. [Google Scholar] [CrossRef]
- Essex, M.J.; Klein, M.H.; Slattery, M.J.; Goldsmith, H.H.; Kalin, N.H. Early risk factors and developmental pathways to chronic high inhibition and social anxiety disorder in adolescence. Am. J. Psychiatry 2009, 167, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Dyson, M.; Klein, D.; Olino, T.; Dougherty, L.; Durbin, C. Social and non-social behavioral inhibition in preschool-age children: Differential associations with parent-reports of temperament and anxiety. Child Psychiatry Hum. Dev. 2011, 42, 390–405. [Google Scholar] [CrossRef]
- Brooker, R.J.; Kiel, E.J.; Buss, K.A. Early social fear predicts kindergarteners’ socially anxious behaviors: Direct associations, moderation by inhibitory control, and differences from nonsocial fear. Emotion 2016, 16, 997–1010. [Google Scholar] [CrossRef]
- Buss, K.A.; Davis, E.L.; Ram, N.; Coccia, M. Dysregulated fear, social inhibition, and respiratory sinus arrhythmia: A replication and extension. Child Dev. 2018, 89, e214–e228. [Google Scholar] [CrossRef]
- Asendorpf, J.B. Development of inhibition during childhood: Evidence for situational specificity and a two-factor model. Dev. Psychol. 1990, 26, 721–730. [Google Scholar] [CrossRef]
- Andersson, K.; Bohlin, G.; Hagekull, B. Early temperament and stranger wariness as predictors of social inhibition in 2-year-olds. Br. J. Dev. Psychol. 1999, 17, 421–434. [Google Scholar] [CrossRef]
- Fox, A.S.; Kalin, N.H. A translational neuroscience approach to understanding the development of social anxiety disorder and its pathophysiology. Am. J. Psychiatry 2014, 171, 1162–1173. [Google Scholar] [CrossRef] [PubMed]
- Geva, R.; Sopher, K.; Kurtzman, L.; Galili, G.; Feldman, R.; Kuint, J. Neonatal brainstem dysfunction risks infant social engagement. Soc. Cogn. Affect. Neurosci. 2013, 8, 158–164. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Volpe, J.J. Brain injury in premature infants: A complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009, 8, 110–124. [Google Scholar] [CrossRef]
- Geva, R.; Schreiber, J.; Segal-Caspi, L.; Markus-Shiffman, M. Neonatal brainstem dysfunction after preterm birth predicts behavioral inhibition. J. Child Psychol. Psychiatry 2014, 55, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, K.; Bora, S.; Woodward, L.J. Social development of children born very preterm: A systematic review. Dev. Med. Child Neurol. 2015, 57, 899–918. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.M.; Champion, P.R.; Woodward, L.J. Social competence of preschool children born very preterm. Early Hum. Dev. 2013, 89, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Delobel-Ayoub, M.; Kaminski, M.; Marret, S.; Burguet, A.; Marchand, L.; N′Guyen, S.; Matis, J.; Thiriez, G.; Fresson, J.; Arnaud, C.; et al. Behavioral outcome at 3 years of age in very preterm infants: The EPIPAGE Study. Pediatrics 2006, 117, 1996. [Google Scholar] [CrossRef]
- Wolke, D.; Baumann, N.; Strauss, V.; Johnson, S.; Marlow, N. Bullying of preterm children and emotional problems at school age: Cross-culturally invariant effects. J. Pediatr. 2015, 166, 1417–1422. [Google Scholar] [CrossRef]
- Gardner, F.; Johnson, A.; Yudkin, P.; Bowler, U.; Hockley, C.; Mutch, L.; Wariyar, U. Behavioral and emotional adjustment of teenagers in mainstream school who were born before 29 weeks’ gestation. Pediatrics 2004, 114, 676–682. [Google Scholar] [CrossRef]
