Dexamethasone Alters Tracheal Aspirate T-Cell Cytokine Production in Ventilated Preterm Infants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Patient Characteristics
2.3. Dexamethasone Treatment and Tracheal Aspirate Sample Collection
2.4. Respiratory Severity Score
2.5. Immunostaining and Flow Cytometric Analysis
2.6. Statistical Analysis
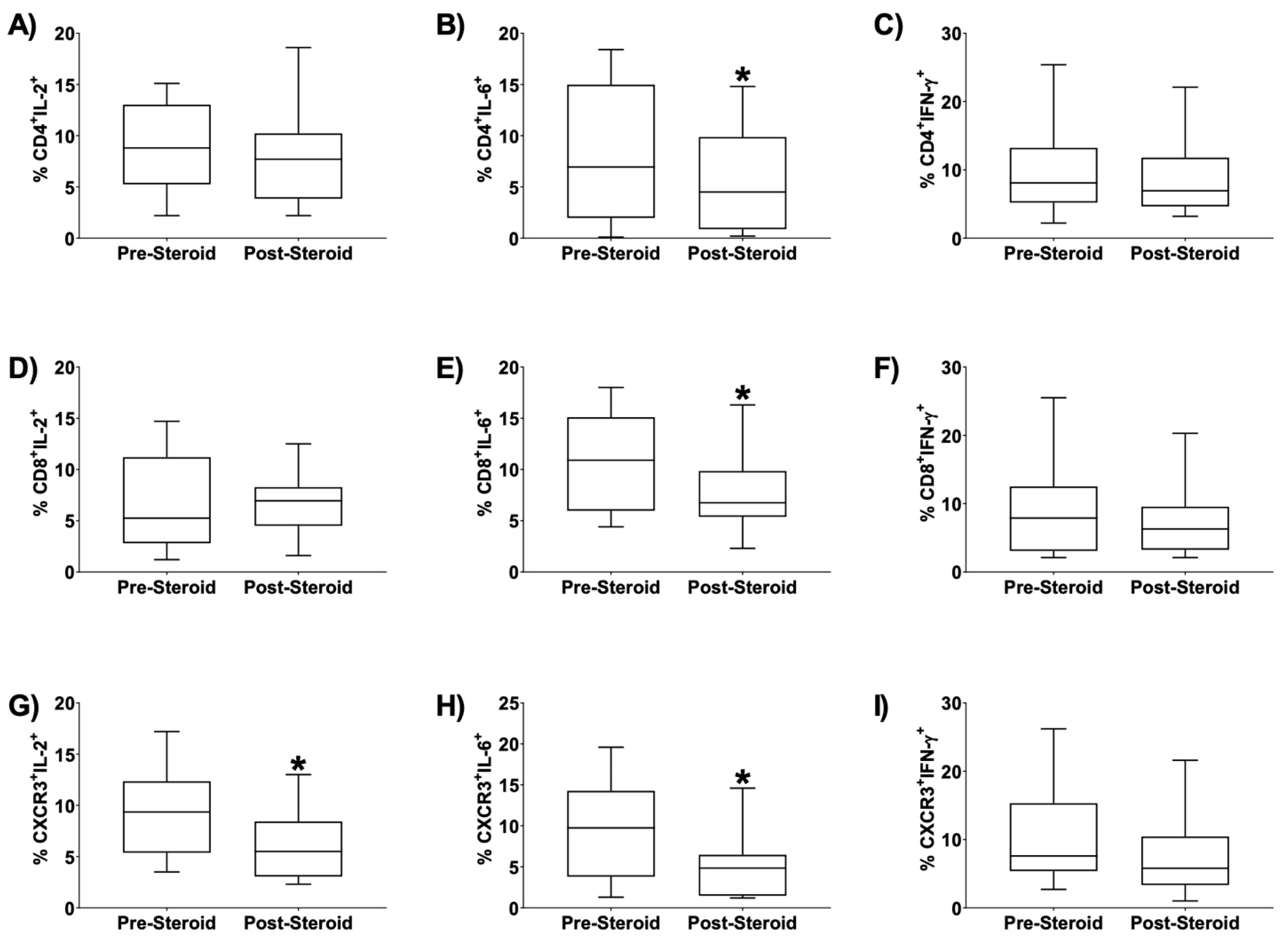
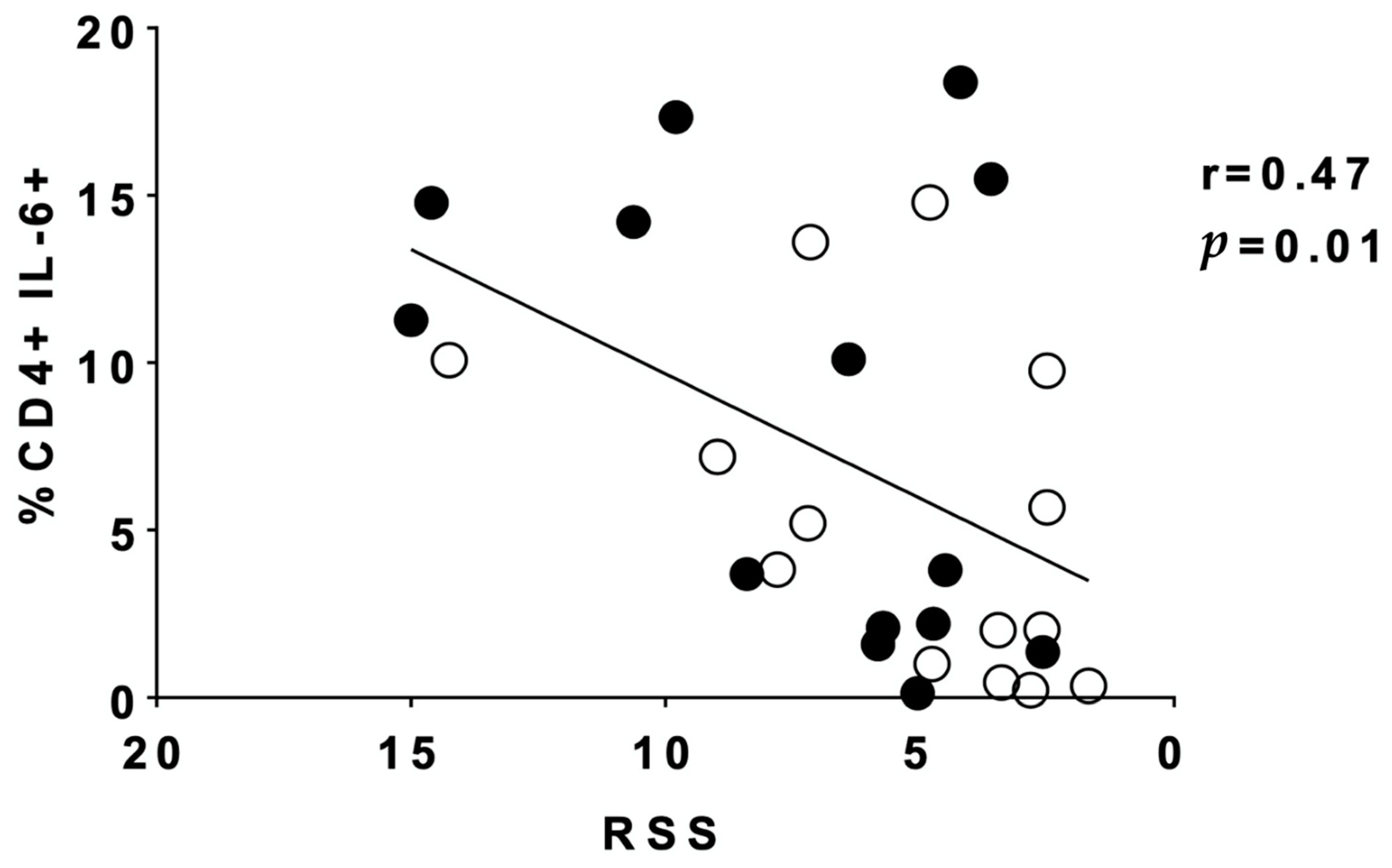
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclosures
References
- Htun, Z.T.; Schulz, E.V.; Desai, R.K.; Marasch, J.L.; McPherson, C.C.; Mastrandrea, L.D.; Jobe, A.H.; Ryan, R.M. Postnatal steroid management in preterm infants with evolving bronchopulmonary dysplasia. J. Perinatol. 2021, 41, 1783–1796. [Google Scholar] [CrossRef] [PubMed]
- Parimi, P.S.; Birnkrant, D.J.; Rao, L.V.; Diaz, G.; Moore, J.J. Effect of dexamethasone on lymphocyte subpopulations in premature infants with bronchopulmonary dysplasia. J. Perinatol. 1999, 19, 347–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoder, M.C., Jr.; Chua, R.; Tepper, R. Effect of dexamethasone on pulmonary inflammation and pulmonary function of ventilator-dependent infants with bronchopulmonary dysplasia. Am. Rev. Respir. Dis. 1991, 143 (5 Pt 1), 1044–1048. [Google Scholar] [CrossRef]
- Ohlsson, A.; Calvert, S.A.; Hosking, M.; Shennan, A.T. Randomized controlled trial of dexamethasone treatment in very-low-birth-weight infants with ventilator-dependent chronic lung disease. Acta Paediatr. 1992, 81, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Michael, Z.; Spyropoulos, F.; Ghanta, S.; Christou, H. Bronchopulmonary Dysplasia: An Update of Current Pharmacologic Therapies and New Approaches. Clin. Med. Insights Pediatr. 2018, 12, 1179556518817322. [Google Scholar] [CrossRef]
- Nath, S.; Reynolds, A.M.; Lakshminrusimha, S.; Ma, C.; Hudak, M.L.; Ryan, R.M. Retrospective analysis of short-term respiratory outcomes of three different steroids used in clinical practice in intubated preterm infants. Am. J. Perinatol. 2019, 37, 1425–1431. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.; Bhandari, V. Pulmonary Biomarkers of Bronchopulmonary Dysplasia. Biomark Insights 2008, 3, 361–373. [Google Scholar] [CrossRef] [Green Version]
