The Influencing Factors for Volume Stability of Ladle Slag
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Material Testing and Analyses
3. Results and Discussion
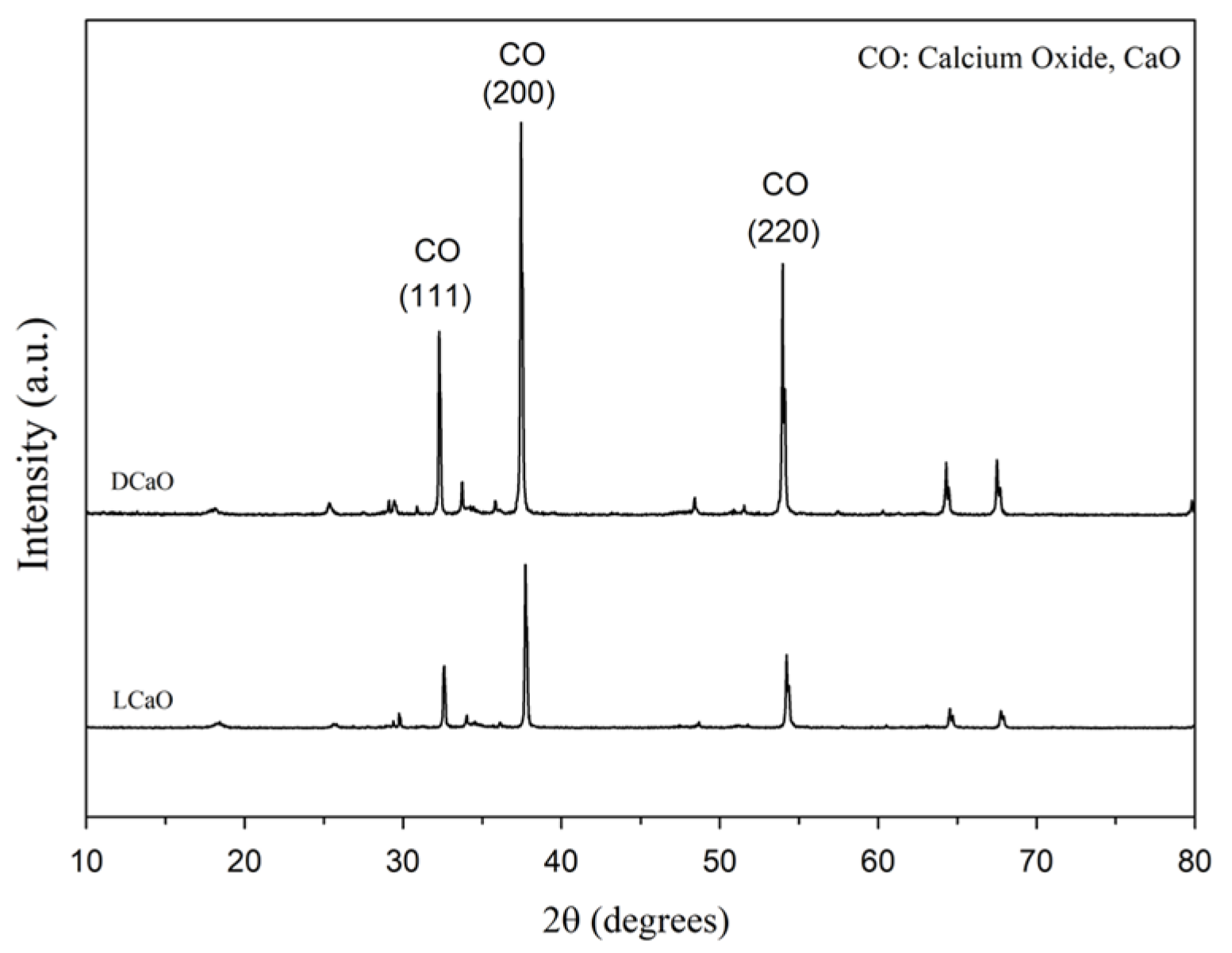
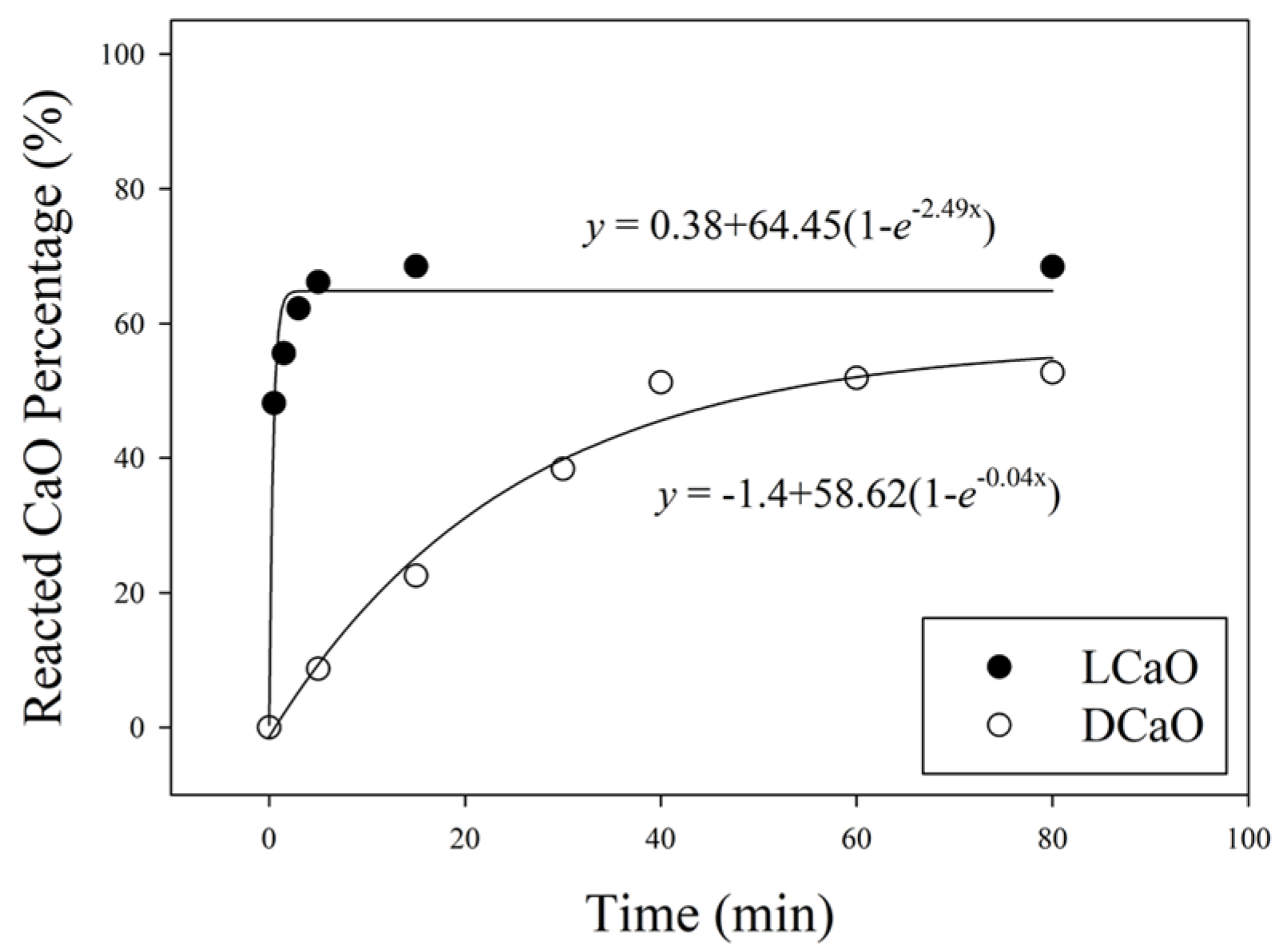
3.1. The Reactivity of Calcinated CaO
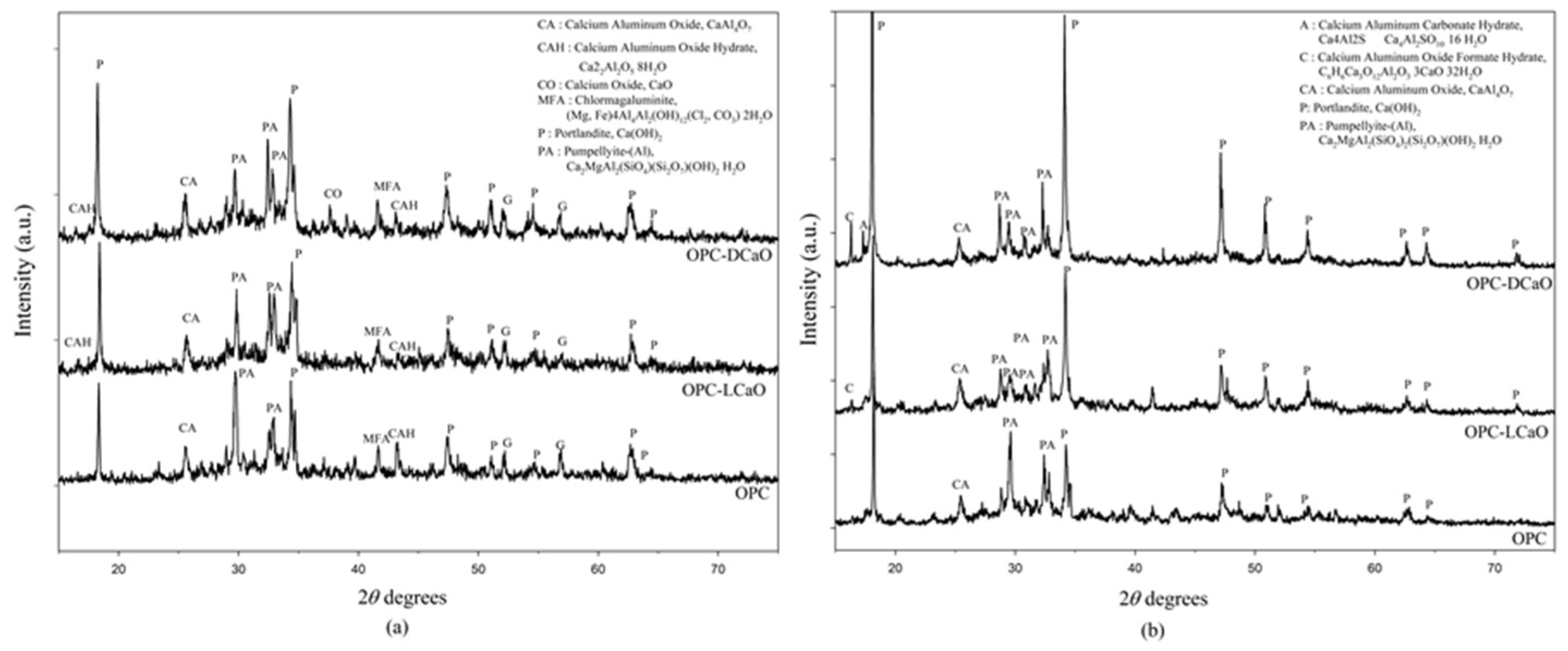
3.2. Different Types of Calcination CaO on Volume Stability
3.3. Stability of Using Ladle Slag and Fly Ash
4. Conclusions
- Ladle slag resulting from a high-temperature process might contain dead burnt CaO (DCaO). The presence of DCaO is the reason that re-using ladle slag causes unsoundness.
- Calcination temperatures may lead to different reactivity of CaO. DCaO (1500 °C) reaction rate is 62 times smaller than light burnt CaO (900 °C). In other words, DCaO would take a longer time to react and is more difficult to hydrate.
- CaO produced under high temperature quickly causes unsoundness problems, and volumetric stability is also related to the type of f-CaO.