- Schmidt, L.A.; Miskovic, V.; Boyle, M.H.; Saigal, S. Shyness and timidity in young adults who were born at extremely low birth weight. Pediatrics 2008, 122, e181–e187. [Google Scholar] [CrossRef] [PubMed]
- Pyhälä, R.; Räikkönen, K.; Pesonen, A.-K.; Heinonen, K.; Hovi, P.; Eriksson, J.G.; Järvenpää, A.-L.; Andersson, S.; Kajantie, E. Behavioral inhibition and behavioral approach in young adults with very low birth weight–The Helsinki study of very low birth weight adults. Personal. Individ. Differ. 2009, 46, 106–110. [Google Scholar] [CrossRef]
- Darlow, B.A.; Horwood, L.J.; Pere-Bracken, H.M.; Woodward, L.J. Psychosocial outcomes of young adults born very low birth weight. Pediatrics 2013, 132, e1521–e1528. [Google Scholar] [CrossRef] [PubMed]
- Jaekel, J.; Baumann, N.; Bartmann, P.; Wolke, D. Mood and anxiety disorders in very preterm/very low birth weight individuals from six to 26 years. J. Child Psychol. Psychiatry 2018, 59, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Eryigit Madzwamuse, S.; Baumann, N.; Jaekel, J.; Bartmann, P.; Wolke, D. Neuro-cognitive performance of very preterm or very low birth weight adults at 26 years. J. Child Psychol. Psychiatry 2015, 56, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Saigal, S.; Day, K.L.; Van Lieshout, R.J.; Schmidt, L.A.; Morrison, K.M.; Boyle, M.H. Health, wealth, social integration, and sexuality of extremely low-birth-weight prematurely born adults in the fourth decade of life. JAMA Pediatr. 2016, 170, 678–686. [Google Scholar] [CrossRef]
- Voegtline, K.M.; Stifter, C.A.; Family Life Project, I. Late-Preterm Birth, Maternal symptomatology, and infant negativity. Infant Behav. Dev. 2010, 33, 545–554. [Google Scholar] [CrossRef] [PubMed]
- De Jong, M.; Verhoeven, M.; van Baar, A.L. School Outcome, Cognitive Functioning, and Behaviour Problems in Moderate and Late Preterm Children and Adults: A Review. Semin. Fetal Neonatal Med. 2012, 17, 163–169. [Google Scholar] [CrossRef]
- Talge, N.M.; Holzman, C.; Wang, J.; Lucia, V.; Gardiner, J.; Breslau, N. Late-preterm birth and its association with cognitive and socioemotional outcomes at 6 years of age. Pediatrics 2010, 126, 1124–1131. [Google Scholar] [CrossRef]
- Van Baar, A.L.; Vermaas, J.; Knots, E.; de Kleine, M.J.K.; Soons, P. Functioning at school age of moderately preterm children born at 32 to 36 weeks’ gestational age. Pediatrics 2009, 124, 251–257. [Google Scholar] [CrossRef]
- Heuser, K.M.; Jaekel, J.; Wolke, D. Origins and predictors of friendships in 6- to 8-year-old children born at neonatal risk. J. Pediatr. 2018, 193, 93–101.e5. [Google Scholar] [CrossRef] [PubMed]
- Landry, S.H.; Chapieski, M.L.; Richardson, M.A.; Palmer, J.; Hall, S. The social competence of children born prematurely: Effects of medical complications and parent behaviors. Child Dev. 1990, 61, 1605–1616. [Google Scholar] [CrossRef] [PubMed]
- Treyvaud, K.; Anderson, V.A.; Howard, K.; Bear, M.; Hunt, R.W.; Doyle, L.W.; Inder, T.E.; Woodward, L.; Anderson, P.J. Parenting behavior is associated with the early neurobehavioral development of very preterm children. Pediatrics 2009, 123, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Poehlmann-Tynan, J.; Gerstein, E.D.; Burnson, C.; Weymouth, L.; Bolt, D.M.; Maleck, S.; Schwichtenberg, A.J. Risk and resilience in preterm children at age 6. Dev. Psychopath. 2014, 27, 843–858. [Google Scholar] [CrossRef] [PubMed]