- Ambalavanan, N.; Carlo, W.A.; D’Angio, C.T.; McDonald, S.A.; Das, A.; Schendel, D.; Thorsen, P.; Higgins, R.D. Cytokines associated with bronchopulmonary dysplasia or death in extremely low birth weight infants. Pediatrics 2009, 123, 1132–1141. [Google Scholar] [CrossRef] [Green Version]
- Tullus, K.; Noack, G.W.; Burman, L.G.; Nilsson, R.; Wretlind, B.; Brauner, A. Elevated cytokine levels in tracheobronchial aspirate fluids from ventilator treated neonates with bronchopulmonary dysplasia. Eur. J. Pediatr. 1996, 155, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Ryan, R.M.; Ahmed, Q.; Lakshminrusimha, S. Inflammatory mediators in the immunobiology of bronchopulmonary dysplasia. Clin. Rev. Allergy Immunol. 2008, 34, 174–190. [Google Scholar] [CrossRef] [PubMed]
- Rocha, G.P.; Guedes, A.; Carvalho, C.; Areias, A.; Ramos, J.P.; Rodrigues, T.; Guimarães, H. Cord Blood Levels of IL-6, IL-8 and IL-10 May Be Early Predictors of Bronchopulmonary Dysplasia in Preterm Newborns Small for Gestational Age. Dis. Markers 2012, 33, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Bourbia, A.; Cruz, M.A.; Rozycki, H.J. NF-kappaB in tracheal lavage fluid from intubated premature infants: Association with inflammation, oxygen, and outcome. Arch. Dis. Child Fetal. Neonatal. Ed. 2006, 91, F36–F39. [Google Scholar] [CrossRef] [Green Version]
- Popova, A.P.; Cui, T.X.; Kaciroti, N.; Goldsmith, A.M.; Linn, M.J.; Pryhuber, G.S.; Hershenson, M.B. Tracheal Aspirate Levels of the Matricellular Protein SPARC Predict Development of Bronchopulmonary Dysplasia. PLoS ONE 2015, 10, e0144122. [Google Scholar] [CrossRef]
- Blosser, E.G.; Randolph, D.A. CD4 T Cells. NeoReviews 2013, 14, e456–e462. [Google Scholar] [CrossRef]
- Ballabh, P.; Simm, M.; Kumari, J.; Krauss, A.N.; Jain, A.; Auld, P.A. Lymphocyte subpopulations in bronchopulmonary dysplasia. Am. J. Perinatol. 2003, 20, 465–475. [Google Scholar] [PubMed]
