Bacillus coagulans BACO-17 Alone or in Combination with Galacto-Oligosaccharide Ameliorates Salmonella-Induced Diarrhea and Intestinal Inflammation
Abstract
:1. Introduction
2. Materials and Methods
2.1. In Vitro Screening of Prebiotics
2.2. Diets and In Vivo Experimental Design
2.3. Analysis of Fecal Bacteria
2.4. Determination of Fecal Moisture
2.5. Diarrhea Index Assessment
2.6. Determination of SCFAs
2.7. Histological Assessment of Colonic Inflammation
2.8. Statistical Analysis
3. Results and Discussion
3.1. In Vitro Prebiotic Activity
3.2. Food Intake and Body Weight Gain
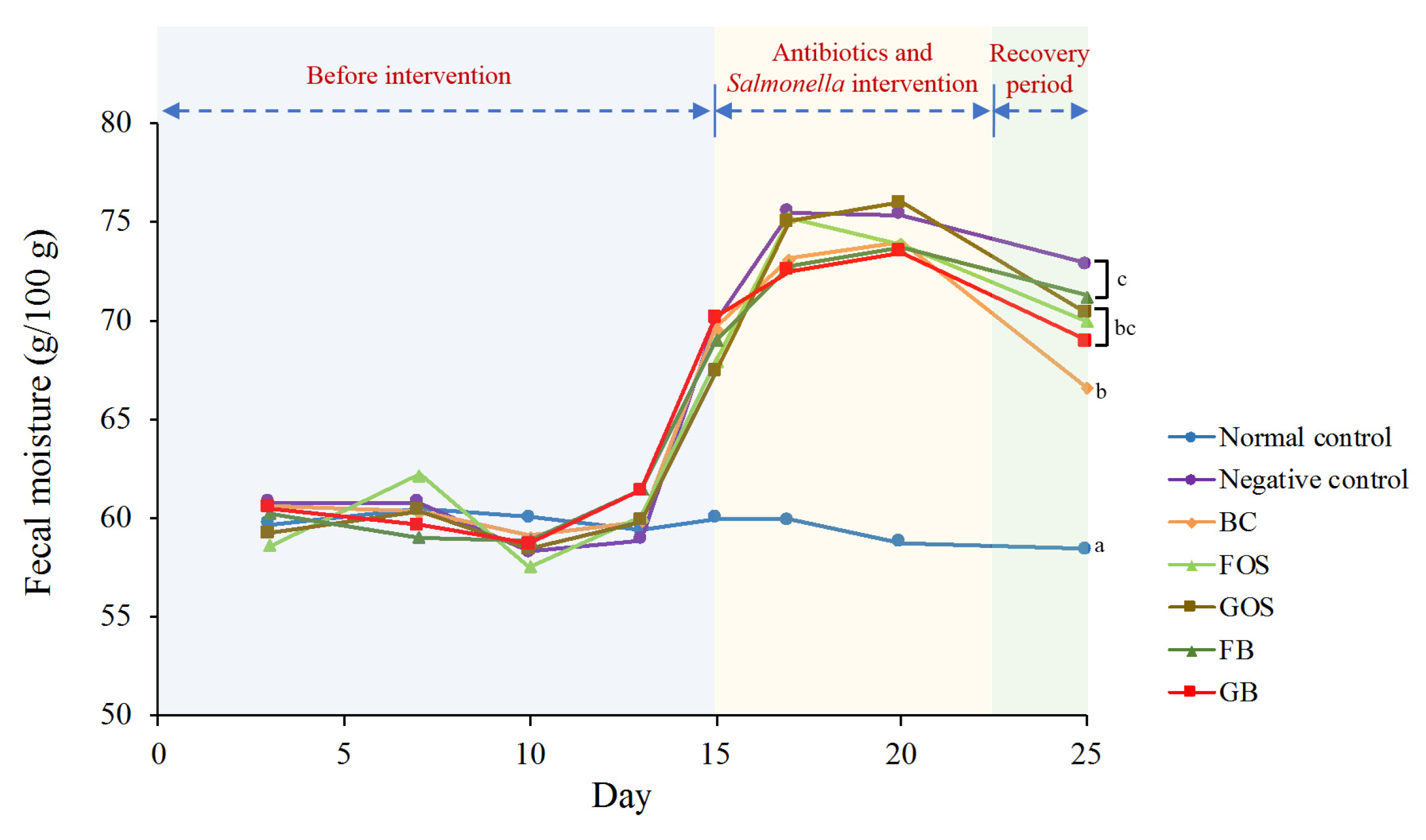
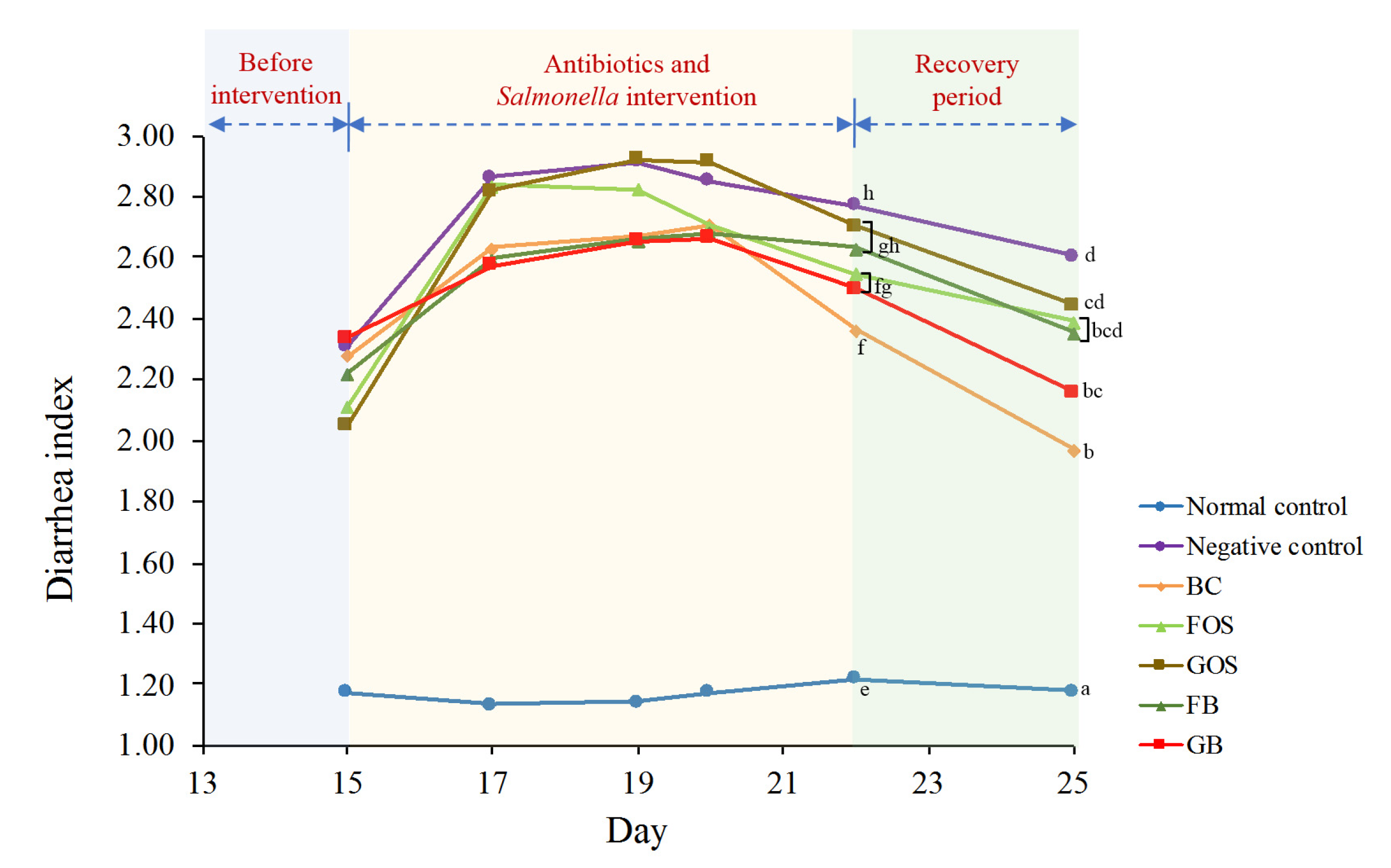
3.3. Assessment of Fecal Moisture and Diarrhea Index
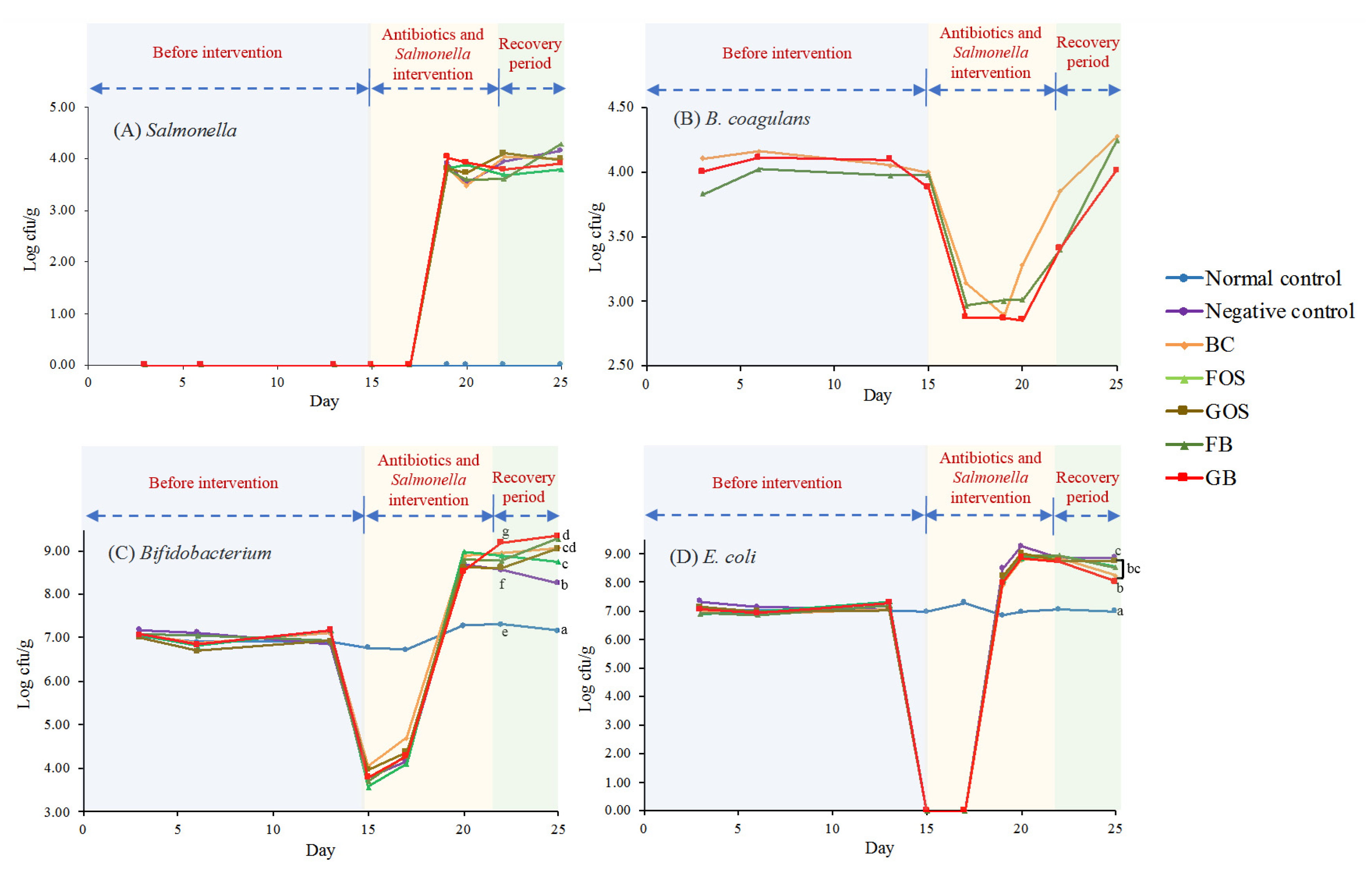
3.4. Changes in Fecal Microflora
3.5. Changes in Fecal SCFAs
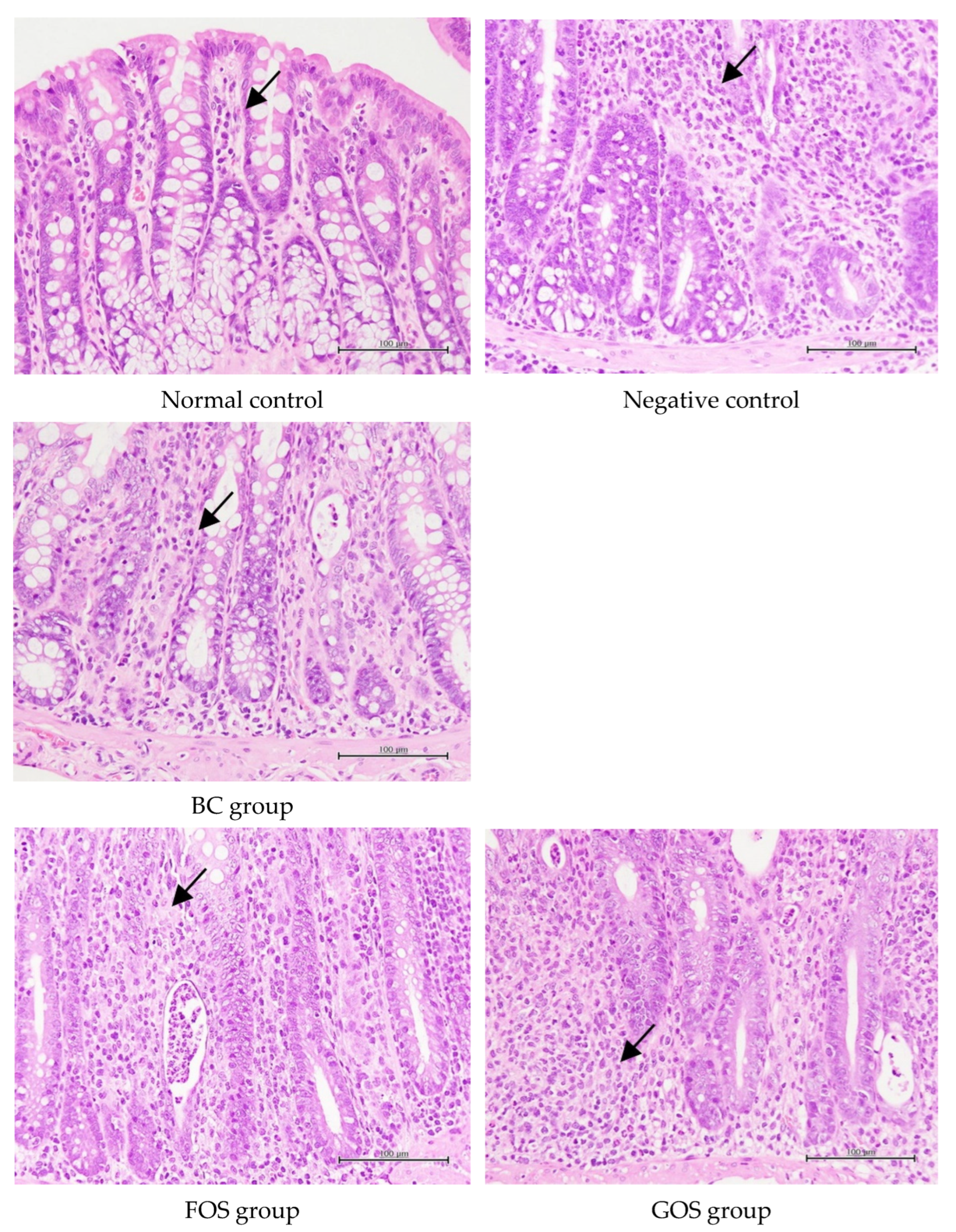
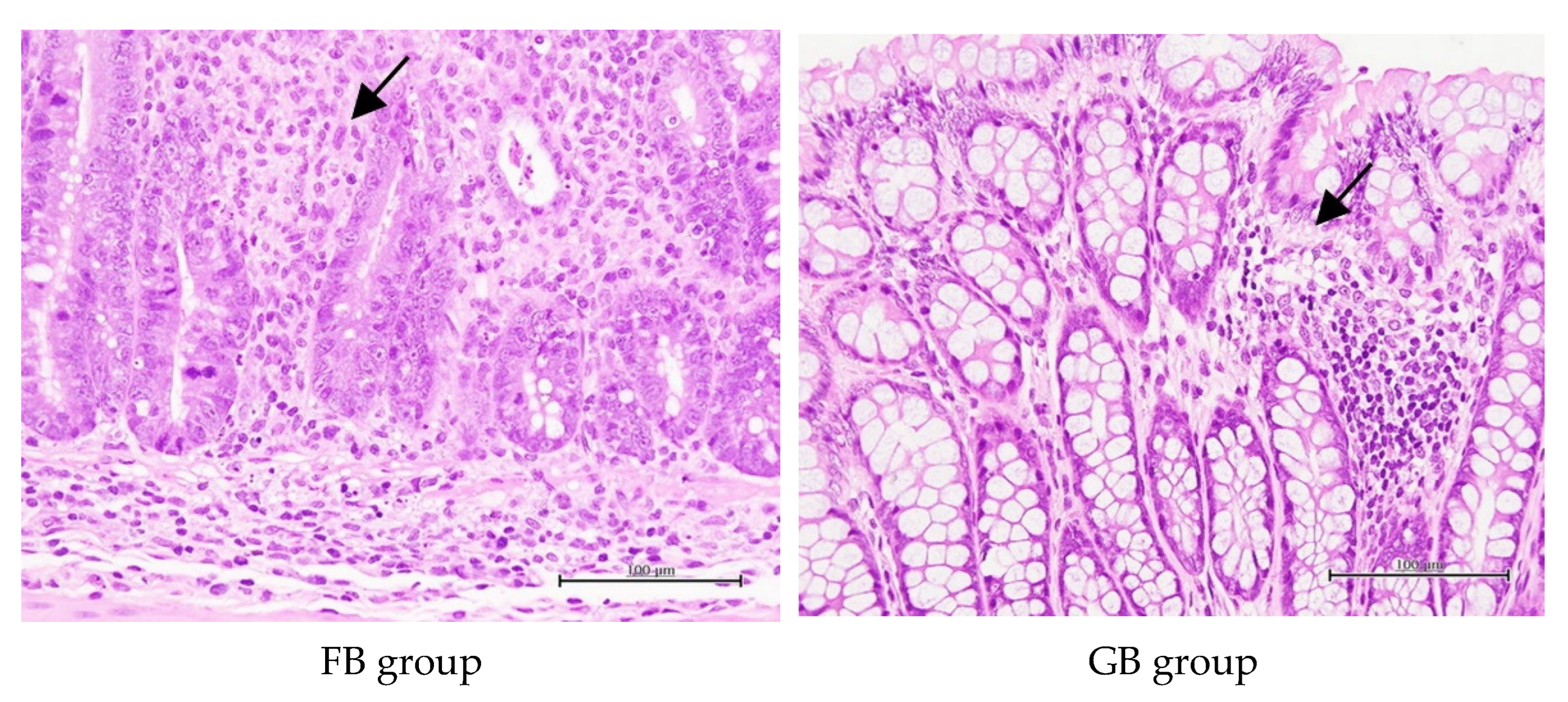
3.6. Histopathological Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bergogne-Berezin, E. Treatment and prevention of antibiotic associated diarrhea. Int. J. Antimicrob. Agents 2000, 16, 521–526. [Google Scholar] [CrossRef]
