Degradation of Selected Antidepressants Sertraline and Citalopram in Ultrapure Water and Surface Water Using Gamma Radiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Irradiation Procedure
2.3. Analytical Methods
2.4. Cytotoxicity Monitoring
3. Results
3.1. Radiolytic Degradation
3.2. Determination of Chemical Radiation Yield
3.3. Effect of CO32−, NO3− and HA
3.4. SER and CIT Degradation in Surface Water
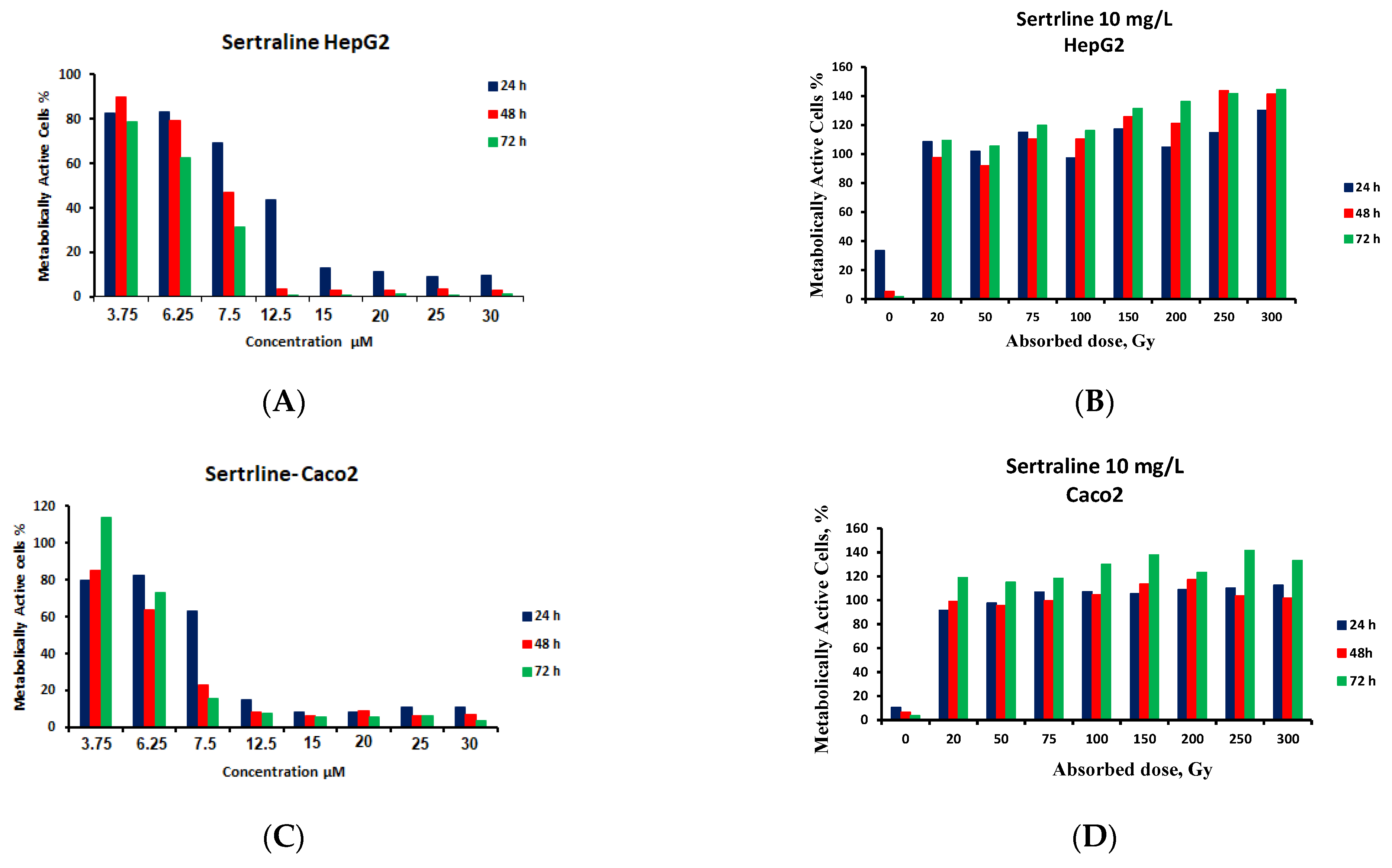
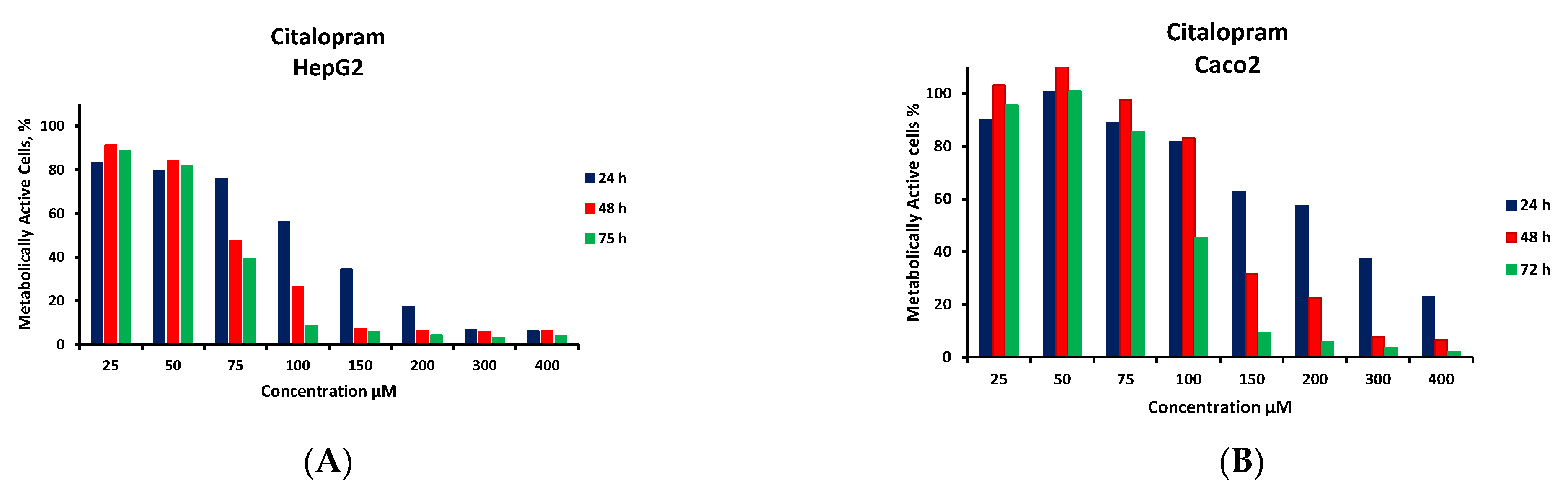
3.5. Cytotoxicity Monitoring
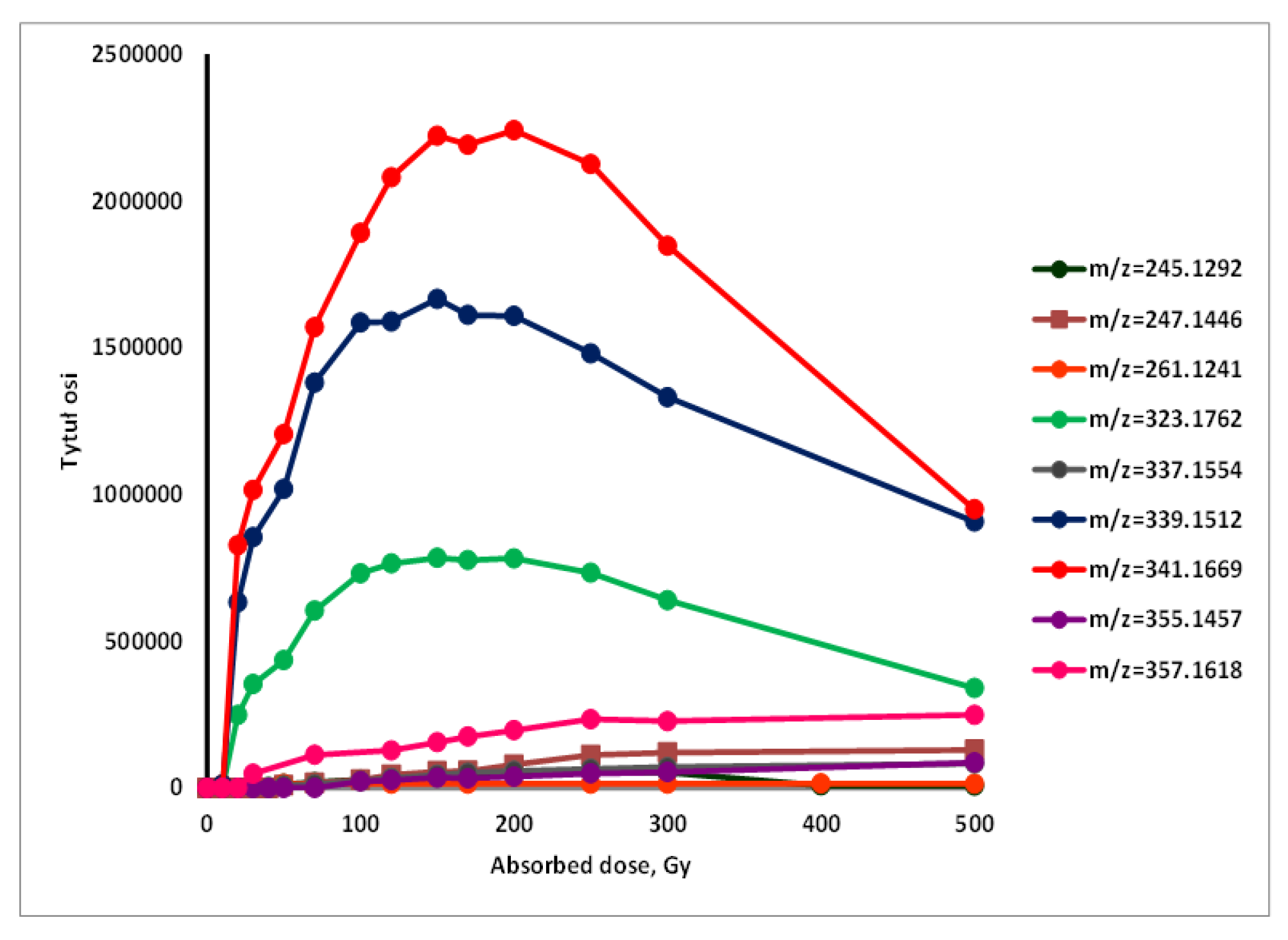
3.6. Preliminary Identification of Degradation Product
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Virkutyte, J.; Varma, R.S.; Jegatheesan, V. Treatment of Micropollutants in Water and Wastewater; IWA Publishing: London, UK, 2010. [Google Scholar]
- Brauer, R.; Alfageh, B.; Blais, J.E.; Chan, E.W.; Chui, C.S.L.; Hayes, J.F.; Man, K.K.C.; Lau, W.C.Y.; Yan, V.K.C.; Beykloo, M.Y.; et al. Psychotropic medicine consumption in 65 countries and regions, 2008–2019: A longitudinal study. Lancet Psychiatry 2021, 8, 1071–1082. [Google Scholar] [CrossRef]
- Rabeea, S.A.; Merchant, H.A.; Khan, M.U.; Kow, C.S.; Hasan, S.S. Surging trends in prescriptions and costs of antidepressents in England amid COVID-19. DARU J. Pharm. Sci. 2021, 29, 217–221. [Google Scholar] [CrossRef]
- Cipriani, A.; Furukawa, T.A.; Salanti, G.; Chaimani, A.; Atkinson, L.Z.; Ogawa, Y.; Leucht, S.; Ruhe, H.G.; Turner, E.H.; Higgins, J.P.T.; et al. Comparative efficacy and acceptability of 21 antidepressant drugs for acute treatment of adults with major depressive disorder. A systematic review and network meta-analysis. Lancet 2018, 391, 1357–1366. [Google Scholar] [CrossRef] [Green Version]
- Sheffler, Z.M.; Abdijadid, S. Antidepressants. In StatPearls; National Center for Biotechnology Information, U.S. National Library of Medicine 8600 Rockville Pike, Treasure Island (FL); StatPearls Publishing: Bethesda, MD, USA, 2021. [Google Scholar]
- Lalji, H.M.; McGrogan, A.; Bailey, S.J. An analysis of antidepressant prescribing trends in England 2015–2019. J. Affect. Disord. Rep. 2021, 6, 100205. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.; Wang, Y.; Ye, X.; Zhu, D.; Shi, X.; He, P. Antidepressant use and expenditure in the treatment of patients with depression: Evidence from China urban medical claim data. J. Affect. Disord. 2022, 296, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Giebułtowicz, J.; Nałęcz-Jawecki, G. Occurrence of antidepressant residues in the sewage-impacted Vistula and Utrata rivers and in tap water in Warsaw (Poland). Ecotoxicol. Environ. Saf. 2014, 104, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Golovko, O.; Kumar, V.; Fedorova, G.; Randak, T.; Grabic, R. Seasonal changes in antibiotics, antidepressants/psychiatric drugs, antihistamines and lipid regulators in a wastewater treatment plant. Chemosphere 2014, 111, 418–426. [Google Scholar] [CrossRef]
- Evans, S.E.; Davies, P.; Lubben, A.; Kasprzyk-Hordern, B. Determination of chiral pharmaceuticals and illicit drugs in wastewater and sludge using microwave assisted extraction, solid-phase extraction and chiral liquid chromatography coupled with tandem mass spectrometry. Anal. Chim. Acta 2015, 882, 112–126. [Google Scholar] [CrossRef] [Green Version]