- Groom, J.R.; Luster, A.D. CXCR3 in T cell function. Exp. Cell Res. 2011, 317, 620–631. [Google Scholar] [CrossRef]
- Scheible, K.M.; Emo, J.; Laniewski, N.; Baran, A.M.; Peterson, D.R.; Holden-Wiltse, J.; Bandyopadhyay, B.; Straw, A.G.; Huyck, H.; Ashton, J.M.; et al. T cell developmental arrest in former premature infants increases risk of respiratory morbidity later in infancy. JCI Insight 2018, 3, e96724. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, Q.; Pryhuber, G.; D’Angelis, C.; Kumar, V.; Lakshminrusimha, S.; Metlay, L. T-Lymphocytes in Human Infants with Bronchopulmonary Dysplasia, E-PAS, 2008.
- Pignatti, P.; Brunetti, G.; Moretto, D.; Yacoub, M.R.; Fiori, M.; Balbi, B.; Balestrino, A.; Cervio, G.; Nava, S.; Moscato, G. Role of the chemokine receptors CXCR3 and CCR4 in human pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2006, 173, 310–317. [Google Scholar] [CrossRef]
- Munshi, U.K.; Niu, J.O.; Siddiq, M.M.; Parton, L.A. Elevation of interleukin-8 and interleukin-6 precedes the influx of neutrophils in tracheal aspirates from preterm infants who develop bronchopulmonary dysplasia. Pediatr. Pulmonol. 1997, 24, 331–336. [Google Scholar] [CrossRef]
- Jobe, A.H.; Bancalari, E. Bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 2001, 163, 1723–1729. [Google Scholar] [CrossRef]
- Doyle, L.W.; Davis, P.G.; Morley, C.J.; McPhee, A.; Carlin, J.B.; Investigators, D.S. Low-dose dexamethasone facilitates extubation among chronically ventilator-dependent infants: A multicenter, international, randomized, controlled trial. Pediatrics 2006, 117, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Schlosser, R.J.; Yawn, J.R.; Mattos, J.L.; Soler, Z.M.; Mulligan, J.K. Sinonasal T-cell expression of cytotoxic mediators granzyme B and perforin is reduced in patients with chronic rhinosinusitis. Am. J. Rhinol. Allergy 2017, 31, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, J.K.; Mulligan, R.M.; Atkinson, C.; Schlosser, R.J. Human sinonasal epithelial cells direct dendritic function and T-cell T helper 1/T helper 2 skewing following Aspergillus exposure. Int. Forum. Allergy Rhinol. 