Safety and Efficacy of Regadenoson for Pediatric Stress Perfusion Cardiac MRI with Quantification of Myocardial Blood Flow
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. CMR Protocol
2.3. Stress Response Data
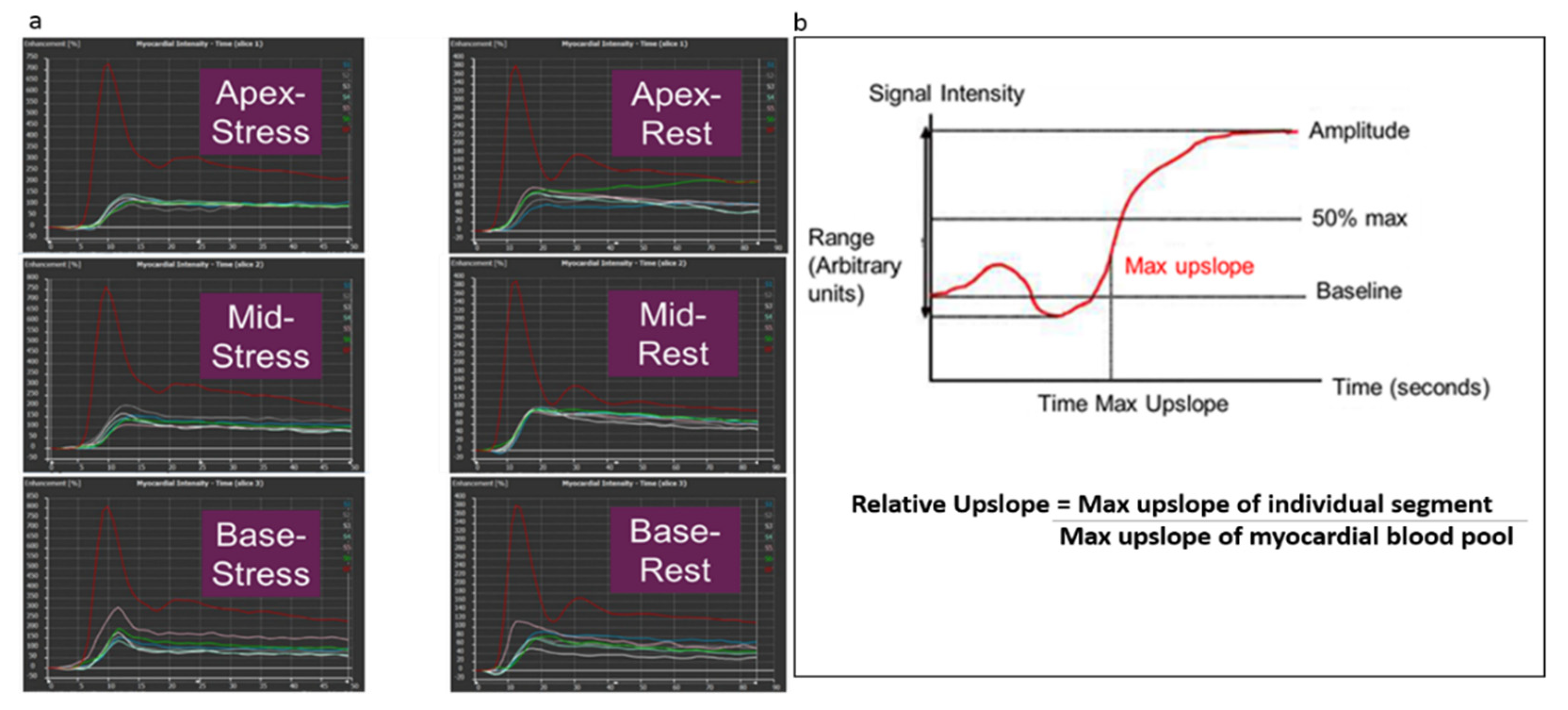
2.4. Image Analysis
2.4.1. Qualitative Myocardial Perfusion Analysis
2.4.2. Semi-Quantitative Myocardial Perfusion Analysis
2.4.3. Quantitative Measures of Cardiac Volume, Function, and Fibrosis
2.4.4. Statistical Analysis
3. Results
3.1. Study Population
3.2. CMR Protocol Data
3.2.1. Hemodynamic Response to Regadenoson
3.2.2. Adverse Events of Regadenoson
3.3. Image Analysis
3.3.1. Qualitative Myocardial Perfusion Analysis
3.3.2. Semi-Quantitative Myocardial Perfusion Analysis
3.3.3. Quantitative Measures of Cardiac Volume, Function, and Fibrosis
3.4. Clinical Course
4. Discussion
4.1. Safety and Efficacy of Regadenoson
4.2. Semiquantitative Assessment of Myocardial Perfusion Reserve
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nandalur, K.R.; Dwamena, B.A.; Choudhri, A.F.; Nandalur, M.R.; Carlos, R.C. Diagnostic performance of stress cardiac magnetic resonance imaging in the detection of coronary artery disease: A meta-analysis. J. Am. Coll. Cardiol. 2007, 50, 1343–1353. [Google Scholar] [CrossRef] [PubMed]
- Klem, I.; Heitner, J.F.; Shah, D.J.; Sketch, M.H., Jr.; Behar, V.; Weinsaft, J.; Cawley, P.; Parker, M.; Elliott, M.; Judd, R.M.; et al. Improved detection of coronary artery disease by stress perfusion cardiovascular magnetic resonance with the use of delayed enhancement infarction imaging. J. Am. Coll. Cardiol. 2006, 47, 1630–1638. [Google Scholar] [CrossRef] [PubMed]
- Schwitter, J.; Wacker, C.M.; van Rossum, A.C.; Lombardi, M.; Al-Saadi, N.; Ahlstrom, H.; Dill, T.; Larsson, H.B.; Flamm, S.D.; Marquardt, M.; et al. MR-IMPACT: Comparison of perfusion-cardiac magnetic resonance with single-photon emission computed tomography for the detection of coronary artery disease in a multicentre, multivendor, randomized trial. Eur. Heart J. 2008, 29, 480–489. [Google Scholar] [CrossRef]
- Greenwood, J.P.; Maredia, N.; Younger, J.F.; Brown, J.M.; Nixon, J.; Everett, C.C.; Bijsterveld, P.; Ridgway, J.P.; Radjenovic, A.; Dickinson, C.J.; et al. Cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary heart disease (CE-MARC): A prospective trial. Lancet 2012, 379, 453–460. [Google Scholar] [CrossRef]
- Schwitter, J.; Nanz, D.; Kneifel, S.; Bertschinger, K.; BÜChi, M.; KnÜSel, P.R.; Marincek, B.; LÜScher, T.F.; Von Schulthess, G.K. Assessment of myocardial perfusion in coronary artery disease by magnetic resonance: A comparison with positron emission tomography and coronary angiography. Circulation 2001, 103, 2230–2235. [Google Scholar] [CrossRef]