- Landry, S.H.; Smith, K.E.; Miller-Loncar, C.L.; Swank, P.R. Predicting cognitive-language and social growth curves from early maternal behaviors in children at varying degrees of biological risk. Dev. Psychol. 1997, 33, 1040–1053. [Google Scholar] [CrossRef] [PubMed]
- Forcada-Guex, M.; Pierrehumbert, B.; Borghini, A.; Moessinger, A.; Muller-Nix, C. Early dyadic patterns of mother–infant interactions and outcomes of prematurity at 18 months. Pediatrics 2006, 118, e107–e114. [Google Scholar] [CrossRef] [PubMed]
- Nomura, Y.; Wickramaratne, P.J.; Pilowsky, D.J.; Newcorn, J.H.; Bruder, B.; Davey, C.; Fifer, W.P.; Brooks-Gunn, J.; Weissman, M.M. Low birthweight and risk of affective disorders & selected medical illness in offspring at high and low risk for depression. Compr. Psychiatry 2007, 48, 470–478. [Google Scholar] [CrossRef]
- Jaekel, J.; Pluess, M.; Belsky, J.; Wolke, D. Effects of maternal sensitivity on low birth weight children’s academic achievement: A test of differential susceptibility versus diathesis stress. J. Child Psychol. Psychiatry 2015, 56, 693–701. [Google Scholar] [CrossRef]
- Wolke, D.; Jaekel, J.; Hall, J.; Baumann, N. Effects of sensitive parenting on the academic resilience of very preterm and very low birth weight adolescents. J. Adolesc. Health 2013, 53, 642–647. [Google Scholar] [CrossRef]
- Clark, C.A.C.; Woodward, L.J.; Horwood, L.J.; Moor, S. Development of emotional and behavioral regulation in children born extremely preterm and very preterm: Biological and social Influences. Child Dev. 2008, 79, 1444–1462. [Google Scholar] [CrossRef]
- Wolke, D.; Strauss, V.; Johnson, S.; Gilmore, C.; Marlow, N.; Jaekel, J. Universal gestational age effects on cognitive and basic mathematic processing: 2 cohorts in 2 countries. J. Pediatr. 2015, 166, 1410–1416.e2. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A. Ein Verfahren zur Messung des Fuer das Bildungsverhalten Relevanten Sozial Status BRSS Ueberarbeitete Fassung; Deutsches Institut fuer Internationale Paedagogische Forschung: Frankfurt, Germany, 1988. [Google Scholar]
- Breeman, L.D.; Jaekel, J.; Baumann, N.; Bartmann, P.; Wolke, D. Neonatal predictors of cognitive ability in adults born very preterm: A prospective cohort study. Dev. Med. Child Neurol. 2017, 59, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Wolke, D.; Schmid, G.; Schreier, A.; Meyer, R. Crying and feeding problems in infancy and cognitive outcome in preschool children born at risk: A prospective population study. J. Dev. Behav. Pediatr. 2009, 30, 226–238. [Google Scholar] [CrossRef] [PubMed]
- Natsuaki, M.N.; Leve, L.D.; Neiderhiser, J.M.; Shaw, D.S.; Scaramella, L.V.; Ge, X.; Reiss, D. Intergenerational transmission of risk for social inhibition: The interplay between parental responsiveness and genetic influences. Dev. Psychopath. 2013, 25, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Pyhälä, R.; Wolford, E.; Kautiainen, H.; Andersson, S.; Bartmann, P.; Baumann, N.; Brubakk, A.-M.; Evensen, K.A.I.; Hovi, P.; Kajantie, E.; et al. Self-reported mental health problems among adults born preterm: A meta-analysis. Pediatrics 2017, 139, e20162690. [Google Scholar] [CrossRef] [PubMed]