- Elshaghabee, F.F.M.; Rokana, N.; Gulhane, R.D.; Sharma, C.; Panwar, H. Bacillus as potential probiotics: Status, concerns, and future perspectives. Front. Microbiol. 2017, 8, 1490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kapse, N.G.; Engineer, A.S.; Gowdaman, V.; Wagh, S.; Dhakephalkar, P.K. Functional annotation of the genome unravels probiotic potential of Bacillus coagulans HS243. Genomics 2019, 111, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Saw, C.Y.; Chang, T.J.; Chen, P.Y.; Dai, F.J.; Lau, Y.Q.; Chen, T.Y.; Chau, C.F. Presence of Bacillus coagulans spores and vegetative cells in rat intestine and feces and their physiological effects. Biosci. Biotechnol. Biochem. 2019, 83, 2327–2333. [Google Scholar] [CrossRef]
- Hun, L. Bacillus coagulans significantly improved abdominal pain and bloating in patients with IBS. Postgrad. Med. 2009, 121, 119–124. [Google Scholar] [CrossRef]
- Sudha, M.R.; Jayanthi, N.; Aasin, M.; Dhanashri, R.D.; Anirudh, T. Efficacy of Bacillus coagulans Unique IS2 in treatment of irritable bowel syndrome in children: A double blind, randomised placebo controlled study. Benef. Microbes 2018, 9, 563–572. [Google Scholar] [CrossRef]
- Maity, C.; Gupta, A.K. A prospective, interventional, randomized, double-blind, placebo-controlled clinical study to evaluate the efficacy and safety of Bacillus coagulans LBSC in the treatment of acute diarrhea with abdominal discomfort. Eur. J. Clin. Pharmacol. 2019, 75, 21–31. [Google Scholar] [CrossRef]