- Silva, L.J.G.; Pereira, A.M.P.T.; Meisel, L.M.; Lino, C.M.; Pena, A. A one-year follow-up analysis of antidepressants in Portuguese wastewaters: Occurrence and fate, seasonal influence and risk assessment. Sci. Total Environ. 2014, 490, 279–287. [Google Scholar] [CrossRef]
- Writer, J.H.; Ferrer, I.; Barber, L.B.; Thurman, E.M. Widespread occurrence of neuroactive pharmaceuticals and metabolites in 24 Minnesota rivers and wastewaters. Sci. Total Environ. 2013, 461, 519–527. [Google Scholar] [CrossRef]
- Fick, J.; Soderstrom, H.; Lindberg, R.H.; Phan, C.; Tysklind, M.; Larsson, D.G.J. Contamination of surface ground and drinking water from pharmaceutical production. Environ. Toxicol. Chem. 2009, 28, 2522–2527. [Google Scholar] [CrossRef]
- Arnnok, P.; Singh, R.R.; Burakham, R.; Perez-Fuenteja, A.; Aga, D.S. Selective uptake and bioacumulation of antidepressants in fish from effluent-impacted Niagara River. Environ. Sci. Technol. 2017, 51, 10652–10662. [Google Scholar] [CrossRef]
- Brooks, B.W.; Chambliss, C.K.; Stanley, J.K.; Ramirez, A.; Banks, K.E.; Johnson, R.D.; Lewis, R.J. Determination of select antidepressants in fish from an effluent-dominated stream. Environ. Toxicol. Chem. 2005, 24, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Kumer, A.; Xagogaraki, I. Pharmaceuticals, personal care products and endocrine disrupting chemicals IN US surface and finished drinking waters: A proposed ranking system. Sci. Total Environ. 2010, 408, 5972–5989. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, C.D.; Chu, S.; Judt, C.; Li, H.; Oakes, K.D.; Servos, M.R.; Andrews, D.M. Antidepressants and their metabolites in municipal wastewater, and downstream exposure in an urban watershed. Environ. Toxicol. Chem. 2010, 29, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Tarcomnicu, I.; van Nuijs, A.L.N.; Simons, W.; Bervoets, L.; Blust, R.; Jorens, P.G.; Neels, H.; Covaci, A. Simultaneous determination of 15 top-prescribed pharmaceuticals and their metabolites in influent wastewater by reversed-phase liquid chromatography coupled to tandem mass spectrometry. Talanta 2011, 83, 795–803. [Google Scholar] [CrossRef]
- Degreef, M.; van Nuijs, A.L.N.; Maudens, K.E. Validation of simple, fast liquid chromatography-tandem mass spectrometry method for the simultaneous quantification of 40 antidepressant drugs or their metabolites in plasma. Clin. Chim. Acta 2018, 485, 243–257. [Google Scholar] [CrossRef]
- Ma, W.; Gao, X.; Guo, H.; Chen, W. Determination of 13 antidepressants in blood by UPLC-MS/MS with supported liquid extraction pretreatment. J. Chromatogr. B 2021, 1171, 122608. [Google Scholar] [CrossRef]
- Kinyua, J.; Covaci, A.; Maho, W.; McCall, A.K.; Neels, H.; van Nuis, A.L. Sewage-based epidemiology in monitoring the use new psychoactive substances: Validation and application of an analytical method using LC-MS/MS. Drug Test. Anal. 2015, 7, 812–818. [Google Scholar] [CrossRef]
- Lin, W.; Huang, Z.; Gao, S.; Luo, Z.; An, W.; Li, P.; Ping, S.; Ren, Y. Evaluating the stability of prescription drugs in municipal wastewater and sewers based on wastewater-based epidemiology. Sci. Total Environ. 2021, 754, 142414. [Google Scholar] [CrossRef]