- Using ASTM C114-18 [24] to evaluate f-CaO content might lead to underestimating up to 20% of DCaO compared to the actual quantity for its low hydration reactivity. On the other hand, evaluating LCaO content would not lead to this problem since it has high hydration reactivity. Therefore, ASTM C114-18 [24] might not be suitable for testing the DCaO content.
- The presence of f-CaO leads to the unsoundness of ladle slag. Therefore, understanding the discrepancy of f-CaO may be the key to the recovery of ladle slag.
- Since the f-CaO content in ladle slag is mainly DCaO with low reactivity, it is hard to quantify and has a high potential to delay hydration and cause expansion. On that account, there is room for further investigation on the method of detecting the quantity of DCaO and enhancing DCaO reactivity in order to reuse ladle slag as a cementitious material.
- The expansion problem could not be judged directly based on the content of ladle slag. It should be based on the content of free CaO and MgO, and the content of free CaO and MgO should be below 2.5% and 1%, respectively.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neville, A.M.; Brooks, J.J. Concrete Technology; Longman Scientific & Technical: London, UK, 1987. [Google Scholar]
- Neville, A.M. Properties of Concrete; Longman: London, UK, 1995. [Google Scholar]
- Geiseler, J. Slag-approved materials for a better future. In Proceedings of the International Symposium on the Utilization of Metallurgical Slag (ISUS 99), Beijing, China, 16–19 November 1999. [Google Scholar]
- Alizadeh, R.; Chini, M.; Ghods, P.; Hoseini, M.; Montazer, S.; Shekarchi, M. Utilization of Electric Arc Furnace Slag as Aggregates in Concrete—Environmental Issue. In Proceedings of the 6th CANMET/ACI International Conference on Recent Advances in Concrete Technology, Bucharest, Romania, June 2003; Volume 1, pp. 451–464. [Google Scholar]
- Papayianni, I.; Anastasiou, E. Production of high-strength concrete using high volume of industrial by-products. Constr. Build. Mater. 2010, 24, 1412–1417. [Google Scholar] [CrossRef]
- Shi, C.; Day, R.L. Early strength development and hydration of alkali-activated blast furnace slag/fly ash blends. Adv. Cem. Res. 1999, 11, 189–196. [Google Scholar] [CrossRef]
- Sun, S. Investigations on steel slag cements. In Collections of Achievements on the Treatment and Applications of Metallurgical Industrial Wastes; Chinese Metallurgical Industry Press: Beijing, China, 1983; pp. 1–71. [Google Scholar]
- Tang, M. An Investigation on Mineral Composition of Steel Slag for Cement Production; Research Report; Nanjing Institute of Chemical Technology: Nanjing, China, 1973. [Google Scholar]
- Emery, J.J. Slag utilization in pavement construction. In Extending Aggregate Resources; ASTM International: West Conshohocken, PA, USA, 1982. [Google Scholar]
- Wang, G.; Wang, Y.; Gao, Z. Use of steel slag as a granular material: Volume expansion prediction and usability criteria. J. Hazard. Mater. 2010, 184, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Mozt, H.; Geiseler, J. Products of steel slags. In Proceedings of the International Conference on the Science and Engineering of Recycling for Environmental Protection, WASCON 2000, Harrogate, UK, 31 May–2 June 2000; pp. 207–220. [Google Scholar]