- Kolida, S.; Gibson, G.R. Synbiotics in health and disease. Annu. Rev. Food Sci. Technol. 2011, 2, 373–393. [Google Scholar] [CrossRef] [Green Version]
- van der Beek, C.M.; Dejong, C.H.; Troost, F.J.; Masclee, A.A.; Lenaerts, K. Role of short-chain fatty acids in colonic inflammation, carcinogenesis, and mucosal protection and healing. Nutr. Rev. 2017, 75, 286–305. [Google Scholar] [CrossRef] [Green Version]
- Nyangale, E.P.; Farmer, S.; Keller, D.; Chernoff, D.; Gibson, G.R. Effect of prebiotics on the fecal microbiota of elderly volunteers after dietary supplementation of Bacillus coagulans GBI-30, 6086. Anaerobe 2014, 30, 75–81. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, B.; Xu, J.; Liu, Y.; Qiu, E.; Li, Z.; Li, Z.; He, Y.; Zhou, H.; Bai, Y.; et al. Bacteroides fragilis protects against antibiotic-associated diarrhea in rats by modulating intestinal defenses. Front. Immunol. 2018, 9, 1040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Ampting, M.T.; Schonewille, A.J.; Vink, C.; Brummer, R.J.M.; van der Meer, R.; Bovee-Oudenhoven, I.M. Damage to the intestinal epithelial barrier by antibiotic pretreatment of Salmonella-infected rats is lessened by dietary calcium or tannic acid. J. Nutr. 2010, 140, 2167–2172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakai, H.; Sagara, A.; Matsumoto, K.; Hasegawa, S.; Sato, K.; Nishizaki, M.; Shoji, T.; Syunji