- Hua, F.L.; Tsang, Y.F.; Chua, H. Progress of water pollution control in Hong Kong. Aquat. Ecosyst. Health Manag. 2008, 11, 225–229. [Google Scholar] [CrossRef]
- Tsang, Y.F. Environmental protection and pollution management in China. In The Entrepreneurial Rise in Southeast Asia: The Quadruple Helix Influence on Technological Innovation; Sindakis, S., Walter, C., Eds.; Palgrave Macmillan: New York, NY, USA, 2015. [Google Scholar]
- Behera, S.K.; Kim, H.W.; Oh, J.E.; Park, H.S. Occurrence and removal of antibiotics, hormones and several other pharmaceuticals and other in wastewater treatment plants of the largest industrial city of Korea. Sci. Total Environ. 2011, 409, 4351–4360. [Google Scholar] [CrossRef]
- Styrishave, B.; Halling-Sorensen, B.; Ingerslev, F. Environmental risk assessment of three selective serotonin reuptake inhibitors in the aquatic environment: A case study including a cocktail scenario. Environ. Toxicol. Chem. 2011, 30, 254–261. [Google Scholar] [CrossRef]
- O’Flynn, D.; Lawler, J.; Yusuf, A.; Parle-McDermott, A.; Harold, D.; Mc Cloughlin, T.; Holland, L.; Regan, F.; White, B. A review of pharmaceutical occurrence and pathways in the aquatic environment in the context of a changing climate and the COVID-19 pandemic. Anal. Methods 2021, 13, 575–594. [Google Scholar] [CrossRef]
- Shi, W.; Han, Y.; Sun, S.; Tang, Y.; Zhou, W.; Du, X.; Liu, G. Immunotoxicites of microplastics and sertraline, alone and in combination, to a bivalent species: Size-dependent interaction and potential toxication mechanism. J. Hazard. Mater. 2020, 396, 122603. [Google Scholar] [CrossRef]
- Horsing, M.; Kosjek, T.; Andersen, H.R.; Heath, E.; Ledin, A. Fate of citalopram during water treatemnt with O3, ClO2, UV and fenton oxidation. Chemosphere 2012, 89, 129–135. [Google Scholar] [CrossRef]
- Rejek, M.; Grzechulska-Damszel, J. Degradation of sertraline in water by suspended and supported TiO2. Pol. J. Chem. Technol. 2018, 20, 107–112. [Google Scholar] [CrossRef] [Green Version]
- Pliego, G.; Xekoukoulotakis, N.; Venieri, D.; Zazo, J.A.; Casas, J.A.; Rodriguez, J.J.; Mantzavinos, D. Complete degradation of the persistent anti-depressant sertraline in aqueous solution by solar photo-Fenton oxidation. J. Chem. Technol. Biotechnol. 2014, 89, 814–818. [Google Scholar] [CrossRef]
- de Lima Perini, J.A.; Nogueira, R.F.P. Zero-valent iron mediated degradation of sertraline-effect of H2O2 addition and application to sewage treatment plant effluent. J. Chem. Technol. Biotechnol. 2016, 91, 276–282. [Google Scholar] [CrossRef]
- Spina, M.; Venancio, W.; Rodrigues-Silva, C.; Pivetta, R.C.; Diniz, V.; Rath, S.; Guimaraes, J.R. Degradation of antidepressant pharmaceuticals by photoperoxidation in diverse water matrices: A highlight in the evaluation of acute and chronic toxicity. Environ. Sci. Pollut. Res. 2021, 28, 24034–24045. [Google Scholar] [CrossRef] [PubMed]
- Osawa, R.A.; Carvalho, A.P.; Monteiro, O.C.; Oliveira, M.C.; Florencio, M.H. Transformation products of citalopram: Identification, wastewater analysis and in silico toxicological assessment. Chemosphere 2019, 217, 858–868. [Google Scholar] [CrossRef]
- Lejeunesse, A.; Blais, M.; Barbeau, B.; Sauve, S.; Gagnon, C. Ozone oxidation of antidepressants in wastewater–Treatment evaluation and characterization of new by-products by LC-QTOF MS. Chem. Cent. J. 2013, 7, 15–25. [Google Scholar] [CrossRef] [Green Version]