- Cosentino-Rocha, L.; Klein, V.C.; Linhares, M.B.M. Effects of preterm birth and gender on temperament and behavior in children. Infant Behav. Dev. 2014, 37, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Wolke, D.; Lereya, S.T. Long-term effects of bullying. Arch. Dis. Child. 2015, 100, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Potijk, M.R.; de Winter, A.F.; Bos, A.F.; Kerstjens, J.M.; Reijneveld, S.A. Higher rates of behavioural and emotional problems at preschool age in children born moderately preterm. Arch. Dis. Child 2012, 97, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Nadeau, L.; Tessier, R. Social adjustment at school: Are children with cerebral palsy perceived more negatively by their peers than other at-risk children? Disabil. Rehabil. 2009, 31, 302–308. [Google Scholar] [CrossRef]
- Montagna, A.; Nosarti, C. Socio-emotional development following very preterm birth: Pathways to psychopathology. Front. Psychol. 2016, 7, 80. [Google Scholar] [CrossRef]
- Healy, E.; Reichenberg, A.; Nam, K.W.; Allin, M.P.G.; Walshe, M.; Rifkin, L.; Murray, R.M.; Nosarti, C. Preterm birth and adolescent social functioning–alterations in emotion-processing brain areas. J. Pediatr. 2013, 163, 1596–1604. [Google Scholar] [CrossRef] [PubMed]
- Tucker, D.M.; Derryberry, D.; Luu, P.; Phan, K.L. Anatomy and physiology of human emotion: Vertical integration of brainstem, limbic, and cortical systems. Neuropsychol. Emot. 2000, 56–79. [Google Scholar]
- Feldman, R. From biological rhythms to social rhythms: Physiological precursors of mother-infant synchrony. Dev. Psychol. 2006, 42, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Levitt, P. Structural and functional maturation of the developing primate brain. J. Pediatr. 2003, 143, 35–45. [Google Scholar] [CrossRef]
- Porges, S.W. The polyvagal theory: Phylogenetic contributions to social behavior. Physiol. Behav. 2003, 79, 503–513. [Google Scholar] [CrossRef]
- Geva, R.; Feldman, R. A neurobiological model for the effects of early brainstem functioning on the development of behavior and emotion regulation in infants: Implications for prenatal and perinatal risk. J. Child Psychol. Psychiatry 2008, 49, 1031–1041. [Google Scholar] [CrossRef] [PubMed]
- Blakemore, S.-J. The social brain in adolescence. Nat. Rev. Neurosci. 2008, 9, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Hack, M.; Taylor, H.G. Perinatal brain injury in preterm infants and later neurobehavioral function. JAMA 2000, 284, 1973–1974. [Google Scholar] [CrossRef] [PubMed]
- Field, T.M. Visual and cardiac responses to animate and inanimate faces by young term and preterm infants. Child Dev. 1979, 50, 188–194. [Google Scholar] [CrossRef]
- Barratt, M.S.; Roach, M.A.; Leavitt, L.A. Early channels of mother-infant communication: Preterm and term infants. J Child Psychol. Psychiatry 1992, 33, 1193–1204. [Google Scholar] [CrossRef]
- De Schuymer, L.; De Groote, I.; Desoete, A.; Roeyers, H. Gaze aversion during social interaction in preterm infants: A function of attention skills? Infant Behav. Dev. 2012, 35, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Harel, H.; Gordon, I.; Geva, R.; Feldman, R. Gaze behaviors of preterm and full-term infants in nonsocial and social contexts of increasing dynamics: Visual recognition, attention regulation, and gaze synchrony. Infancy 2011, 16, 69–90. [Google Scholar] [CrossRef]
- Feldman, R.; Eidelman, A.I. Neonatal state organization, neuromaturation, mother-infant interaction, and cognitive development in small-for-gestational-age premature infants. Pediatrics 2006, 118, e869–e878. [Google Scholar] [CrossRef] [PubMed]
