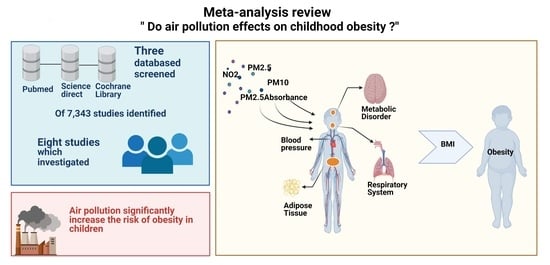
Effect of Air Pollution on Obesity in Children: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources and Search Strategy
2.2. Study Selection
2.3. Outcome Measures
- (1)
- The World Health Organization (WHO) 2007 Growth Charts: overweight is a z-score of > + 1 (equivalent to BMI 25 kg/m2) and obesity is a z-score of > + 2 (equivalent to a BMI equal to 30 kg/m2) [15].
- (2)
- The Centers for Disease Control and Prevention (CDC) BMI growth charts: normal weight ≤ 85th percentile, overweight = 85th to 95th percentile, and obesity ≥ 95th percentile [16].
- (3)
- Body Mass Index (BMI) cutoffs by age and sex in accordance with the International Obesity Task Force (IOTF) at the age of 18 [17].
- (4)
- The Chinese national standard Screening for Overweight and Obesity among School-age Children and Adolescents calculated using BMI and cutoff by sex and age group [18].
- (5)
- The national Obesity Observatory and the population cut-off taken as greater than the 85th percentile [19].
2.4. Data Extraction and Quality Assessment
2.5. Data Synthesis and Statistical Analysis
2.6. Sensitivity and Subgroup Analysis
3. Results
3.1. General Information
3.2. Results of the Meta-Analysis
3.3. Sensitivity and Subgroup Analysis
3.4. Publication Bias of Included Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization Home Page. Available online: http://www.who.int/kobecentre/measuring/urban-global-report/en/ (accessed on 20 June 2020).
- Kim, J.S.; Chen, Z.; Aldreate, T.L.; Toledo-Corral, C.; Lurmann, F.; Berhane, K.; Gilloland, F.D. Associations of air pollution, obesity and cariometabolic health in young adults: The Meta-AIR study. Environ. Int. 2019, 133, p105180. [Google Scholar] [CrossRef]
- An, R.; Ji, M.; Yan, H.; Guan, C. Impact of ambient air pollution on obesity: A systematic review. IJO 2018, 42, 1112–1126. [Google Scholar] [CrossRef]
- Han, J.C.; Lawlor, D.A.; Kimm, S.Y. Childhood obesity. Lancet 2010, 375, 1737–1748. [Google Scholar] [CrossRef]
- Nicole, W. Obesogens: An environmental link to obesity. Environ. Health Perspect. 2012, 120, 63–68. [Google Scholar]
- Toledo-Corral, C.; Alderete, T.; Habre, R.; Berhane, K.; Lurmann, F.; Weigensberg, M.; Goran, M.; Gilliland, F. Effects of air pollution exposure on glucose metabolism in Los Angeles minority children. Pediatr. Obes. 2018, 13, 54–62. [Google Scholar] [CrossRef] [Green Version]
- Begg, C.B.; Berlin, J.A. Publication bias and dissemination of clinical research. J. Natl. Cancer Inst. 1989, 81, 107–115. [Google Scholar] [CrossRef]