- Gornik, T.; Vozic, A.; Heath, E.; Trontelj, J.; Roskar, R.; Zigon, D.; Vione, D.; Kosjek, T. Determination and photodegradation of sertraline residues in aqueous environment. Environ. Pollut. 2020, 256, 113431. [Google Scholar] [CrossRef]
- Jimenez-Holgado, C.; Calza, P.; Fabbri, D.; Bello, F.D.; Medana, C.; Sakkas, V. Investigation of the aquatic photolytic and photocatalytic degradation of citalopram. Molecules 2021, 26, 5331. [Google Scholar] [CrossRef]
- Calza, P.; Jiminez-Holgado, C.; Coha, M.; Chrimatopoulos, C.; Bello, F.D.; Medana, C.; Sakkas, V. Study of the photoinduced transformations of sertralinie in aqueous media. Sci. Total Environ. 2021, 756, 143805. [Google Scholar] [CrossRef] [PubMed]
- Trojanowicz, M.; Bojanowska-Czajka, A.; Szreder, T.; Męczyńska-Wielgosz, S.; Bobrowski, K.; Fornal, E.; Nichipor, H. Application of ionizing radiation for removal of endocrine disrupotor bisphenol A from waters and wastewaters. Chem. Eng. J. 2021, 403, 126169. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical Review of rate constants for reaction of hydrated electrons, hydrogen atoms and hydroxyl radicals OH/O in aqueous solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef] [Green Version]
- Bojanowska-Czajka, A.; Kciuk, G.; Gumiela, M.; Borowiecka, S.; Nałęcz-Jawecki, G.; Koc, A.; Garcia-Reyes, J.F.; Ozbay, D.S.; Trojanowicz, M. Analytical, toxicological and kinetic investigation of decomposition of the drug diclofenac in waters and wastes using gamma radiation. Environ. Sci. Pollut. Res. 2015, 22, 20255–20270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basfar, A.A.; Mohamed, K.A.; Al-Abduly, A.J.; Al-Shahrani, A.A. Radiolytic degradation of atrazine aqueous solution containing humic substances. Ecotoxicol. Environ. Saf. 2009, 72, 948–953. [Google Scholar] [CrossRef] [PubMed]
- Trojanowicz, M.; Bobrowski, K.; Szreder, T.; Bojanowska-Czajka, A. Gamma-ray, X-ray and electron beam processes. In Advanced Oxidation Processes for Wastewater Treatment; Ameta, S.C., Ameta, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 257–331. [Google Scholar]
- Henry, T.B.; Kwon, J.-W.; Armbrust, K.L.; Black, M.C. Acute and chronic toxicity of five selective serotonin reuptake inhibitors in Ceriodaphnia dubia. Environ. Toxicol. Chem. 2004, 23, 2229–2233. [Google Scholar] [CrossRef] [PubMed]
- Minagh, E.; Hernan, R.; O’Rourke, K.; Lyng, F.M.; Davoren, M. Aquatic ecotoxicity of the selective serotonin reuptake inhibitor sertraline hydrochloride in a battery of fresh water test species. Ecotoxicol. Environ. Saf. 2009, 72, 434–440. [Google Scholar] [CrossRef] [PubMed]
- de Gage, S.B.; Collin, C.; Le-Tri, T.; Pariente, A.; Begaud, B.; Verdoux, H.; Dray-Spira, R.; Zureik, M. Antidepressants and Hepatotoxicity: A short study among 5 million individuals registers in the French National Health Insurance. CNS Drugs 2018, 32, 673–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almansour, M.I.; Jarrar, Y.B.; Jarrar, B.M. In vivo investigation on the chronic hepatotoxicity induced by sertraline. Environ. Toxicol. Pharmacol. 2018, 61, 107–115. [Google Scholar] [CrossRef]