- Nosarti, C.; Nam, K.W.; Walshe, M.; Murray, R.M.; Cuddy, M.; Rifkin, L.; Allin, M.P.G. Preterm birth and structural brain alterations in early adulthood. Neuroimage Clin. 2014, 6, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Spittle, A.J.; Treyvaud, K.; Doyle, L.W.; Roberts, G.; Lee, K.J.; Inder, T.E.; Cheong, J.L.Y.; Hunt, R.W.; Newnham, C.A.; Anderson, P.J. Early emergence of behavior and social-emotional problems in very preterm infants. J. Am. Acad. Child Adoles. Psychiatry 2009, 48, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Dollar, J.M.; Stifter, C.A.; Buss, K.A. Exuberant and inhibited children: Person-centered profiles and links to social adjustment. Dev. Psychol. 2017, 53, 1222–1229. [Google Scholar] [CrossRef] [PubMed]
- Stifter, C.A.; Putnam, S.; Jahromi, L. Exuberant and inhibited toddlers: Stability of temperament and risk for problem behavior. Dev. Psychopathol. 2008, 20, 401–421. [Google Scholar] [CrossRef]
- Hirshfeld-Becker, D.R.; Biederman, J.; Calltharp, S.; Rosenbaum, E.D.; Faraone, S.V.; Rosenbaum, J.F. Behavioral inhibition and disinhibition as hypothesized precursors to psychopathology: Implications for pediatric bipolar disorder. Biol. Psychiatry 2003, 53, 985–999. [Google Scholar] [CrossRef]
- Derryberry, D.; Rothbart, M.K. Reactive and effortful processes in the organization of temperament. Dev. Psychopath. 1997, 9, 633–652. [Google Scholar] [CrossRef]
- Cloninger, C.R. A unified biosocial theory of personality and its role in the development of anxiety states. Psychiatr. Dev. 1986, 3, 167–226. [Google Scholar]
- Mathewson, K.J.; Chow, C.H.T.; Dobson, K.G.; Pope, E.I.; Schmidt, L.A.; Van Lieshout, R.J. Mental health of extremely low birth weight survivors: A systematic review and meta-analysis. Psychol. Bull. 2017, 143, 347–383. [Google Scholar] [CrossRef] [PubMed]
- Eryigit-Madzwamuse, S.; Strauss, V.; Baumann, N.; Bartmann, P.; Wolke, D. Personality of adults who were born very preterm. Arch. Dis. Child.-Fetal Neonatal Ed. 2015, 100, F524–F529. [Google Scholar] [CrossRef] [PubMed]
- Sonuga-Barke, E.J.S.; Kennedy, M.; Kumsta, R.; Knights, N.; Golm, D.; Rutter, M.; Maughan, B.; Schlotz, W.; Kreppner, J. Child-to-adult neurodevelopmental and mental health trajectories after early life deprivation: The young adult follow-up of the longitudinal English and Romanian Adoptees study. Lancet 2017, 389, 1539–1548. [Google Scholar] [CrossRef]
- Bruce, J.; Tarullo, A.R.; Gunnar, M.R. Disinhibited social behavior among internationally adopted children. Dev. Psychopath. 2009, 21, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Jaekel, J.; Eryigit-Madzwamuse, S.; Wolke, D. Preterm toddlers’ inhibitory control abilities predict attention regulation and academic achievement at age 8 years. J. Pediatr. 2016, 169, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Bilgin, A.; Wolke, D. Regulatory problems in very preterm and full-term infants over the first 18 months. J. Dev. Behav. Pediatr. 2016, 37, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Degnan, K.A.; Hane, A.A.; Henderson, H.A.; Moas, O.L.; Reeb-Sutherland, B.C.; Fox, N.A. Longitudinal stability of temperamental exuberance and social–emotional outcomes in early childhood. Dev. Psychol. 2011, 47, 765–780. [Google Scholar] [CrossRef] [PubMed]