- Biko, D.M.; Collins, R.T., 2nd; Partington, S.L.; Harris, M.; Whitehead, K.K.; Keller, M.S.; Fogel, M.A. Magnetic Resonance Myocardial Perfusion Imaging: Safety and Indications in Pediatrics and Young Adults. Pediatr. Cardiol. 2018, 39, 275–282. [Google Scholar] [CrossRef]
- Hauser, M.; Bengel, F.M.; Kuhn, A.; Sauer, U.; Zylla, S.; Braun, S.L.; Nekolla, S.G.; Oberhoffer, R.; Lange, R.; Schwaiger, M.; et al. Myocardial blood flow and flow reserve after coronary reimplantation in patients after arterial switch and ross operation. Circulation 2001, 103, 1875–1880. [Google Scholar] [CrossRef]
- Secinaro, A.; Ntsinjana, H.; Tann, O.; Schuler, P.K.; Muthurangu, V.; Hughes, M.; Tsang, V.; Taylor, A.M. Cardiovascular magnetic resonance findings in repaired anomalous left coronary artery to pulmonary artery connection (ALCAPA). J. Cardiovasc. Magn. Reson. 2011, 13, 27. [Google Scholar] [CrossRef]
- Mavrogeni, S.; Papadopoulos, G.; Douskou, M.; Kaklis, S.; Seimenis, I.; Baras, P.; Nikolaidou, P.; Bakoula, C.; Karanasios, E.; Manginas, A.; et al. Magnetic resonance angiography is equivalent to X-ray coronary angiography for the evaluation of coronary arteries in Kawasaki disease. J. Am. Coll. Cardiol. 2004, 43, 649–652. [Google Scholar] [CrossRef]
- Duran, S.R.; Huffaker, T.; Dixon, B.; Gooty, V.; Abou Zahr, R.; Arar, Y.; Greer, J.S.; Butts, R.J.; Hussain, M.T. Feasibility and safety of quantitative adenosine stress perfusion cardiac magnetic resonance imaging in pediatric heart transplant patients with and without coronary allograft vasculopathy. Pediatr. Radiol. 2021, 51, 1311–1321. [Google Scholar] [CrossRef]
- Kazmirczak, F.; Nijjar, P.S.; Zhang, L.; Hughes, A.; Chen, K.-H.A.; Okasha, O.; Martin, C.M.; Akçakaya, M.; Farzaneh-Far, A.; Shenoy, C. Safety and prognostic value of regadenoson stress cardiovascular magnetic resonance imaging in heart transplant recipients. J. Cardiovasc. Magn. Reson. 2019, 21, 9. [Google Scholar] [CrossRef] [PubMed]
- Freed, B.H.; Narang, A.; Bhave, N.M.; Czobor, P.; Mor-Avi, V.; Zaran, E.R.; Turner, K.M.; Cavanaugh, K.P.; Chandra, S.; Tanaka, S.M.; et al. Prognostic value of normal regadenoson stress perfusion cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2013, 15, 108. [Google Scholar] [CrossRef]
- Noel, C.V.; Krishnamurthy, R.; Moffett, B.; Krishnamurthy, R. Myocardial stress perfusion magnetic resonance: Initial experience in a pediatric and young adult population using regadenoson. Pediatr. Radiol. 2017, 47, 280–289. [Google Scholar] [CrossRef]
- Gordi, T.; Frohna, P.; Sun, H.L.; Wolff, A.; Belardinelli, L.; Lieu, H. A population pharmacokinetic/pharmacodynamic analysis of regadenoson, an adenosine A2A-receptor agonist, in healthy male volunteers. Clin. Pharmacokinet. 2006, 45, 1201–1212. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, J.C.; Doan, T.T.; Loar, R.W.; Pednekar, A.S.; Trivedi, P.M.; Masand, P.M.; Noel, C.V. Myocardial Stress Perfusion MRI Using Regadenoson: A Weight-based Approach in Infants and Young Children. Radiol. Cardiothorac. Imaging 2019, 1, e190061. [Google Scholar] [CrossRef] [PubMed]