2011, 1, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, J.K.; Pasquini, W.N.; Carroll, W.W.; Williamson, T.; Reaves, N.; Patel, K.J.; Mappus, E.; Schlosser, P.J.; Atkinson, C. Dietary vitamin D3 deficiency exacerbates sinonasal inflammation and alters local 25(OH)D3 metabolism. PLoS ONE 2017, 12, e0186374. [Google Scholar] [CrossRef]
- O’Connell, B.P.; Schlosser, R.J.; Wentzel, J.L.; Nagel, W.; Mulligan, J.K. Systemic monocyte-derived dendritic cells and associated Th2 skewing in chronic rhinosinusitis. Otolaryngol. Head Neck Surg. 2014, 150, 312–320. [Google Scholar] [CrossRef]
- Psaltis, A.J.; Schlosser, R.J.; Yawn, J.R.; Henriquez, O.; Mulligan, J.K. Characterization of B-cell subpopulations in patients with chronic rhinosinusitis. Int. Forum. Allergy Rhinol. 2013, 3, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Eldredge, L.C.; Creasy, R.S.; Presnell, S.; Debley, J.S.; Juul, S.E.; Mayock, D.E.; Ziegler, S.F. Infants with evolving bronchopulmonary dysplasia demonstrate monocyte-specific expression of IL-1 in tracheal aspirates. Am. J. Physiol. Lung Cell Mol. Physiol. 2019, 317, L49–L56. [Google Scholar] [CrossRef] [PubMed]
- Harijith, A.; Choo-Wing, R.; Cataltepe, S.; Yasumatsu, R.; Aghai, Z.H.; Janer, J.; Andersson, S.; Homer, R.J.; Bhandari, V. A role for matrix metalloproteinase 9 in IFNgamma-mediated injury in developing lungs: Relevance to bronchopulmonary dysplasia. Am. J. Respir. Cell Mol. Biol. 2011, 44, 621–630. [Google Scholar] [CrossRef]
- Pease, J.E. Designing small molecule CXCR3 antagonists. Expert. Opin. Drug Discov. 2017, 12, 159–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrews, S.P.; Cox, R.J. Small Molecule CXCR3 Antagonists. J. Med. Chem. 2016, 59, 2894–2917. [Google Scholar] [CrossRef]