Horie, S.; Nakagawa, T.; Tokuyama, S.; et al. 5-Fluorouracil induces diarrhea with changes in the expression of inflammatory cytokines and aquaporins in mouse intestines. PLoS ONE 2013, 8, e54788. [Google Scholar] [CrossRef]
- Aleisa, A.M.; Al-Rejaie, S.S.; Abuohashish, H.M.; Ola, M.S.; Parmar, M.Y.; Ahmed, M.M. Pretreatment of Gymnema sylvestre revealed the protection against acetic acid-induced ulcerative colitis in rats. BMC Complement. Altern. Med. 2014, 14, 49. [Google Scholar] [CrossRef] [Green Version]
- Cano Roca, C.L. Characterization of Commercial Probiotics: Antibiotic Resistance, Acid and Bile Resistance, and Prebiotic Utilization. Master’s Thesis, University of Nebraska, Lincoln, NE, USA, 2014. [Google Scholar]
- Tulstrup, M.V.L.; Christensen, E.G.; Carvalho, V.; Linninge, C.; Ahrné, S.; Højberg, O.; Licht, T.R.; Bahl, M.I. Antibiotic treatment affects intestinal permeability and gut microbial composition in Wistar rats dependent on antibiotic class. PLoS ONE 2015, 10, e0144854. [Google Scholar] [CrossRef] [PubMed]
- Tulstrup, M.V.L.; Roager, H.M.; Thaarup, I.C.; Frandsen, H.L.; Frøkiær, H.; Licht, T.R.; Bahl, M.I. Antibiotic treatment of rat dams affects bacterial colonization and causes decreased weight gain in pups. Commun. Biol. 2018, 1, 145. [Google Scholar] [CrossRef] [PubMed]