- Wolke, D.; Eryigit-Madzwamuse, S.; Gutbrod, T. Very preterm/very low birthweight infants’ attachment: Infant and maternal characteristics. Arch. Dis. Child.-Fetal Neonatal Ed. 2014, 99, F70–F75. [Google Scholar] [CrossRef]
- Bilgin, A.; Wolke, D. Maternal Sensitivity in Parenting Preterm Children: A Meta-analysis. Pediatrics 2015, 136, e177–e193. [Google Scholar] [CrossRef]
- Feldman, R.; Eidelman, A.I. Maternal postpartum behavior and the emergence of infant–mother and infant–father synchrony in preterm and full-term infants: The role of neonatal vagal tone. Dev. Psychobiol. 2007, 49, 290–302. [Google Scholar] [CrossRef]
- Wijnroks, L. Maternal recollected anxiety and mother–infant interaction in preterm infants. Infant Ment. Health J. 1999, 20, 393–409. [Google Scholar] [CrossRef]
- Muller-Nix, C.; Forcada-Guex, M.; Pierrehumbert, B.; Jaunin, L.; Borghini, A.; Ansermet, F. Prematurity, maternal stress and mother–child interactions. Early Hum. Dev. 2004, 79, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Grunau, R.E. Neonatal pain in very preterm infants: Long-term effects on brain, neurodevelopment and pain reactivity. Rambam Maimonides Med. J. 2013, 4, e0025. [Google Scholar] [PubMed]
- Newnham, C.A.; Milgrom, J.; Skouteris, H. Effectiveness of a modified mother–infant transaction program on outcomes for preterm infants from 3 to 24 months of age. Infant Behav. Dev. 2009, 32, 17–26. [Google Scholar] [CrossRef]
- Guilherme Monte Cassiano, R.; Gaspardo, C.M.; Cordaro Bucker Furini, G.; Martinez, F.E.; Martins Linhares, M.B. Impact of neonatal risk and temperament on behavioral problems in toddlers born preterm. Early Hum. Dev. 2016, 103, 175–181. [Google Scholar] [CrossRef]
- Klein, V.C.; Gaspardo, C.M.; Martinez, F.E.; Martins Linhares, M.B. Neonatal characteristics and temperament predict behavior problems in children born preterm. J. Hum. Growth Dev. 2015, 25, 331–340. [Google Scholar] [CrossRef]
- Perricone, G.; Morales, M.R. The temperament of preterm infant in preschool age. Ital. J. Pediatr. 2011, 37, 4. [Google Scholar] [CrossRef]
- Moore, T.; Hennessy, E.M.; Myles, J.; Johnson, S.J.; Draper, E.S.; Costeloe, K.L.; Marlow, N. Neurological and developmental outcome in extremely preterm children born in England in 1995 and 2006: The EPICure studies. BMJ Br. Med. J. 2012, 345, e7961. [Google Scholar] [CrossRef]
- Pierrat, V.; Marchand-Martin, L.; Arnaud, C.; Kaminski, M.; Resche-Rigon, M.; Lebeaux, C.; Bodeau-Livinec, F.; Morgan, A.S.; Goffinet, F.; Marret, S.; et al. Neurodevelopmental outcome at 2 years for preterm children born at 22 to 34 weeks’ gestation in France in 2011: EPIPAGE-2 cohort study. Br. Med. J. 2017, 358, j3448. [Google Scholar] [CrossRef]
- Cheong, J.L.Y.; Anderson, P.J.; Burnett, A.C.; Roberts, G.; Davis, N.; Hickey, L.; Carse, E.; Doyle, L.W.; Victorian Infant Collaborative Study, G. Changing neurodevelopment at 8 years in children born extremely preterm since the 1990s. Pediatrics 2017, 139, e20164086. [Google Scholar] [CrossRef]
- Twilhaar, E.S.; de Kieviet, J.F.; Aarnoudse-Moens, C.S.H.; van Elburg, R.M.; Oosterlaan, J. Academic performance of children born preterm: A meta-analysis and meta-regression. Arch. Dis. Child.-Fetal Neonatal Ed. 2017, 103, F322–F330. [Google Scholar] [CrossRef] [PubMed]
