Plain Radiographic Analysis of Laryngeal Dimensions in Young Children: Normal versus Croup
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design, Setting, and Population
2.2. Definitions of Laryngeal Levels
2.3. Measurement of Laryngeal Dimensions
2.4. Comparison with Age-Matched Croup Population
2.5. Statistical Analysis
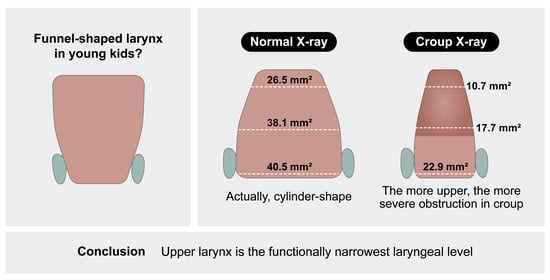
3. Results
3.1. Baseline Characteristics
3.2. Measurement of Laryngeal Dimensions
3.3. Comparison with Age-Matched Croup Population
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eckenhoff, J.E. Some anatomic considerations of the infant larynx influencing endotracheal anesthesia. Anesthesiology 1951, 12, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Litman, R.S.; Weissend, E.E.; Shibata, D.; Westesson, P.L. Developmental changes of laryngeal dimensions in unparalyzed, sedated children. Anesthesiology 2003, 98, 41–45. [Google Scholar] [CrossRef]
- Dalal, P.G.; Murray, D.; Messner, A.H.; Feng, A.; McAllister, J.; Molter, D. Pediatric laryngeal dimensions: An age-based analysis. Anesth. Analg. 2009, 108, 1475–1479. [Google Scholar] [CrossRef] [PubMed]
- Wani, T.M.; Bissonnette, B.; Rafiq Malik, M.; Hayes, D., Jr.; Ramesh, A.S.; Al Sohaibani, M.; Tobias, J.D. Age-based analysis of pediatric upper airway dimensions using computed tomography imaging. Pediatr. Pulmonol. 2016, 51, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Mizuguchi, S.; Motomura, Y.; Maki, J.; Baba, R.; Ichimiya, Y.; Tokuda, K.; Kaku, N.; Takada, H.; Maehara, Y.; Ohga, S. Tracheal Size and Morphology on the Reconstructed CT Imaging. Pediatr. Crit. Care Med. 2019, 20, e366–e371. [Google Scholar] [CrossRef] [PubMed]
- Holzki, J.; Brown, K.A.; Carroll, R.G.; Coté, C.J. The anatomy of the pediatric airway: Has our knowledge changed in 120 years? A review of historic and recent investigations of the anatomy of the pediatric larynx. Paediatr. Anaesth. 2018, 28, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Holzki, J.F.; Laschat, M.; Puder, C. The pediatric larynx: A complicated organ. Anesth. Analg. 2010, 110, 1509–1510. [Google Scholar] [CrossRef] [PubMed]
- Berkovits, R. Comment on the pediatric larynx. Anesth. Analg. 2010, 110, 1510. [Google Scholar] [CrossRef]
- Khine, H.H.; Corddry, D.H.; Kettrick, R.G.; Martin, T.M.; McCloskey, J.J.; Rose, J.B.; Theroux, M.C.; Zagnoev, M. Comparison of cuffed and uncuffed endotracheal tubes in young children during general anesthesia. Anesthesiology 1997, 86, 627–631. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.; Dullenkopf, A.; Fischer, J.E.; Keller, C.; Gerber, A.C. Prospective randomized controlled multi-centre trial of cuffed or uncuffed endotracheal tubes in small children. Br. J. Anaesth. 2009, 103, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, B. Prolonged intubation injuries of the larynx: Endoscopic diagnosis, classification, and treatment. Ann. Otol. Rhinol. Laryngol. Suppl. 1993, 160, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Holzki, J.; Laschat, M.; Puder, C. Iatrogenic damage to the pediatric airway. Mechanisms and scar development. Paediatr Anaesth 2009, 19 (Suppl. 1), 131–146. [Google Scholar] [CrossRef] [PubMed]