- Chen, S.; Wu, Q.; Li, X.; Li, D.; Fan, M.; Ren, Z.; Bryant, M.; Mei, N.; Ning, B.; Guo, L. The role of hepatic cytochrome P450s in the cytotoxicity of sertraline. Arch. Toxicol. 2020, 94, 2401–2411. [Google Scholar] [CrossRef]
- Bavadekar, S.; Panchal, P.; Hanbashi, A.; Vansal, S. Cytotoxic effects of selective serotonin- and serotonin-norepinephrine reuptake inhibitors on human metastatic breast cancer cell line, MCF-7 (842.3). FASEB J. 2014, 28, 842. [Google Scholar] [CrossRef]
- Minguez, L.; Halm-Lemeille, M.-P.; Costil, K.; Bureau, R.; Lebel, J.-M.; Serpentini, A. Assement of cytotoxicity and immunomodulatory properties of four antidepressants on primary cultures of abalone hemocytes (Haliotis tuberculate). Aquat. Toxicol. 2014, 153, 3–11. [Google Scholar] [CrossRef]
- Ilgin, S.; Dagasan, F.; Donmez, D.B.; Baysal, M.; Ekliglu, O.A. Evaluation of the hepatotoxic potential of citalopram in rats. J. Fac. Pharm. Istanb. Univ. 2019, 50, 188–194. [Google Scholar]
- Ahmadian, E.; Eftekhari, A.; Fard, J.K.; Babaei, H.; Nayebi, A.M.; Mohammadnejad, D.; Eghbal, M.A. In vitro and in vivo evaluation of the mechanisms of citalopram-induced hepatotoxicity. Arch. Pharm. Res. 2017, 40, 1296–1313. [Google Scholar] [CrossRef]
- Jimenez-Holgado, C.; Sakkas, V.; Richard, C. Phototransformation of three psychoactive drugs in presence of sedimental water extractable organic matter. Molecules 2021, 26, 2466. [Google Scholar] [CrossRef]
- Jakimska, A.; Śliwka-Kaszyńska, M.; Nagórski, P.; Kot-Wasik, A.; Namieśnik, J. Environmental Fate of two psychiatric drugs, dizaepam and sertraline: Phototransformation and investigation of their photoproducts in natural waters. J. Chromatogr. Sep. Tech. 2014, 5, 253. [Google Scholar]
- Shen, L.Q.; Beach, E.S.; Xiang, Y.; Tshudy, D.J.; Khanina, N.; Horwitz, C.P.; Bier, M.E.; Collins, T.J. Rapid, biomimetic degradation in water of the persistent drug sertraline by TAML Catalysts and hydrogen peroxide. Environ. Sci. Technol. 2011, 45, 7882–7887. [Google Scholar] [CrossRef] [PubMed]
- Beretsou, V.G.; Psoma, A.K.; Gago-Ferrero, P.; Aalizadeh, R.; Fenner, K.; Thomaidis, N.S. Identification of biotransformation products of citalopram formed in activated sludge. Water Res. 2016, 103, 205–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]

 ) solution saturated with argon in the presence of t-butanol at pH 7-solvated electrons predominate, (●) solution saturated with N2O •OH radicals predominate, (
) solution saturated with argon in the presence of t-butanol at pH 7-solvated electrons predominate, (●) solution saturated with N2O •OH radicals predominate, (  ,●) aerated neutral solution with reactive species •OH, and O2•−/HO2•.
,●) aerated neutral solution with reactive species •OH, and O2•−/HO2•.
 ) solution saturated with argon in the presence of t-butanol at pH 7-solvated electrons predominate, (●) solution saturated with N2O •OH radicals predominate, (
) solution saturated with argon in the presence of t-butanol at pH 7-solvated electrons predominate, (●) solution saturated with N2O •OH radicals predominate, (  ,●) aerated neutral solution with reactive species •OH, and O2•−/HO2•.
,●) aerated neutral solution with reactive species •OH, and O2•−/HO2•.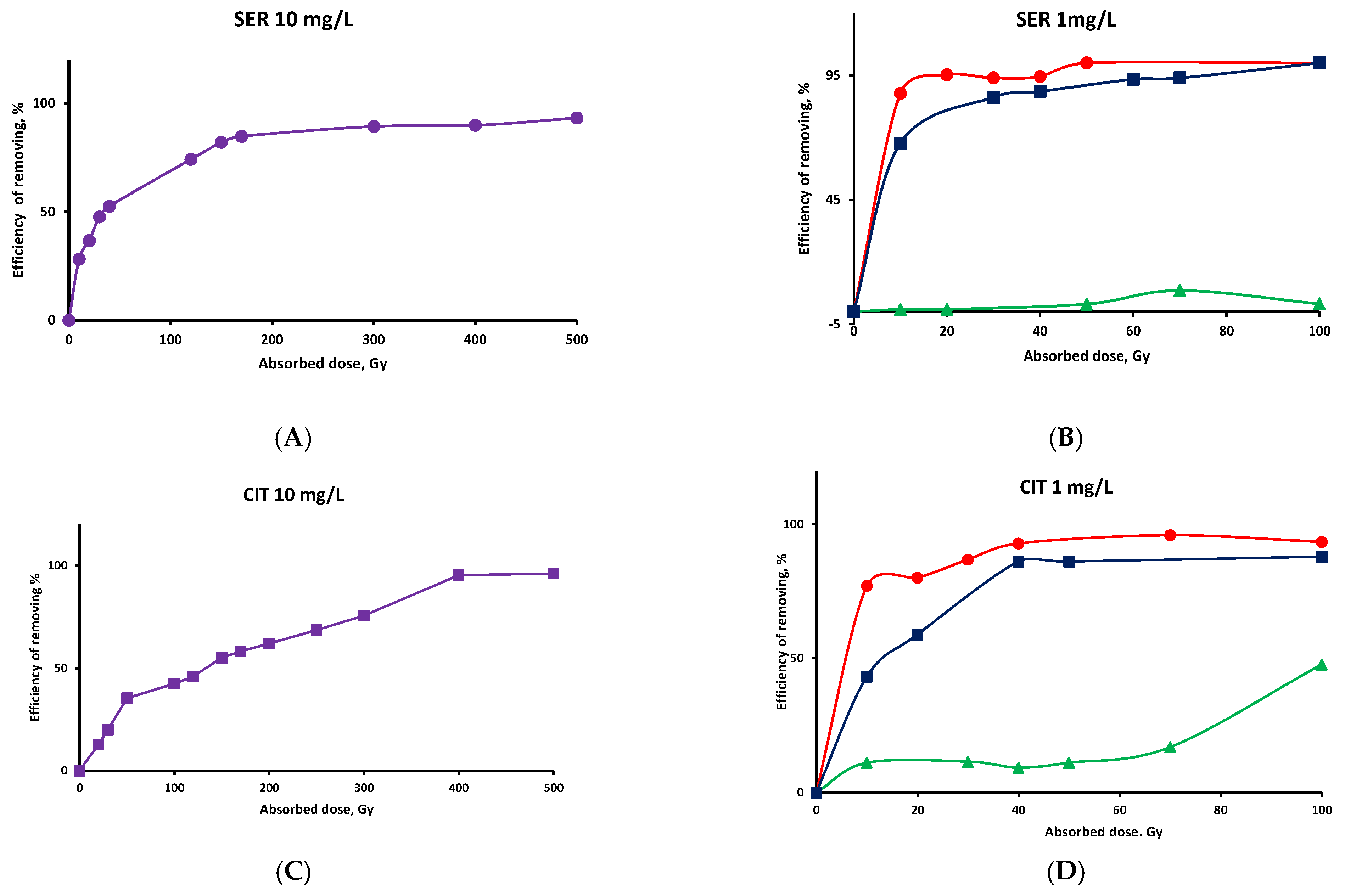
 )-Cl− formation, (
)-Cl− formation, (  )-NO3− formation, (
)-NO3− formation, (  )-NO2− formation.