- Rojas, M.F.; Sánchez De Rojas, M.I. Chemical assessment of the electric arc furnace slag as construction material: Expansive compounds. Cem. Concr. Res. 2004, 34, 1881–1888. [Google Scholar] [CrossRef]
- Pellegrino, C.; Gaddo, V. Mechanical and durability characteristics of concrete containing EAF slag as aggregate. Cem. Concr. Compos. 2009, 31, 663–671. [Google Scholar] [CrossRef]
- Gieseler, J.; Schlosser, R. Investigation concerning the structure and properties of steel slags. In Proceedings of the 3rd International Conference on Molten Slags and Fluxes, Glasgow, Scotland, 27–29 June 1988. [Google Scholar]
- Kaewmanee, K.; Krammart, P.; Sumranwanich, T.; Choktaweekarn, P.; Tangtermsirikul, S. Effect of free lime content on properties of cement-fly ash mixtures. Constr. Build. Mater. 2013, 38, 829–836. [Google Scholar] [CrossRef]
- Suito, H.; Yokomaku, T.; Hayashida, Y.; Takahashi, Y. Effect of free lime on disintegration of LD slags. Tetsu-to-Hagané 1977, 63, 2316–2325. [Google Scholar] [CrossRef] [Green Version]
- Nawaz, A.; Julnipitawong, P.; Krammart, P.; Tangtermsirikul, S. Effect and limitation of free lime content in cement-fly ash mixtures. Constr. Build. Mater. 2016, 102, 515–530. [Google Scholar] [CrossRef]
- Tsimas, S.; Moutsatsou-Tsima, A. High-calcium fly ash as the fourth constituent in concrete: Problems, solutions and perspectives. Cem. Concr. Compos. 2005, 27, 231–237. [Google Scholar] [CrossRef]
- Hassibi, M. An Overview of Lime Slaking and Factors that Affect the Process. In Proceedings of the 3rd International Sorbalit Symposium, New Orleans, LA, USA, 3–5 November 1999; pp. 2–20. [Google Scholar]
- Gao, P.W.; Xu, S.Y.; Chen, X.; Li, J.; Lu, X.L. Research on autogenous volume deformation of concrete with MgO. Constr. Build. Mater. 2013, 40, 998–1001. [Google Scholar] [CrossRef]
- Boynton, R.S. Chemistry and Technology of Lime and Limestone; Wiley: New York, NY, USA, 1980. [Google Scholar]
- Boldyrev, V.V. Reactivity of solids: Past, present and future. J. Therm. Anal. 1993, 40, 1041–1062. [Google Scholar] [CrossRef]
- Kneller, W.A.; Gupta, J.; Borkowski, M.L.; Dollimore, D. Determination of original free lime content of weathered iron and steel slags by thermogravimetric analysis. Transp. Res. Rec. 1994, 1434. Available online: https://trid.trb.org/view/409659 (accessed on 1 November 2021).
- ASTM International. C114-18 Standard Test Methods for Chemical Analysis of Hydraulic Cement; ASTM International: West Conshohocken, PA, USA, 2018. [Google Scholar]
- European Standard. EN 450 Fly Ash for Concrete—Definitions, Requirement and Quality Control; European Standard: Brussels, Belgium, 1994. [Google Scholar]
- ASTM International. C151/C151M-18 Standard Test Method for Autoclave Expansion of Hydraulic Cement; ASTM International: West Conshohocken, PA, USA, 2018. [Google Scholar]
- Wang, X.Y.; Xue, Z.L.; Li, J.L. Investigation of the reactivity and grain size of lime calcined at extra-high temperatures by flash heating. J. South. African Inst. Min. Metall. 2016, 116, 1159–1164. [Google Scholar] [CrossRef] [Green Version]
- Berent, K.; Komarek, S.; Lach, R.; Pyda, W. The Effect of Calcination Temperature on the Structure and Performance of Nanocrystalline Mayenite Powders. Materials 2019, 12, 3476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamad, S.F.S.; Mohamad, S.; Jemaat, Z. Study of calcination condition on decomposition of calcium carbonate in waste cockle shell to calcium oxide using thermal gravimetric analysis. ARPN J. Eng. Appl. Sci. 2016, 11, 9917–9921. [Google Scholar]