- Darras, K.E.; Roston, A.T.; Yewchuk, L.K. Imaging Acute Airway Obstruction in Infants and Children. Radiographics 2015, 35, 2064–2079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, W.C.; Hsu, Y.L.; Chen, C.Y.; Peng, Y.C.; Chen, J.N.; Fu, Y.C.; Chang, Y.J.; Lee, E.P.; Lin, M.J.; Wu, H.P. Initial radiographic tracheal ratio in predicting clinical outcomes in croup in children. Sci. Rep. 2019, 9, 17893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sirisopana, M.; Saint-Martin, C.; Wang, N.N.; Manoukian, J.; Nguyen, L.H.; Brown, K.A. Novel measurements of the length of the subglottic airway in infants and young children. Anesth. Analg. 2013, 117, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Salour, M. The steeple sign. Radiology 2000, 216, 428–429. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.K.; Ahuja, G.S.; Wiedmann, M.; Osann, K.E.; Su, E.; Heidari, A.E.; Jing, J.C.; Qu, Y.; Lazarow, F.; Wang, A.; et al. Long-Range Optical Coherence Tomography of the Neonatal Upper Airway for Early Diagnosis of Intubation-related Subglottic Injury. Am. J. Respir. Crit. Care Med. 2015, 192, 1504–1513. [Google Scholar] [CrossRef] [Green Version]
- Wani, T.M.; Rafiq, M.; Akhter, N.; AlGhamdi, F.S.; Tobias, J.D. Upper airway in infants-a computed tomography-based analysis. Paediatr. Anaesth. 2017, 27, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Zhu, S.Y.; Luo, F.; Gao, Y.; Yang, X.Y. High-frequency sonographic measurements of true and false vocal cords. J. Ultrasound. Med. 2010, 29, 1023–1030. [Google Scholar] [CrossRef]



| Dimension | Glottis | Subglottis | Cricoid | p * |
|---|---|---|---|---|
| AP diameter, mm | 9.8 ± 1.5 | 8.5 ± 1.4 | 7.4 ± 1.5 | <0.001 |
| Transverse diameter, mm | 3.4 ± 1.8 | 5.6 ± 1.0 | 6.8 ± 1.0 | <0.001 |
| AP-to-transverse ratio † | 2.9:1 | 1.5:1 | 1.1:1 | NA |
| CSA, mm2 | 26.5 ± 15.1 | 38.1 ± 11.2 | 40.5 ± 12.5 | <0.001 |
| Dimension | Study Population (n = 401) | Croup Population (n = 401) | p * |
|---|---|---|---|
| Glottis | |||
| AP diameter, mm | 9.8 ± 1.5 | 7.1 ± 1.6 | <0.001 |
| Transverse diameter, mm | 3.4 ± 1.8 | 1.8 ± 1.2 | <0.001 |
| CSA, mm2 * | 26.5 ± 15.1 | 10.7 ± 8.5 | <0.001 |
| Subglottis | |||
| AP diameter, mm | 8.5 ± 1.4 | 6.3 ± 1.6 | <0.001 |
| Transverse diameter, mm | 5.6 ± 1.0 | 3.4 ± 1.1 | <0.001 |
| CSA, mm2 † | 38.1 ± 11.2 | 17.7 ± 8.9 | <0.001 |
| Cricoid | |||
| AP diameter, mm | 7.4 ± 1.5 | 5.5 ± 1.5 | <0.001 |
| Transverse diameter, mm | 6.8 ± 1.0 | 5.0 ± 1.3 | <0.001 |
| CSA, mm2 * | 40.5 ± 12.5 | 22.9 ± 11.0 | <0.001 |
| Narrowing, % | Glottis | Subglottis | Cricoid | p |
|---|---|---|---|---|
| AP diameter | 28.2 (14.3–38.2) | 25.1 (11.9–37.8) | 25.6 (12.9–38.0) | NS * |
| Transverse diameter | 50.0 (21.4–68.6) | 41.4 (26.9–52.6) | 27.4 (14.7–39.8) | <0.001 † |
| CSA | 63.2 (42.7–79.3) | 56.6 (38.5–70.4) | 46.1 (29.5–60.0) | <0.001 † |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Park, J.-E.; Kim, J.-H. Plain Radiographic Analysis of Laryngeal Dimensions in Young Children: Normal versus Croup. Children 2022, 9, 1532. https://doi.org/10.3390/children9101532
Kim Y, Park J-E, Kim J-H. Plain Radiographic Analysis of Laryngeal Dimensions in Young Children: Normal versus Croup. Children. 2022; 9(10):1532. https://doi.org/10.3390/children9101532
Chicago/Turabian StyleKim, Youngdae, Ji-Eun Park, and Jung-Heon Kim. 2022. "Plain Radiographic Analysis of Laryngeal Dimensions in Young Children: Normal versus Croup" Children 9, no. 10: 1532. https://doi.org/10.3390/children9101532