- Alderete, T.L.; Habre, R.; Toledo-Corral, C.M.; Berhane, K.; Chen, Z.; Lurmann, F.W.; Weigensberg, M.J.; Goran, M.I.; Gilliland, F.D. Longitudinal associations between ambient air pollution with insulin sensitivity, β-cell function, and adiposity in Los Angeles Latino children. Diabetes 2017, 66, 1789–1796. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.S.; Alderete, T.L.; Chen, Z.; Lurmann, F.; Rappaport, E.; Habre, R.; Berhane, K.; Gilliland, F.D. Longitudinal associations of in utero and early life near-roadway air pollution with trajectories of childhood body mass index. Environ. Health 2018, 17, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McConnell, R.; Gilliland, F.; Goran, M.; Allayee, H.; Hricko, A.; Mittelman, S. Does near-roadway air pollution contribute to childhood obesity? Pediatr. Obes. 2016, 11, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, G.; Nachman, R.M.; Sun, Q.; Zhang, X.; Koehler, K.; Chen, Z.; Hong, X.; Wang, G.; Caruso, D.; Zong, G. Individual and joint effects of early-life ambient PM2.5 exposure and maternal prepregnancy obesity on childhood overweight or obesity. Environ. Health Perspect. 2017, 125, 067005. [Google Scholar] [CrossRef]
- Nikolić, M.; Stanković, A.; Jović, S.; Kocić, B.; Bogdanović, D. Effects of air pollution on growth in schoolchildren. Coll Antropol. 2014, 38, 493–497. [Google Scholar] [PubMed]
- Jerrett, M.; McConnell, R.; Chang, C.R.; Wolch, J.; Reynolds, K.; Lurmann, F.; Gilliland, F.; Berhane, K. Automobile traffic around the home and attained body mass index: A longitudinal cohort study of children aged 10–18 years. Prev. Med. 2010, 50, S50–S58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borghi, E.; de Onis, M.; Garza, C.; Van den Broeck, J.; Frongillo, E.A.; Grummer-Strawn, L.; Van Buuren, S.; Pan, H.; Molinari, L.; Martorell, R. Construction of the World Health Organization child growth standards: Selection of methods for attained growth curves. Stat. Med. 2006, 25, 247–265. [Google Scholar] [CrossRef]
- Growth Chart Training. Available online: http://www.Cdc.Gov/nccdphp/dnpao/growthcharts/resources/sas.Htm (accessed on 25 June 2020).
- Cole, T.J.; Lobstein, T. Extended international (IOTF) body mass index cut-offs for thinness, overweight and obesity. Pediatr. Obes. 2012, 7, 284–294. [Google Scholar] [CrossRef]
- Guo, Q.; Xue, T.; Jia, C.; Wang, B.; Cao, S.; Zhao, X.; Zhang, Q.; Zhao, L.; Zhang, J.J.; Duan, X. Association between exposure to fine particulate matter and obesity in children: A national representative cross-sectional study in China. Environ. Int. 2020, 143, 105950. [Google Scholar] [CrossRef]
- National Obesity Observatory. A Simple Guide to Classifying Body Mass Index in Children. Available online: https://khub.net/documents/31798783/32039025/A+simple+guide+to+classifying+body+mass+index+in+children/ced23256-6f8d-43c7-9f44-222e2beebf97?version=1.0 (accessed on 12 August 2020).
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 12 August 2020).