)-NO2− formation.
 )-Cl− formation, (
)-Cl− formation, (  )-NO3− formation, (
)-NO3− formation, (  )-NO2− formation.
)-NO2− formation.
 )-F- formation, (
)-F- formation, (  )-NO3− formation, (
)-NO3− formation, (  )-NO2− formation.
)-NO2− formation.
 )-F- formation, (
)-F- formation, (  )-NO3− formation, (
)-NO3− formation, (  )-NO2− formation.
)-NO2− formation.
 ) solution before irradiation aerated, (
) solution before irradiation aerated, (  ) solution before irradiation saturated with Ar and in the presence of tert-butanol.
) solution before irradiation saturated with Ar and in the presence of tert-butanol.
 ) solution before irradiation aerated, (
) solution before irradiation aerated, (  ) solution before irradiation saturated with Ar and in the presence of tert-butanol.
) solution before irradiation saturated with Ar and in the presence of tert-butanol.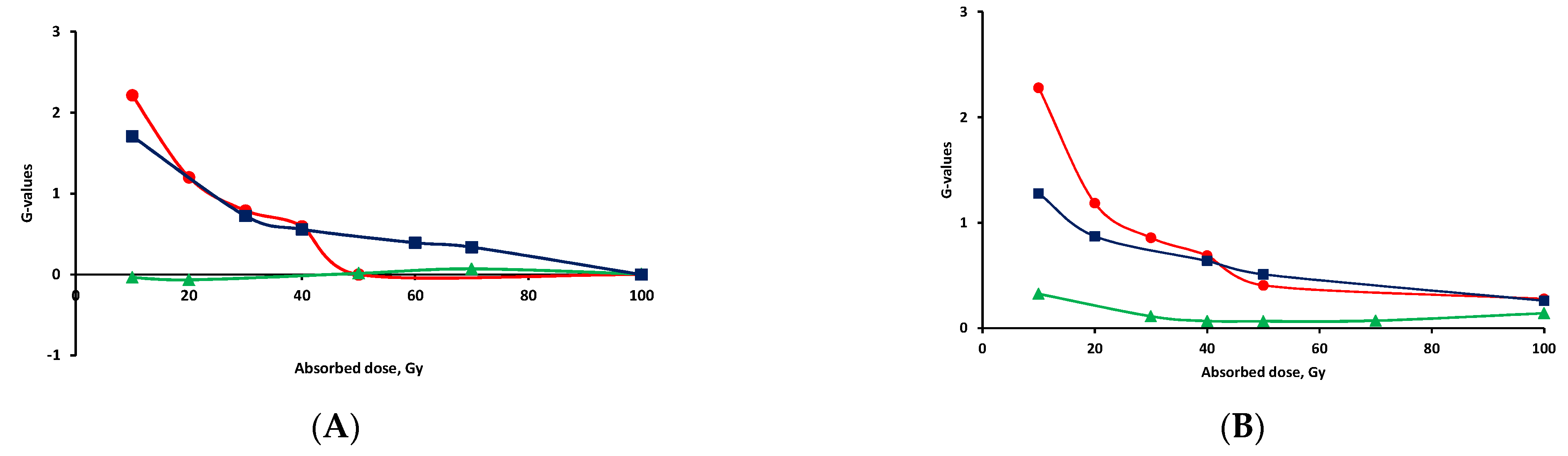
 ) sertraline 1 mg/L in ultrapure (Milli-Q) water, (●) sertraline 1 mg/L in the presence of 10 mg/L CO32−, (
) sertraline 1 mg/L in ultrapure (Milli-Q) water, (●) sertraline 1 mg/L in the presence of 10 mg/L CO32−, (  ) sertraline 1 mg/L in the presence of 10 mg/L NO3−, (
) sertraline 1 mg/L in the presence of 10 mg/L NO3−, (  ) sertraline 1 mg/L in the presence of 10 mg HA; (B) (
) sertraline 1 mg/L in the presence of 10 mg HA; (B) (  ) citalopram 1 mg/L in ultrapure (Milli-Q) water, (●) citalopram 1 mg/L in the presence of 10 mg/L CO32−, (
) citalopram 1 mg/L in ultrapure (Milli-Q) water, (●) citalopram 1 mg/L in the presence of 10 mg/L CO32−, (  ) citalopram 1 mg/L in the presence of 10 mg/L NO3−, (
) citalopram 1 mg/L in the presence of 10 mg/L NO3−, (  ) citalopram mg/L in the presence of 10 mg HA.
) citalopram mg/L in the presence of 10 mg HA.
 ) sertraline 1 mg/L in ultrapure (Milli-Q) water, (●) sertraline 1 mg/L in the presence of 10 mg/L CO32−, (
) sertraline 1 mg/L in ultrapure (Milli-Q) water, (●) sertraline 1 mg/L in the presence of 10 mg/L CO32−, (  ) sertraline 1 mg/L in the presence of 10 mg/L NO3−, (
) sertraline 1 mg/L in the presence of 10 mg/L NO3−, (  ) sertraline 1 mg/L in the presence of 10 mg HA; (B) (
) sertraline 1 mg/L in the presence of 10 mg HA; (B) (  ) citalopram 1 mg/L in ultrapure (Milli-Q) water, (●) citalopram 1 mg/L in the presence of 10 mg/L CO32−, (
) citalopram 1 mg/L in ultrapure (Milli-Q) water, (●) citalopram 1 mg/L in the presence of 10 mg/L CO32−, (  ) citalopram 1 mg/L in the presence of 10 mg/L NO3−, (
) citalopram 1 mg/L in the presence of 10 mg/L NO3−, (  ) citalopram mg/L in the presence of 10 mg HA.