- Qamar, T.R.; Syed, F.; Nasir, M.; Rehman, H.; Zahid, M.N.; Liu, R.H.; Iqbal, S. Novel combination of prebiotics galacto-oligosaccharides and inulin-inhibited aberrant crypt foci formation and biomarkers of colon cancer in Wistar rats. Nutrients 2016, 8, 465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majeed, M.; Natarajan, S.; Sivakumar, A.; Ali, F.; Pande, A.; Majeed, S.; Karri, S.K. Evaluation of anti-diarrhoeal activity of Bacillus coagulans MTCC 5856 and its effect on gastrointestinal motility in Wistar rats. Int. J. Pharma Bio Sci. 2016, 7, 311. [Google Scholar]
- Ara, K.; Meguro, S.; Hase, T.; Tokimitsu, I.; Otsuji, K.; Kawai, S.; Ito, S.; Iino, H. Effect of spore-bearing lactic acid-forming bacteria (Bacillus coagulans SANK 70258) administration on the intestinal environment, defecation frequency, fecal characteristics and dermal characteristics in humans and rats. Microb. Ecol. Health Dis. 2002, 14, 4–13. [Google Scholar] [CrossRef]
- Bovee-Oudenhoven, I.M.J.; Ten Bruggencate, S.J.M.; Lettink-Wissink, M.L.G.; Van der Meer, R. Dietary fructo-oligosaccharides and lactulose inhibit intestinal colonisation but stimulate translocation of salmonella in rats. Gut 2003, 52, 1572–1578. [Google Scholar] [CrossRef] [Green Version]