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [Green Version]
- Egger, M.; Smith, G.D. Meta-analysis bias in location and selection of studies. BMJ 1998, 316, 61–66. [Google Scholar] [CrossRef]
- Sterne, J.A.; Egger, M. Funnel plots for detecting bias in meta-analysis: Guidelines on choice of axis. J. Clin. Epidemiol. 2001, 54, 1046–1055. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Dong, G.H.; Qian, Z.; Liu, M.M.; Wang, D.; Ren, W.H.; Flick, L.H.; Fu, J.; Wang, J.; Chen, W.; Simckes, M. Ambient air pollution and the prevalence of obesity in Chinese children: The seven northeastern cities study. Obesity 2014, 22, 795–800. [Google Scholar] [CrossRef] [Green Version]
- Fioravanti, S.; Cesaroni, G.; Badaloni, C.; Michelozzi, P.; Forastiere, F.; Porta, D. Traffic-related air pollution and childhood obesity in an Italian birth cohort. Environ. Res. 2018, 160, 479–486. [Google Scholar] [CrossRef]
- Bont, D.J.; Casas, M.; Barrera-Gómez, J.; Cirach, M.; Rivas, I.; Valvi, D.; Álvarez, M.; Dadvand, P.; Sunyer, J.; Vrijheid, M. Ambient air pollution and overweight and obesity in school-aged children in Barcelona, Spain. Environ. Int. 2019, 125, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Bloemsma, L.D.; Wijga, A.H.; Klompmaker, J.O.; Janssen, N.A.; Smit, H.A.; Koppelman, G.H.; Brunekreef, B.; Lebret, E.; Hoek, G.; Gehring, U. The associations of air pollution, traffic noise and green space with overweight throughout childhood: The PIAMA birth cohort study. Environ. Res. 2019, 169, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Wilding, S.; Ziauddeen, N.; Smith, D.; Roderick, P.; Chase, D.; Alwan, N.A. Are environmental area characteristics at birth associated with overweight and obesity in school-aged children? Findings from the SLOPE (Studying Lifecourse Obesity PrEdictors) population-based cohort in the south of England. BMC Med. 2020, 18, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Vrijheid, M.; Fossati, S.; Maitre, L.; Márquez, S.; Roumeliotaki, T.; Agier, L.; Andrusaityte, S.; Cadiou, S.; Casas, M.; de Castro, M. Early-life environmental exposures and childhood obesity: An exposome-wide approach. Environ. Health Perspect. 2020, 128, 067009. [Google Scholar] [CrossRef]
- An, R.; Zhang, S.; Ji, M.; Guan, C. Impact of ambient air pollution on physical activity among adults: A systematic review and meta-analysis. Perspect. Public Health 2018, 138, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yavar, Z.; Verdin, M.; Ying, Z.; Mihai, G.; Kampfrath, T.; Wang, A.; Zhong, M.; Lippmann, M.; Chen, L.-C. Effect of early particulate air pollution exposure on obesity in mice: Role of p47phox. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2518–2527. [Google Scholar] [CrossRef] [Green Version]
- Lawrence, W.R.; Yang, M.; Zhang, C.; Liu, R.-Q.; Lin, S.; Wang, S.-Q.; Liu, Y.; Ma, H.; Chen, D.-H.; Zeng, X.-W. Association between long-term exposure to air pollution and sleep disorder in Chinese children: The Seven Northeastern Cities study. Sleep 2018, 41, zsy122. [Google Scholar] [CrossRef]