) citalopram mg/L in the presence of 10 mg HA.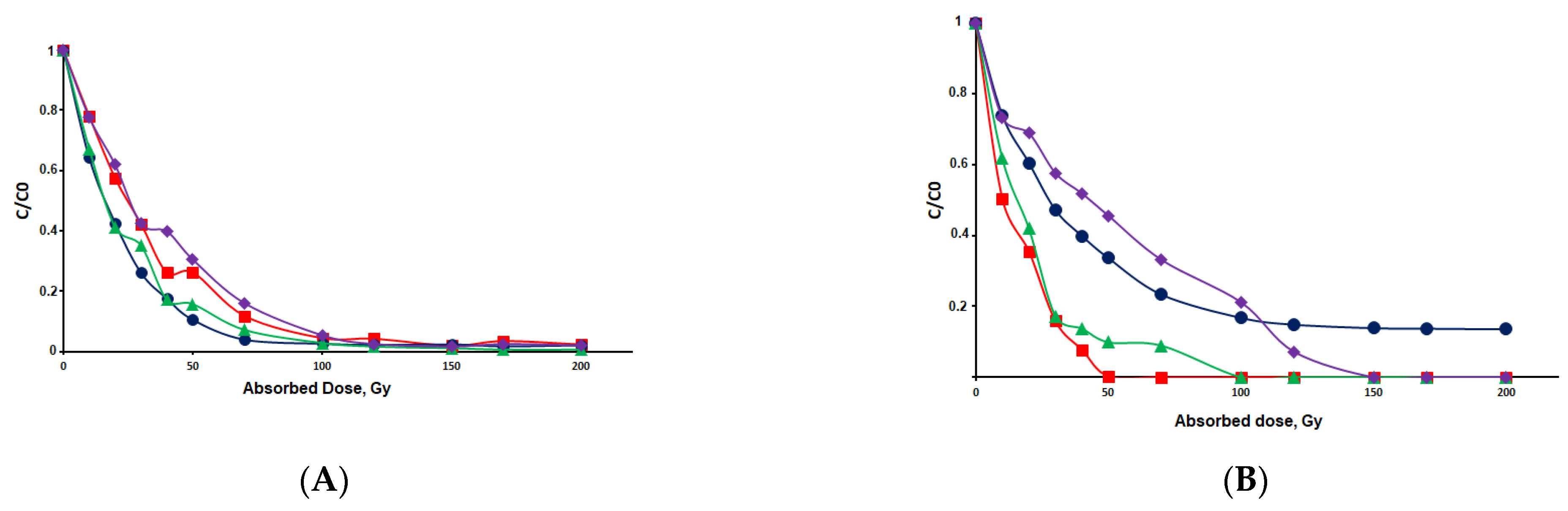
 ) Vistula river from Warsaw, (
) Vistula river from Warsaw, (  ) Rokitnica river from Czubin, (●) ultrapure (Milli-Q) water.
) Rokitnica river from Czubin, (●) ultrapure (Milli-Q) water.
 ) Vistula river from Warsaw, (
) Vistula river from Warsaw, (  ) Rokitnica river from Czubin, (●) ultrapure (Milli-Q) water.
) Rokitnica river from Czubin, (●) ultrapure (Milli-Q) water.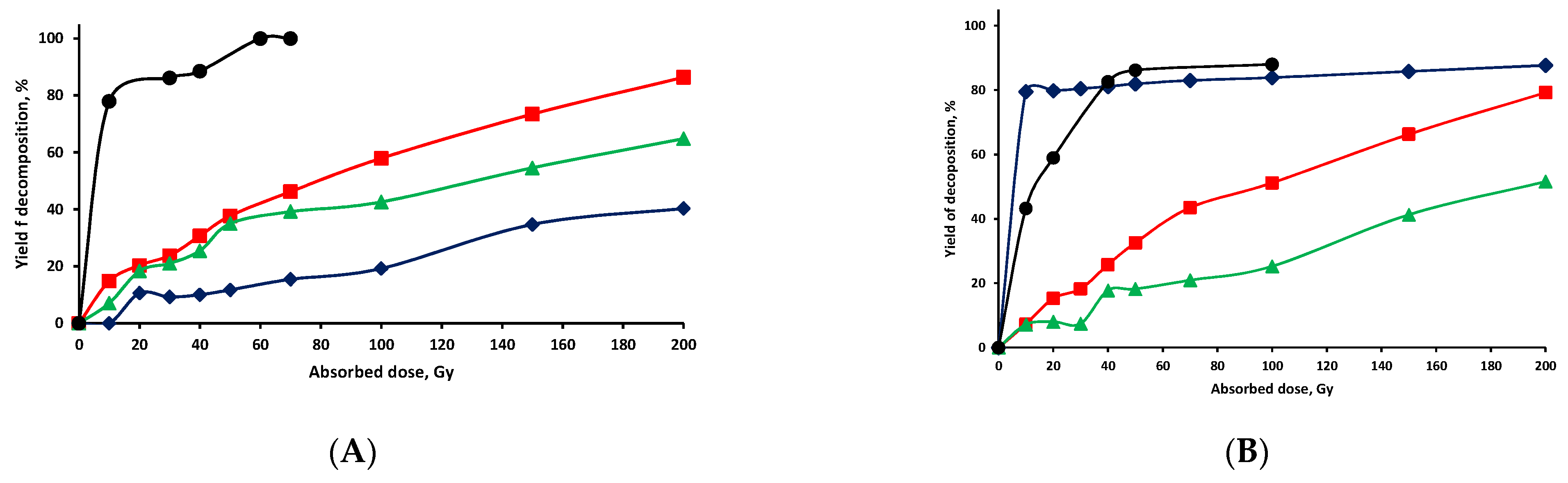

| Compound | Type of AOP/R | Conditions of Treatment | Treated Media | Initial Concentration | Yield of Decomposition | Ref. |
|---|---|---|---|---|---|---|
| Citalopram | Photochemical | 254 nm 254 nm + 0.42 mmol/L H2O2 | Ultrapure Water 1. Ultrapure water 2. Drinking tap Water 3. Surface Water | 25 µg L−1 25 µg L−1 | 60% at 30 min 100% at 30 min 90% at 30 min 90% at 60 min | [33] |
| Citalopram | Chlorination Photochemical | Sodium hypochlorite solution 5 mg/L free chlorine 254 nm | Raw water Raw water | 0.5 mg L−1 0.5 mg L−1 | 100% at 30 min 100% at 175 min | [34] |
| Citalopram Sertraline Citalopram Sertraline | Ozonation | 5 mg L−1 ozone 9 mg L−1 ozone | Primary-treated effluent Primary-treated effluent | 186 ng L−1 14 ng L−1 148 ng L−1 9.4 ng L−1 | 34% 100% 62% 100% | [35] |
| Sertraline | Photochemical | pH 5.5 pH 7.0 pH 12.0 pH 5.5 pH 7.0 pH 12.0 | Surface water Surface water | 10 µg L−1 1 mg L−1 | T1/2 > 60 h T1/2 = 11.6 h T1/2 = 5.78 h T1/2 > 60 h T1/2 = 28.9 h T1/2 = 11.6 h | [36] |
| Sertraline | Photo-Fenton oxidation | Dark Fenton + 40% of the stoichiometric H2O2 dose and 5 mg L−1 Fe2+ | Distilled water | 50 mg L−1 | TOC 90% reduction | [31] |
| Ciatlopram | Ozonation UV radiation | 400 mg L−1 Polychromatic light (an enhanced emission between 250 and 190 nm) | Distilled water pH 7 Distilled water pH 7 | 100 µg L−1 100 µg L−1 | 85% at 20 min (max) 100% at 15 min | [29] |
| Citalopram | Natural sunlight Simulated solar irradiation Photocatalytic | Solar irradiation (outdor) Xenon lamp 1500 W 295–400 nm TiO2 400 mg L−1 | Milli Q Lake water WWTP Milli Q Lake water WWTP Milli Q | 10 mg L−1 10 mg L−1 10 mg L−1 10 mg L−1 10 mg L−1 10 mg L−1 20 mg L−1 | T1/2 = 3456.74 h T1/2 = 693.15 h T1/2 = 462.10 h T1/2 = 61.89 h T1/2 = 25.77 h T1/2 = 23.42 h 100% at 30 min 90% TOC reduction at 5 h | [37] |
| Sertraline | Simulated solar irradiation | Xenon arc lamp 1500 W 300–800 nm | Milli Q | 1 mg L−1 | 60% at 65 min | [38] |
| No. | m/z | Chemical Structure | Degradation Process | Refrences | Gamma Radiation |
|---|---|---|---|---|---|
| 1. | 176.1071 |  | Photocatalytic process | [38] | + |
| 2. | 186.1279 | 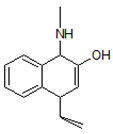 | Photocatalytic process | [38] | + |
| 3 | 188.1072 |  | Photocatalytic process | [38] | + |
| 4. | 214.1228 | 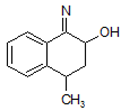 | Photocatalytic process | [38] | + |