- Christopher, A.B.; Quinn, R.E.; Zoulfagharian, S.; Matisoff, A.J.; Cross, R.R.; Xue, H.; Campbell-Washburn, A.; Olivieri, L.J. Motion-corrected cardiac MRI is associated with decreased anesthesia exposure in children. Pediatr. Radiol. 2020, 50, 1709–1716. [Google Scholar] [CrossRef]
- Kellman, P.; Chefd’hotel, C.; Lorenz, C.H.; Mancini, C.; Arai, A.E.; McVeigh, E.R. High spatial and temporal resolution cardiac cine MRI from retrospective reconstruction of data acquired in real time using motion correction and resorting. Magn. Reson. Med. 2009, 62, 1557–1564. [Google Scholar] [CrossRef]
- Lockie, T.; Ishida, M.; Perera, D.; Chiribiri, A.; De Silva, K.; Kozerke, S.; Marber, M.; Nagel, E.; Rezavi, R.; Redwood, S.; et al. High-resolution magnetic resonance myocardial perfusion imaging at 3.0-Tesla to detect hemodynamically significant coronary stenoses as determined by fractional flow reserve. J. Am. Coll. Cardiol. 2011, 57, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Wohrle, J.; Nusser, T.; Merkle, N.; Kestler, H.A.; Grebe, O.C.; Marx, N.; Hoher, M.; Kochs, M.; Hombach, V. Myocardial perfusion reserve in cardiovascular magnetic resonance: Correlation to coronary microvascular dysfunction. J. Cardiovasc. Magn. Reson. 2006, 8, 781–787. [Google Scholar] [CrossRef]
- Husain, N.; Watanabe, K.; Berhane, H.; Gupta, A.; Markl, M.; Rigsby, C.K.; Robinson, J.D. Multi-parametric cardiovascular magnetic resonance with regadenoson stress perfusion is safe following pediatric heart transplantation and identifies history of rejection and cardiac allograft vasculopathy. J. Cardiovasc. Magn. Reson. 2021, 23, 135. [Google Scholar] [CrossRef]
- Schulz-Menger, J.; Bluemke, D.A.; Bremerich, J.; Flamm, S.D.; Fogel, M.A.; Friedrich, M.G.; Kim, R.J.; von Knobelsdorff-Brenkenhoff, F.; Kramer, C.M.; Pennell, D.J.; et al. Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) board of trustees task force on standardized post processing. J. Cardiovasc. Magn. Reson. 2013, 15, 35. [Google Scholar] [CrossRef] [PubMed]
- Schulz-Menger, J.; Bluemke, D.A.; Bremerich, J.; Flamm, S.D.; Fogel, M.A.; Friedrich, M.G.; Kim, R.J.; von Knobelsdorff-Brenkenhoff, F.; Kramer, C.M.; Pennell, D.J.; et al. Standardized image interpretation and post-processing in cardiovascular magnetic resonance—2020 update: Society for Cardiovascular Magnetic Resonance (SCMR): Board of Trustees Task Force on Standardized Post-Processing. J. Cardiovasc. Magn. Reson. 2020, 22, 19. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.C.; Messroghli, D.R.; Kellman, P.; Piechnik, S.K.; Robson, M.D.; Ugander, M.; Gatehouse, P.D.; Arai, A.E.; Friedrich, M.G.; Neubauer, S.; et al. Myocardial T1 mapping and extracellular volume quantification: A Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of Cardiology consensus statement. J. Cardiovasc. Magn. Reson. 2013, 15, 92. [Google Scholar] [CrossRef] [PubMed]