- Keith, S.W.; Redden, D.T.; Katzmarzyk, P.T.; Boggiano, M.M.; Hanlon, E.C.; Benca, R.M.; Ruden, D.; Pietrobelli, A.; Barger, J.L.; Fontaine, K. Putative contributors to the secular increase in obesity: Exploring the roads less traveled. Int. J. Obes. 2006, 30, 1585–1594. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Georas, S.; Alexis, N.; Fritz, P.; Xia, T.; Williams, M.A.; Horner, E.; Nel, A. A work group report on ultrafine particles (American Academy of Allergy, Asthma & Immunology): Why ambient ultrafine and engineered nanoparticles should receive special attention for possible adverse health outcomes in human subjects. J. Allergy Clin. Immunol. 2016, 138, 386–396. [Google Scholar] [PubMed] [Green Version]
- Chen, C.; Liu, C.; Chen, R.; Wang, W.; Li, W.; Kan, H.; Fu, C. Ambient air pollution and daily hospital admissions for mental disorders in Shanghai, China. Sci. Total Environ. 2018, 613, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Bolton, J.L.; Smith, S.H.; Huff, N.C.; Gilmour, M.I.; Foster, W.M.; Auten, R.L.; Bilbo, S.D. Prenatal air pollution exposure induces neuroinflammation and predisposes offspring to weight gain in adulthood in a sex-specific manner. FASEB J. 2012, 26, 4743–4754. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Yue, P.; Deiuliis, J.A.; Lumeng, C.N.; Kampfrath, T.; Mikolaj, M.B.; Cai, Y.; Ostrowski, M.C.; Lu, B.; Parthasarathy, S. Ambient air pollution exaggerates adipose inflammation and insulin resistance in a mouse model of diet-induced obesity. Circulation 2009, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazaheri, M.; Clifford, S.; Jayaratne, R.; Megat Mokhtar, M.A.; Fuoco, F.; Buonanno, G.; Morawska, L. School children’s personal exposure to ultrafine particles in the urban environment. Environ. Sci. Technol. 2014, 48, 113–120. [Google Scholar] [CrossRef] [Green Version]
- Pañella, P.; Casas, M.; Donaire-Gonzalez, D.; Garcia-Esteban, R.; Robinson, O.; Valentín, A.; Gulliver, J.; Momas, I.; Nieuwenhuijsen, M.; Vrijheid, M. Ultrafine particles and black carbon personal exposures in asthmatic and non-asthmatic children at school age. Indoor Air 2017, 27, 891–899. [Google Scholar] [CrossRef] [Green Version]
- Huang, F.; Li, X.; Wang, C.; Xu, Q.; Wang, W.; Luo, Y.; Tao, L.; Gao, Q.; Guo, J.; Chen, S. PM2.5 spatiotemporal variations and the relationship with meteorological factors during 2013—2014 in Beijing, China. PLoS ONE 2015, 10, e0141642. [Google Scholar]
- Haberzettl, P.; O’Toole, T.E.; Bhatnagar, A.; Conklin, D.J. Exposure to fine particulate air pollution causes vascular insulin resistance by inducing pulmonary oxidative stress. Environ. Health Perspect. 2016, 124, 1830–1839. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.; Zhang, J.; Li, Z.; Gow, A.; Chung, K.F.; Hu, M.; Sun, Z.; Zeng, L.; Zhu, T.; Jia, G. Chronic exposure to air pollution particles increases the risk of obesity and metabolic syndrome: Findings from a natural experiment in Beijing. FASEB J. 2016, 30, 2115–2122. [Google Scholar] [CrossRef]
- Cyrys, J.; Heinrich, J.; Hoek, G.; Meliefste, K.; Lewné, M.; Gehring, U.; Bellander, T.; Fischer, P.; van Vliet, P.; Brauer, M. Comparison between different traffic-related particle indicators: Elemental carbon (EC), PM2.5 mass, and absorbance. J. Expo. Sci. Environ. Epidemiol. 2003, 13, 134–143. [Google Scholar] [CrossRef] [Green Version]
- Gray, H.A.; Cass, G.R. Source contributions to atmospheric fine carbon particle concentrations. Atmos. Environ. 1998, 32, 3805–3825. [Google Scholar] [CrossRef]
- Obot, C.J.; Morandi, M.T.; Beebe, T.P., Jr.; Hamilton, R.F.; Holian, A. Surface components of airborne particulate matter induce macrophage apoptosis through scavenger receptors. Toxicol. Appl. Pharmacol. 2002, 184, 98–106. [Google Scholar] [CrossRef]