- Miao, S.; Tang, B.; Liu, H.; Wang, Z.; Shi, Y.; Dong, Y.; Liu, W.; Qin, C.; Ren, H. CXCR3 blockade combined with cyclosporine A alleviates acute graft-versus-host disease by inhibiting alloreactive donor T cell responses in a murine model. Mol. Immunol. 2018, 94, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Kelsen, S.G.; Aksoy, M.O.; Yang, Y.; Shahabuddin, S.; Litvin, J.; Safadi, F.; Rogers, T.J. The chemokine receptor CXCR3 and its splice variant are expressed in human airway epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2004, 287, L584–L591. [Google Scholar] [CrossRef] [PubMed]

| Sex | |
| Male, n (%) | 10 (71.4%) |
| Female, n (%) | 4 (28.6%) |
| Race | |
| White, n (%) | 3 (21.4%) |
| Black, n (%) | 9 (64.3%) |
| Not specified, n (%) | 2 (14.3%) |
| Birth Weight, g (SD) | 772 (208) |
| Weight at Treatment, g (SD) | 1157 (452) |
| Birth Gestational Age (range) | 25 6/7 weeks (23 1/7–27 3/7 weeks) |
| Treatment Postmenstrual Age (range) | 29 0.5/7 weeks (24 6/7–37 6/7 weeks) |
| 1st sample to dexamethasone interval (d), (SD) | 0.7 (1.1) |
| Dexamethasone initiation to 2nd sample interval (d), (SD) | 2.8 (0.58) |
| Respiratory Severity Score (RSS) | |
| Pre-treatment RSS (SD) | 7.21 (3.94) |
| Post-treatment RSS (SD) | 5.28 (3.47) * |
| RSS reduction (SD) | 1.94 (1.74) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yazdi, S.M.; Patel, E.U.; Richardson, C.D.; Hardy, K.T.; Baatz, J.E.; Mulligan, J.K.; Ryan, R.M. Dexamethasone Alters Tracheal Aspirate T-Cell Cytokine Production in Ventilated Preterm Infants. Children 2021, 8, 879. https://doi.org/10.3390/children8100879
Yazdi SM, Patel EU, Richardson CD, Hardy KT, Baatz JE, Mulligan JK, Ryan RM. Dexamethasone Alters Tracheal Aspirate T-Cell Cytokine Production in Ventilated Preterm Infants. Children. 2021; 8(10):879. https://doi.org/10.3390/children8100879
Chicago/Turabian StyleYazdi, Siamak M., Ekta U. Patel, Colby D. Richardson, K. Thomas Hardy, John E. Baatz, Jennifer K. Mulligan, and Rita M. Ryan. 2021. "Dexamethasone Alters Tracheal Aspirate T-Cell Cytokine Production in Ventilated Preterm Infants" Children 8, no. 10: 879. https://doi.org/10.3390/children8100879