- Komsi, N.; Räikkönen, K.; Pesonen, A.-K.; Heinonen, K.; Keskivaara, P.; Järvenpää, A.-L.; Strandberg, T.E. Continuity of temperament from infancy to middle childhood. Infant Behav. Dev. 2006, 29, 494–508. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.; Anderson, P.; Battin, M.; Bowen, J.; Brown, N.; Callanan, C.; Campbell, C.; Chandler, S.; Cheong, J.; Darlow, B.; et al. Long term follow up of high risk children: Who, why and how? BMC Pediatr. 2014, 14. [Google Scholar] [CrossRef] [PubMed]
- Webster-Stratton, C.; Reid, M.J. Treating conduct problems and strengthening social and emotional competence in young children: The Dina Dinosaur treatment program. J. Emot. Behav. Disord. 2003, 11, 130–143. [Google Scholar] [CrossRef]
- Suarez, G.L.; Morales, S.; Metcalf, K.; Pérez-Edgar, K.E. Perinatal complications are associated with social anxiety: Indirect effects through temperament. Infant Child Dev. 2019, 28, e2130. [Google Scholar] [CrossRef]
- Miranda, L.; Jaekel, J.; Heuser, K.M.; Wolke, D. Developmental cascades of social inhibition and friendships in preterm and full-term children. Presented at the Society for Research in Child Development (SRCD) Biennial Meeting, Baltimore, MD, USA, 22 March 2010. [Google Scholar]



| Background Characteristics | Very Preterm 32 w GA n = 229 | Moderately Preterm 32–33 w GA n = 90 | Late Preterm 34–36 w GA n = 205 | Early Term 37–38 w GA n = 199 | Full Term 39–41 w GA n = 591 | F/X2 (df), p Value |
|---|---|---|---|---|---|---|
| Gestational age in weeks M(SD) | 29.5 (1.5) | 32.5 (0.5) | 35.1 (0.8) | 37.6 (0.5) | 40.0 (0.7) | F (4,1309) = 6687.2 *** |
| Birth weight in grams M(SD) | 1,287(323) | 1,640 (375) | 2,219 (547) | 2,827 (559) | 3,407 (504) | F (4,1309) = 951.3 *** |
| Child sex (% male) | 57.2% | 47.8 % | 50.2% | 48.2% | 50.1% | X2 (4,1314) = 4.7 |
| SES (1 = high, 6 = low) M(SD) | 3.5 (1.5) | 3.5 (1.6) | 3.4 (1.6) | 3.3 (1.6) | 3.4 (1.5) | F (4,1309) = 0.7 |
| Good parent–infant relationship | 50.7% | 58.9% | 62.9% | 66.3% | 70.1% | X2 (4,1314) = 28.7 *** |
| Very Preterm 32 w GA n = 229 | Moderately Preterm 32–33 w GA n = 90 | Late Preterm 34–36 w GA n = 205 | Early Term 37–38 w GA n = 199 | Full Term 39–41 w GA n = 591 | X2(df), p Value | |
|---|---|---|---|---|---|---|
| Nonverbal Inhibitiona | 41.26(8) *** | |||||
| Disinhibited (%) | 32.30% | 27.80% | 21.50% | 24.60% | 17.40% | |
| Normal response (%) | 41.00% | 42.20% | 53.70% | 51.30% | 62.40% | |
| Inhibited (%) | 26.60% | 30.00% | 24.90% | 24.10% | 20.10% | |
| Verbal Inhibitionb | 30.37(8) *** | |||||
| Disinhibited (%) | 31.40% | 26.70% | 21.00% | 23.10% | 17.10% | |
| Normal response (%) | 43.70% | 47.80% | 50.20% | 50.80% | 60.40% | |
| Inhibited (%) | 24.90% | 25.60% | 28.80% | 26.10% | 22.50% |
| Variable | B | SE B | Wald | df | OR | 95% CI | |
|---|---|---|---|---|---|---|---|
| Disinhibited 180 s | Intercept | −0.86 *** | 0.24 | 13.03 | 1 | ||
| Sex (male) | 0.27 | 0.14 | 3.50 | 1 | 1.31 | 0.99–1.73 | |
| Lower SES | −0.18 *** | 0.05 | 15.19 | 1 | 0.84 | 0.77–0.92 | |
| Lower gestational age | 0.24 *** | 0.05 | 27.77 | 1 | 1.27 | 1.17–1.40 | |
| Good parenting | −0.27 | 0.15 | 3.33 | 1 | 0.77 | 0.57–1.02 | |
| Inhibited >188 s | Intercept | −1.04 *** | 0.24 | 18.55 | 1 | ||
| Sex (male) | −0.36 ** | 0.14 | 6.79 | 1 | 0.70 | 0.53–0.91 | |
| Lower SES | −0.02 | 0.05 | 0.27 | 1 | 0.98 | 0.90–1.07 | |
| Lower gestational age | 0.19 *** | 0.05 | 17.09 | 1 | 1.21 | 1.11–1.32 | |
| Good parenting | 0.01 | 0.15 | 0.00 | 1 | 1.01 | 0.76–1.35 | |