- Taheri, S.; Lin, L.; Austin, D.; Young, T.; Mignot, E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLOS Med. 2004, 1, e62. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.; Mukerjee, S.; Kovalcik, K.; Sams, E.; Stallings, C.; Hudgens, E.; Scott, J.; Krantz, T.; Neas, L. Near-road measurements for nitrogen dioxide and its association with traffic exposure zones. Atmos. Pollut. Res. 2015, 6, 1082–1086. [Google Scholar] [CrossRef]
- Sutherland, E.R.; Goleva, E.; Strand, M.; Beuther, D.A.; Leung, D.Y. Body mass and glucocorticoid response in asthma. Am. J. Respir. Crit. Care Med. 2008, 178, 682–687. [Google Scholar] [CrossRef] [Green Version]
- Forno, E.; Lescher, R.; Strunk, R.; Weiss, S.; Fuhlbrigge, A.; Celedón, J.C.; Group, C.A.M.P.R. Decreased response to inhaled steroids in overweight and obese asthmatic children. J. Allergy Clin. Immunol. 2011, 127, 741–749. [Google Scholar] [CrossRef] [Green Version]



| Study (Year, Country) | Study Design | Pollutant (Average Exposure) | Exposure Assessment | Follow-Up (Years) | Subjects (Mean Age: Male, %) | BMI Reference | Adjustment |
|---|---|---|---|---|---|---|---|
| Dong G.H., et al. (2014, China) [25] | Prospective cohort study | PM10: 124.2 μg/m3 NO2: 19.5 ppb SO2: 17.6 ppb O3: 27.4 ppb | Followed by the State Environmental protection Administration of China (1992). The daily average concentration of air pollution was calculated based on data from days for which at least 75% valid 1-h values were available from each monitor. | 3 | 8.4 years, 50.4% | BMI (The Centers for Disease Control and Prevention (CDC) BMI growth charts) | Did not adjust for any specific factors |
| Mao G. et al. (2017, China) [11] | Prospective cohort study | PM2.5: 1st trimester: 3.49 mg2m3 2nd trimester: 3.42 mg2m3 3rd trimester: 3.64 mg2m3 Whole pregnancy: 3.17 mg2m3 First 2 years of life: 3.01 mg2m3 | The PROC GEOCODE procedure of SAS 9.4 (SAS Institute Inc.) was used and matched to the nearest monitor using ArcGIS 10.2 (Esri). | 3 | 6.7 years, 41.36% | BMI the SAS Program for the 2000 CDC Growth Charts provided by the Centers for Disease Control and Prevention (CDC) 2011. | Maternal age at delivery, race/ethnicity, education level, smoking status during pregnancy, diabetes, marital status, household income per year, maternal prepregnancy body mass index (MPBMI), season of delivery, preterm birth, birth weight, and breastfeeding |
| Fioravanti S. et al. (2018, Italy) [26] | Cohort study | PM2.5: 5 μg/m3 PM10: 10 μg/m3 PMcoarse: 5 μg/m3 PMabsorbance: 10 μg/m3 NOx: 20 μg/m3 NO2: 10 μg/m3 | Land-use regression models (LUR) | 4–8 | 50 months, 50.6% | BMI (WHO Growth reference 2007) | Maternal educational level, paternal educational level, maternal pre-pregnancy BMI, maternal smoking during pregnancy, maternal age at delivery, gestational age, child’s birthweight, breastfeeding duration, and age at weaning. |
| Bont J.D. et al. (2019, Spain) [27] | Cross-sectional study | PM2.5: 2.7 μg/m3 PM10: 5.6 μg/m3 PMcoarse: 3.7 μg/m3 NO2: 13.7 μg/m3 | Land-use regression models (LUR) | 0.5 | 8.4 years, 57% | BMI (WHO Growth reference 2007) | Parental education, employment status, and country of birth, maternal smoking during pregnancy, child’s adoption status, exposure to environmental tobacco smoke (ETS) at home, number of siblings, and physical activity |