- Amorim, C.; Silvério, S.C.; Cardoso, B.B.; Alves, J.I.; Pereira, M.A.; Rodrigues, L.R. In vitro assessment of prebiotic properties of xylooligosaccharides produced by Bacillus subtilis 3610. Carbohydr. Polym. 2020, 229, 115460. [Google Scholar] [CrossRef] [PubMed]
- Haldar, L.; Gandhi, D.N. Effect of oral administration of Bacillus coagulans B37 and Bacillus pumilus B9 strains on fecal coliforms, Lactobacillus and Bacillus spp. in rat animal model. Vet. World 2016, 9, 766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holota, Y.; Dovbynchuk, T.; Kaji, I.; Vareniuk, I.; Dzyubenko, N.; Chervinska, T.; Zakordonets, L.; Stetska, V.; Ostapchenko, L.; Serhiychuk, T.; et al. The long-term consequences of antibiotic therapy: Role of colonic short-chain fatty acids (SCFA) system and intestinal barrier integrity. PLoS ONE 2019, 14, e0220642. [Google Scholar] [CrossRef] [PubMed]
- Polishchuk, N.N.; Bachurin, H.V.; Cherkovska, O.S.; Zinych, O.L.; Lazaryk, O.L.; Bezugly, M.B. Salmonella-induced changes of the rat intestinal microbiota. Russ. J. Infect. Immun. 2021, 11, 865–874. [Google Scholar] [CrossRef]
- Mekonnen, S.A.; Merenstein, D.; Fraser, C.M.; Marco, M.L. Molecular mechanisms of probiotic prevention of antibiotic-associated diarrhea. Curr. Opin. Biotechnol. 2020, 61, 226–234. [Google Scholar] [CrossRef]