| LR χ2 | 70.09 *** | ||||||
| Variable | B | SE B | Wald | df | OR | 95% CI | |
|---|---|---|---|---|---|---|---|
| Disinhibited 180 s | Intercept | −0.99 *** | 0.24 | 16.91 | 1 | ||
| Sex (male) | 0.40 ** | 0.05 | 7.80 | 1 | 1.50 | 1.13–1.98 | |
| Lower SES | −0.13 ** | 0.05 | 7.80 | 1 | 0.88 | 0.81–0.96 | |
| Lower gestational age | 0.21 *** | 0.05 | 20.75 | 1 | 1.23 | 1.13–1.35 | |
| Good parenting | −0.36 * | 0.15 | 6.02 | 1 | 0.70 | 0.52–0.93 | |
| Inhibited >227 s | Intercept | −1.45 *** | 0.25 | 34.76 | 1 | ||
| Sex (male) | 0.01 | 0.14 | 0.00 | 1 | 1.01 | 0.77–1.31 | |
| Lower SES | 0.16 ** | 0.05 | 11.68 | 1 | 1.17 | 1.07–1.28 | |
| Lower gestational age | 0.10 * | 0.05 | 4.99 | 1 | 1.11 | 1.01–1.21 | |
| Good parenting | −0.19 | 0.14 | 1.73 | 1 | 0.83 | 0.63–1.10 | |
| LR χ2 | 68.87 *** | ||||||
| Very Preterm 32 Weeks GA n = 229 | Moderately Preterm 32–33 Weeks GA n = 90 | Late Preterm 34–36 Weeks GA n = 205 | Early Term 37–38 Weeks GA n = 199 | Full Term 39–41 Weeks GA n = 591 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PIRI | Poor | Good | Poor | Good | Poor | Good | Poor | Good | Poor | Good |
| Disinhibited (%) | 36.3% | 26.7% | 35.1% | 20.8% | 19.7% | 21.7% | 28.4% | 20.5% | 18.1% | 16.7% |
| Normal (%) | 39.8% | 47.4% | 35.1% | 56.6% | 50.0% | 50.4% | 43.3% | 54.4% | 57.1% | 61.8% |
| Inhibited (%) | 23.9% | 25.9% | 29.7% | 22.6% | 30.3% | 27.9% | 28.4% | 25.0% | 24.9% | 21.5% |
| Variable | B | SE B | Wald | df | OR | 95% CI | |
|---|---|---|---|---|---|---|---|
| Disinhibited 180 s | Intercept | −1.09 | 0.20 | 29.39 | 1 | ||
| Sex (male) | 0.40 ** | 0.14 | 7.78 | 1 | 1.49 | 1.13–1.98 | |
| Lower SES | −0.12 ** | 0.05 | 6.93 | 1 | 0.89 | 0.81–0.97 | |
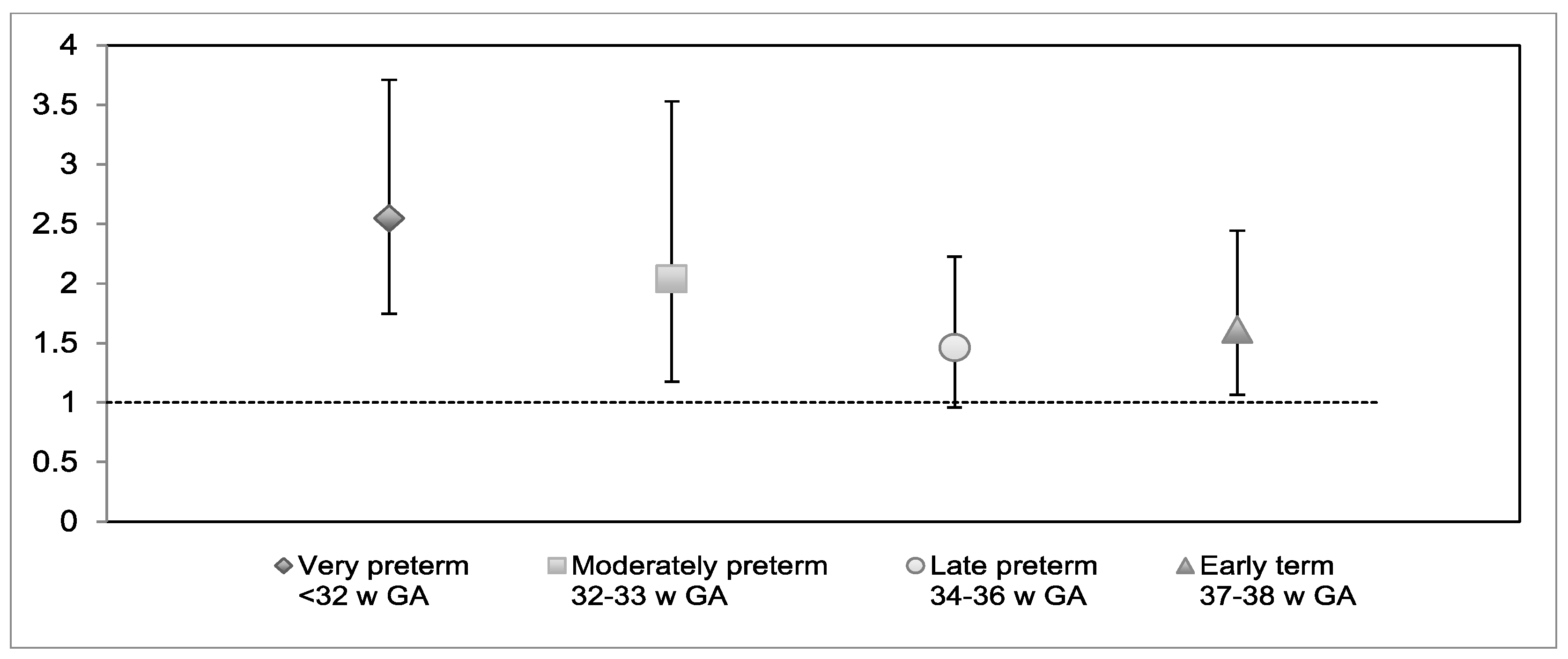
| Very preterm | 0.93 *** | 0.19 | 23.31 | 1 | 2.54 | 1.74–3.70 | |
| Moderately preterm | 0.71 * | 0.28 | 6.37 | 1 | 2.03 | 1.17–3.52 | |
| Late preterm | 0.38 | 0.22 | 3.08 | 1 | 1.46 | 0.96–2.22 | |
| Early term | 0.48 * | 0.21 | 5.04 | 1 | 1.61 | 1.06–2.44 | |
| Inhibited >227 s | Intercept | −1.57 *** | 0.21 | 58.24 | 1 | ||
| Sex (male) | 0.01 | 0.14 | 0.00 | 1 | 1.01 | 0.77–1.32 | |
| Lower SES | 0.16 *** | 0.05 | 12.87 | 1 | 1.18 | 1.08–1.29 | |
| Very preterm | 0.41 * | 0.20 | 4.32 | 1 | 1.50 | 1.02–2.20 | |
| Moderately preterm | 0.34 | 0.28 | 1.47 | 1 | 1.40 | 0.81–2.42 | |
| Late preterm | 0.44 * | 0.19 | 5.27 | 1 | 1.56 | 1.07–2.28 | |
| Early term | 0.34 | 0.20 | 2.82 | 1 | 1.40 | 0.95–2.07 | |
| LR χ2 | 67.02 *** | ||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
M. Reyes, L.; Jaekel, J.; Wolke, D. Effects of Gestational Age and Early Parenting on Children’s Social Inhibition at 6 Years. Children 2019, 6, 81. https://doi.org/10.3390/children6070081
M. Reyes L, Jaekel J, Wolke D. Effects of Gestational Age and Early Parenting on Children’s Social Inhibition at 6 Years. Children. 2019; 6(7):81. https://doi.org/10.3390/children6070081
Chicago/Turabian StyleM. Reyes, Lucia, Julia Jaekel, and Dieter Wolke. 2019. "Effects of Gestational Age and Early Parenting on Children’s Social Inhibition at 6 Years" Children 6, no. 7: 81. https://doi.org/10.3390/children6070081
APA StyleM. Reyes, L., Jaekel, J., & Wolke, D. (2019). Effects of Gestational Age and Early Parenting on Children’s Social Inhibition at 6 Years. Children, 6(7), 81. https://doi.org/10.3390/children6070081