| Bloemsma L.D. et al. (2019, the Netherlands) [28] | Cohort study | PM2.5: 1.17 μg/m3 PM2.5absorbance: 2.5 × 10−5 μg/m3 PM10: 1.06 μg/m3 NO2: 8.9 μg/m3 | Land-use regression models (LUR) | 17 | The youngest 2.5–3.5 and the oldest 16–19, 51.9% | BMI (International Obesity Task Force cutoffs) | Age, sex, maternal and paternal levels of education, maternal smoking during pregnancy, parental smoking in the child’s home and neighborhood, SES, and region |
| Guo Q. et al. (2020, China) [18] | Cross sectional study | PM2.5: 59.8 μg/m3 | A machine-learning model with satellite remote sensing measurements and historical emission inventories as input | 5 | 6–18 years, 48.29% | BMI (The Chinese national standard Screening for Overweight and Obesity among School-age Children and Adolescents | (1) Sociodemographic factors, including gender, age category, urbanity, region, economic level, day or boarding school, and educational level and occupation of mother; (2) dietary intake, including total water intake, food intake, and beverages intake; (3) time-activity patterns; and (4) indicators of indoor air pollution, including household cooking fuel type, household heating fuel type, school heating fuel type, home ventilation time, and secondhand smoke duration |
| Wilding S. et al. (2020, England) [29] | Cross sectional study | PM2.5: 13.1 μg/m3 PM10: 18.6 μg/m3 NOx: 40.2 μg/m3 | Average level of background air pollution modelled on an annual basis. The annual mean for each metric is provided for 1 km grids across the United Kingdom. A spatially weighted average for each Lower and Middle layer Super Output Areas for PM5, PM10, and NOx was calculated based on levels of background air pollution modelled by the UK Department for Environment Food and Rural Affairs | - | 7–11 years, did not report | BMI (The National Obesity Observatory and population cut-off used in NCMP reports. | Did not adjust for any specific factors |
| Vrijheid M. et al. (2020, the United Kingdom, France, Spain, Lithuania, Norway, and Greece) [30] | Cohort study | Outdoor PM2.5absorbance: 0.41 × 10−5/m−1 Indoor PM2.5absorbance: 0.50 × 10−5/m−1 Indoor NO2: 92.8 μg/m3 | Land-use regression models (LUR) | 6–9 | 8.1 years, 54.7% | BMI (WHO Growth reference 2007) | Sex, maternal BMI, maternal education, maternal age at conception, parity, and prenatal country of origin. |
| Study | Selection | Comparability | Outcome | Score | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 1 | 1 | 2 | 3 | ||
| Dong, et al. (2014) [25] | * | * | * | * | ** | * | * | * | 9 |
| Mao, et al. (2017) [11] | * | * | * | * | ** | * | * | * | 9 |
| Fioravanti, et. al. (2018) [26] | * | * | * | * | ** | * | * | * | 9 |
| Bloemsma, et al. (2019) [28] | * | * | * | * | ** | * | * | * | 9 |
| Vrijheid, et al. (2020) [30] | * | * | * | * | ** | * | * | * | 9 |
| Study | Selection | Comparability | Outcome | Score | ||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 1 | 1 | 2 | ||
| Bont, et al. (2019) [27] | * | * | * | * | ** | * | * | 8 |
| Guo, et al. (2020) [18] | * | * | * | * | ** | * | * | 8 |
| Wilding, et al. (2020) [29] | * | * | * | * | ** | * | * | 8 |
| Characteristic | Model | Area of Exposure | Period of Follow-Up | ||||
|---|---|---|---|---|---|---|---|