| 5. | 272.1207 | 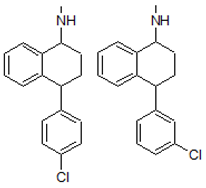 | 1. Photocatalytic process 2. Photolysis | [38] [54] | + |
| 6. | 288.1555 | 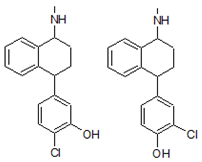 | 1. Photocatalytic process 2. Photolysis | [38] [54] | + |
| 7. | 304.0660 | 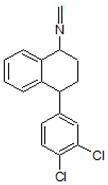 | 1. Photocatalytic process 2. Photolysis | [38] [54] | + |
| 8. | 352.0507 | 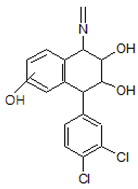 | Photocatalytic process | [38] | + |
| 9. | 291.0337 |  | TAML activator/H2O2 | [55] | + |
| 10. | 322.0768 |  | Photocatalytic process | [38] | + |
| 11. | 338.0715 |  | Photocatalytic process | [38] | + |
| 12. | 354.0661 |  | Photocatalytic process | [38] | + |
| No. | m/z | Chemical Structure | Degradation Process | References | Gamma |
|---|---|---|---|---|---|
| 1. | 245.1292 | 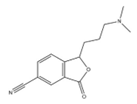 | UV-radiation Photocatalytic degradation Photodegradation in the presence of WEOM (Water Extractable Organic Matter) | [34] [37] [54] | + |
| 2. | 247.1446 |  | Photocatalytic degradation | [37] | + |
| 3. | 261.1241 | 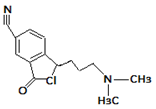 | Photocatalytic degradation | [37] | + |
| 4. | 323.1762 | 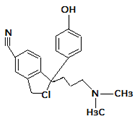 | Photocatalytic degradation Photodegradation in the presence of WEOM (Water Extractable Organic Matter) | [37] [54] | + |
| 5. | 337.1554 | 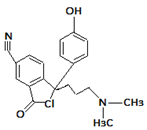 | Photocatalytic degradation | [37] | + |
| 6. | 339.1512 | 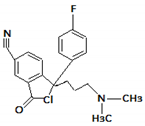 | UV, Cl, H2O Photocatalytic degradation Photodegradation in the presence of WEOM (Water Extractable Organic Matter) | [34] [37] [54] | + |
| 7. | 341.1669 | 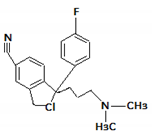 | UV Photocatalytic degradation Photodegradation in the presence of WEOM (Water Extractable Organic Matter) | [34] [37] [54] | + |
| 8. | 355.1457 | 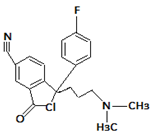 | Cl Photocatalytic degradation Photodegradation in the presence of WEOM (Water Extractable Organic Matter | [34] [37] [54] | + |
| 9. | 357.1618 |  | Photocatalytic degradation | [37] | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bojanowska-Czajka, A.; Pyszynska, M.; Majkowska-Pilip, A.; Wawrowicz, K. Degradation of Selected Antidepressants Sertraline and Citalopram in Ultrapure Water and Surface Water Using Gamma Radiation. Processes 2022, 10, 63. https://doi.org/10.3390/pr10010063
Bojanowska-Czajka A, Pyszynska M, Majkowska-Pilip A, Wawrowicz K. Degradation of Selected Antidepressants Sertraline and Citalopram in Ultrapure Water and Surface Water Using Gamma Radiation. Processes. 2022; 10(1):63. https://doi.org/10.3390/pr10010063
Chicago/Turabian StyleBojanowska-Czajka, Anna, Marta Pyszynska, Agnieszka Majkowska-Pilip, and Kamil Wawrowicz. 2022. "Degradation of Selected Antidepressants Sertraline and Citalopram in Ultrapure Water and Surface Water Using Gamma Radiation" Processes 10, no. 1: 63. https://doi.org/10.3390/pr10010063