- Bhave, N.M.; Freed, B.H.; Yodwut, C.; Kolanczyk, D.; Dill, K.; Lang, R.M.; Mor-Avi, V.; Patel, A.R. Considerations when measuring myocardial perfusion reserve by cardiovascular magnetic resonance using regadenoson. J. Cardiovasc. Magn. Reson. 2012, 14, 89. [Google Scholar] [CrossRef] [PubMed]
- Andrikopoulou, E.; Hage, F.G. Correction to: Adverse effects associated with regadenoson myocardial perfusion imaging. J. Nucl. Cardiol. 2018, 25, 1732. [Google Scholar] [CrossRef]
- Vasu, S.; Bandettini, W.P.; Hsu, L.Y.; Kellman, P.; Leung, S.; Mancini, C.; Shanbhag, S.M.; Wilson, J.; Booker, O.J.; Arai, A.E. Regadenoson and adenosine are equivalent vasodilators and are superior than dipyridamole- a study of first pass quantitative perfusion cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2013, 15, 85. [Google Scholar] [CrossRef]
- Johnson, N.P.; Gould, K.L. Regadenoson Versus Dipyridamole Hyperemia for Cardiac PET Imaging. JACC Cardiovasc. Imaging 2015, 8, 438–447. [Google Scholar] [CrossRef]
- Miller, C.A.; Hsu, L.Y.; Ta, A.; Conn, H.; Winkler, S.; Arai, A.E. Quantitative pixel-wise measurement of myocardial blood flow: The impact of surface coil-related field inhomogeneity and a comparison of methods for its correction. J. Cardiovasc. Magn. Reson. 2015, 17, 11. [Google Scholar] [CrossRef]
- Nagel, E.; Klein, C.; Paetsch, I.; Hettwer, S.; Schnackenburg, B.; Wegscheider, K.; Fleck, E. Magnetic resonance perfusion measurements for the noninvasive detection of coronary artery disease. Circulation 2003, 108, 432–437. [Google Scholar] [CrossRef]
- Narang, A.; Blair, J.E.; Patel, M.B.; Mor-Avi, V.; Fedson, S.E.; Uriel, N.; Lang, R.M.; Patel, A.R. Myocardial perfusion reserve and global longitudinal strain as potential markers of coronary allograft vasculopathy in late-stage orthotopic heart transplantation. Int. J. Cardiovasc. Imaging 2018, 34, 1607–1617. [Google Scholar] [CrossRef]
- Ntsinjana, H.N.; Tann, O.; Hughes, M.; Derrick, G.; Secinaro, A.; Schievano, S.; Muthurangu, V.; Taylor, A.M. Utility of adenosine stress perfusion CMR to assess paediatric coronary artery disease. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Ismail, T.F.; Hsu, L.-Y.; Greve, A.M.; Gonçalves, C.; Jabbour, A.; Gulati, A.; Hewins, B.; Mistry, N.; Wage, R.; Roughton, M.; et al. Coronary microvascular ischemia in hypertrophic cardiomyopathy—A pixel-wise quantitative cardiovascular magnetic resonance perfusion study. J. Cardiovasc. Magn. Reson. 2014, 16, 49. [Google Scholar] [CrossRef] [PubMed]




| Clinical Diagnosis | Number |
|---|---|