| Fixed-Effect Model | Random-Effects Model | Asia | Europe | Less than 4 Years | More than 4 Years | ||
| PM2.5 | |||||||
| Adjusted OR (95% CI) | 1.05 (1.01–1.09) | 1.05 (1.01–1.09) | 1.11 (1.04–1.17) | 1.02 (0.97–1.07) | 1.03 (0.98–1.08) | 1.08 (1.02–1.14) | |
| Heterogeneity | I2 value (%) | 47.2 | 47.2 | 0.0 | 18.2 | 24.5 | 62.5 |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| PM10 | |||||||
| Adjusted OR (95% CI) | 1.06 (1.04–1.10) | 1.07 (1.04–1.10) | N/A | 1.04 (1.01–1.07) | 1.07 (1.04–1.10) | 1.01 (0.89–1.12) | |
| Heterogeneity | I2 value (%) | 71.9 | 71.9 | N/A | 0.0 | 84.6 | 0.0 |
| p-value | <0.001 | <0.001 | N/A | <0.001 | <0.001 | <0.001 | |
| PMcoarse | |||||||
| Adjusted OR (95% CI) | 1.06 (0.94–1.19) | 1.07 (0.95–1.20) | N/A | 1.07 (0.95–1.20) | 1.08 (0.95–1.22) | 0.96 (0.68–1.36) | |
| Heterogeneity | I2 value (%) | 0.0 | 0.0 | N/A | 0.0 | – | – |
| p-value | <0.001 | 0.291 | N/A | 0.291 | 0.228 | 0.817 | |
| PM2.5abs | |||||||
| Adjusted OR (95% CI) | 1.22 (1.04–1.41) | 1.23 (1.06–1.43) | N/A | 1.23 (1.06–1.43) | 1.31 (0.97–1.77) | 1.21 (1.01–1.44) | |
| Heterogeneity | I2 value (%) | 0.0 | 0.0 | N/A | 0.0 | – | 0.0 |
| p-value | <0.001 | 0.007 | N/A | 0.007 | 0.076 | 0.036 | |
| NO2 | |||||||
| Adjusted OR (95% CI) | 1.09 (1.04–1.15) | 1.10 (1.04–1.16) | 1.13 (1.04–1.23) | 1.08 (1.01–1.16) | 1.09 (1.03–1.15) | 1.24 (1.04–1.47) | |
| Heterogeneity | I2 value (%) | 47.6 | 55.2 | – | 66.5 | 41.1 | 66.8 |
| p-value | <0.001 | <0.001 | 0.003 | 0.032 | 0.014 | 0.003 | |
| NOx | |||||||
| Adjusted OR (95% CI) | 1.00 (0.99–1.02) | 1.00 (0.99–1.02) | N/A | 1.00 (0.99–1.01) | 1.00 (0.99–1.01) | 1.02 (0.84–1.24) | |
| Heterogeneity | I2 value (%) | 0.0 | 0.0 | N/A | 0.0 | 0.0 | 0.0 |
| p-value | <0.001 | 0.571 | N/A | 0.423 | 0.428 | 0.839 | |
| Pollutant | Begg’s Test | Egger’s Test | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Z | p-Value | Std_Eff | Coef. | Std. Err. | t | p > ltl | [95% Conf. Interval] | p-Value | |
| PM2.5 | 0.00 | 1.00 | slope | 0.062 | 0.048 | 1.3 | 0.262 | −0.07 to 0.19 | 0.839 |
| bias | −0.023 | 1.05 | −0.22 | 0.84 | −3.14 to 2.69 | ||||
| PM10 | −0.024 | 1.00 | slope | 0.68 | 0.49 | 1.38 | 0.26 | −0.88 to 0.22 | 0.929 |
| bias | −0.15 | 1.54 | −0.10 | 0.93 | −5.07 to 4.78 | ||||
| PMcoarse | 0.00 | 1.00 | slope | 0.24 | – | – | – | – | – |
| bias | −2.53 | – | – | – | – | ||||
| PM2.5absorbance | 0.00 | 1.00 | slope | 0.15 | 0.10 | 1.44 | 0.39 | −1.17 to 1.47 | 0.684 |
| bias | 0.41 | 0.76 | 0.54 | 0.68 | −9.28 to 10.11 | ||||
| NO2 | 0.34 | 0.73 | slope | 0.04 | 0.09 | 0.47 | 0.69 | −0.36 to 0.45 | 0.651 |
| bias | 0.97 | 1.84 | 0.53 | 0.651 | −6.96 to 8.91 | ||||
| NOx | 0.00 | 1.00 | slope | 0.01 | – | – | – | – | – |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parasin, N.; Amnuaylojaroen, T.; Saokaew, S. Effect of Air Pollution on Obesity in Children: A Systematic Review and Meta-Analysis. Children 2021, 8, 327. https://doi.org/10.3390/children8050327
Parasin N, Amnuaylojaroen T, Saokaew S. Effect of Air Pollution on Obesity in Children: A Systematic Review and Meta-Analysis. Children. 2021; 8(5):327. https://doi.org/10.3390/children8050327
Chicago/Turabian StyleParasin, Nichapa, Teerachai Amnuaylojaroen, and Surasak Saokaew. 2021. "Effect of Air Pollution on Obesity in Children: A Systematic Review and Meta-Analysis" Children 8, no. 5: 327. https://doi.org/10.3390/children8050327