- Mu, S.B.; Sun, Z.Y.; Su, X.P. A study on the microstructure and expanding mechanism of highly free-calcium oxide cements. J Wuhan Univ. Technol. 2001, 23, 27–30. [Google Scholar]
- Deng, M.; Hong, D.; Lan, X.; Tang, M. Mechanism of expansion in hardened cement pastes with hard-burnt free lime. Cem. Concr. Res. 1995, 25, 440–448. [Google Scholar] [CrossRef]
- Halder, B.; Tandon, V.; Tarquin, A.; Ramana, C. Influence of coal fly ash on mechanical properties of mortar consisting of total dissolved solids. In Proceedings of the World of Coal Ash Conference, Lexington, KY, USA, 21–24 October 2009; pp. 22–25. [Google Scholar]
- Wang, Q.; Wang, D.; Zhuang, S. The soundness of steel slag with different free CaO and MgO contents. Constr. Build. Mater. 2017, 151, 138–146. [Google Scholar] [CrossRef]





| Ladle Slag | Fly Ash | ||||
|---|---|---|---|---|---|
| Elements (wt.%) | Ca | 29.25 | Elements (wt.%) | Ca | 14.94 |
| Al | 2.24 | Si | 17.82 | ||
| Mg | 9.93 | Al | 8.11 | ||
| Fe | 2.83 | Mg | 1.38 | ||
| Si | 4.77 | Fe | 4.23 | ||
| Heavy Metals (mg/kg) | Mn | 7435 | Na | 0.52 | |
| K | 0.62 | ||||
| Heavy Metals (mg/kg) | Mn | 406 | |||
| Pb | 123 | ||||
| Cu | 56 | ||||
| As | 500 | ||||
| Ti | 3633 | ||||
| Ba | 673 | ||||
| LCaO | DCaO | ||||
|---|---|---|---|---|---|
| Time (min) | Ca(OH)2 (wt.%) | CaO (wt.%) | Time (min) | Ca(OH)2 (wt.%) | CaO (wt.%) |
| 0.5 | 52.38 | 39.64 | 5 | 10.44 | 7.90 |
| 1.5 | 59.04 | 44.68 | 15 | 25.94 | 19.63 |
| 3 | 64.63 | 48.91 | 30 | 41.65 | 31.51 |
| 5 | 68.00 | 51.46 | 40 | 53.24 | 40.29 |
| 15 | 70.09 | 53.04 | 60 | 53.61 | 40.57 |
| 80 | 70.34 | 53.23 | 80 | 54.5 | 41.13 |
| Type of Calcination CaO | f-CaO in OPC(wt.%) | Measured f-CaO(wt.%) | Recovery Percentage |
|---|---|---|---|
| OPC-LCaO | 1.63 ± 0.03 | 6.63 ± 0.02 | 101% |
| OPC-DCaO | 1.63 ± 0.03 | 5.29 ± 0.02 | 80% |
| Specimen | Length Change (%) | Volume Stability 1 | Ca(OH)2 Content (%) | |
|---|---|---|---|---|
| Before Autoclaving | After Autoclaving | |||
| OPC | 0.10 ± 0.003 | Sound | 10.77 ± 1.03 | 14.06 ± 2.1 |
| OPC-LCaO | 0.17 ± 0.005 | Sound | 14.1 ± 1.33 | 18.43 ± 1.88 |
| OPC-DCaO | Crack | Unsound | 13.36 ± 1.27 | 20.23 ± 2.03 |
| Specimen | Length Change (%) | Volume Stability 1 | Ca(OH)2 Content (%) | |
|---|---|---|---|---|
| Before Autoclaving | After Autoclaving | |||
| OPC | 0.10 ± 0.003 | Sound | 10.77 ± 0.52 | 14.06 ± 1.1 |
| OPLS | Crack | Unsound | 12.58 ± 1.33 | 23.05 ± 1.88 |
| OPFA | 0.02 ± 0.005 | Sound | 7.75 ± 0.04 | 7.99 ± 0.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, T.-H.; Chen, Y.-L.; Wang, H.-P.; Chang, J.-E. The Influencing Factors for Volume Stability of Ladle Slag. Processes 2022, 10, 92. https://doi.org/10.3390/pr10010092
Lu T-H, Chen Y-L, Wang H-P, Chang J-E. The Influencing Factors for Volume Stability of Ladle Slag. Processes. 2022; 10(1):92. https://doi.org/10.3390/pr10010092
Chicago/Turabian StyleLu, Tung-Hsuan, Ying-Liang Chen, Hong-Paul Wang, and Juu-En Chang. 2022. "The Influencing Factors for Volume Stability of Ladle Slag" Processes 10, no. 1: 92. https://doi.org/10.3390/pr10010092