| Status post orthotopic heart transplant | 16 |
| Coronary aneurysms | 10 |
| - Isolated coronary aneurysm with mitral regurgitation | - 1 |
| Status post coronary artery revision | 8 |
| - D-TGA status post arterial switch | - 3 |
| - Anomalous RCA from left sinus s/p unroofing | - 3 |
| - Anomalous LCA from right sinus s/p unroofing and creation of a neo-ostium | - 1 |
| - Congenital aortic stenosis s/p Ross | - 1 |
| Abnormal coronary artery anatomy, no intervention | 3 |
| - Single coronary artery with intramural LAD | - 1 |
| - Single coronary artery with LAD between aorta and PA | - 1 |
| - Anomalous LCA from non-coronary sinus | - 1 |
| Anginal chest pain with abnormal ECG | 1 |
| Patient | Diagnosis | Stress Perfusion Defect | Rest Perfusion Defect | Wall Motion Abnormality | Late Gadolinium Enhancement |
|---|---|---|---|---|---|
| 1 | Kawasaki | Yes | No | No | No |
| 2 | DTGA s/p ASO | Yes | No | Yes | No |
| 3 | AAOLCA from right sinus s/p surgery | Yes | No | No | No |
| 4 | Kawasaki | Yes | No | No | No |
| Patients with Perfusion Defect (n = 4) | Patients without Perfusion Defect s/p Orthotopic Heart Transplant (n = 16) | Patients without Perfusion Defect Non-Transplant (n = 18) | ||
|---|---|---|---|---|
| Global MPRI | 1.13 ± 0.27 | 0.75 ± 0.22 | 0.92 ± 0.23 | p = 0.03 * |
| Segmental MPRI | 0.78 ± 0.22 | 0.99 ± 0.36 | p = 0.07 |
| Patients with Stress Perfusion Deficits | Tests Performed Post Perfusion | ||||||
|---|---|---|---|---|---|---|---|
| Diagnosis | Indication | CTA | Cath | NM Stress | EST | Outcome | |
| 1 | AAOLCA from right sinus s/p surgery | exertional chest pain | No | * LAD bridge | Normal | Normal | Surgical myocardial bridge unroofing |
| 2 | Kawasaki | Coronary aneurysm with LAD occlusion | No | No | Normal | Normal | No intervention |
| 3 | D-TGA s/p ASO | Acutely angulated and compressed reimplanted LMCA | No | No | Normal | Started on aspirin | |
| 4 | Kawasaki | Coronary aneurysm with LAD occlusion | No | No | Normal | Normal | No intervention |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, S.G.; Husain, N.; Rigsby, C.K.; Robinson, J.D. Safety and Efficacy of Regadenoson for Pediatric Stress Perfusion Cardiac MRI with Quantification of Myocardial Blood Flow. Children 2022, 9, 1332. https://doi.org/10.3390/children9091332
Patel SG, Husain N, Rigsby CK, Robinson JD. Safety and Efficacy of Regadenoson for Pediatric Stress Perfusion Cardiac MRI with Quantification of Myocardial Blood Flow. Children. 2022; 9(9):1332. https://doi.org/10.3390/children9091332
Chicago/Turabian StylePatel, Shivani G., Nazia Husain, Cynthia K. Rigsby, and Joshua D. Robinson. 2022. "Safety and Efficacy of Regadenoson for Pediatric Stress Perfusion Cardiac MRI with Quantification of Myocardial Blood Flow" Children 9, no. 9: 1332. https://doi.org/10.3390/children9091332