- Cao, J.; Yu, Z.; Liu, W.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Probiotic characteristics of Bacillus coagulans and associated implications for human health and diseases. J. Funct. Foods 2020, 64, 103643. [Google Scholar] [CrossRef]
- Kleessen, B.; Hartmann, L.; Blaut, M. Oligofructose and long-chain inulin: Influence on the gut microbial ecology of rats associated with a human faecal flora. Br. J. Nutr. 2001, 86, 291–300. [Google Scholar] [CrossRef] [Green Version]
- Kempf, F.; Menanteau, P.; Rychlik, I.; Kubasová, T.; Trotereau, J.; Virlogeux-Payant, I.; Schaeffer, S.; Schouler, C.; Drumo, R.; Guitton, E.; et al. Gut microbiota composition before infection determines the Salmonella super- and low-shedder phenotypes in chicken. Microb. Biotechnol. 2020, 13, 1611–1630. [Google Scholar] [CrossRef]
- Stecher, B.; Denzler, R.; Maier, L.; Bernet, F.; Sanders, M.J.; Pickard, D.J.; Barthel, M.; Westendorf, A.M.; Krogfelt, K.A.; Walker, A.W.; et al. Gut inflammation can boost horizontal gene transfer between pathogenic and commensal Enterobacteriaceae. Proc. Natl. Acad. Sci. USA 2012, 109, 1269–1274. [Google Scholar] [CrossRef] [Green Version]
- Alonso, V.R.; Guarner, F. Linking the gut microbiota to human health. Br. J. Nutr. 2013, 109, S21–S26. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Gibson, G.R.; Walton, G.E. An in vitro approach to study effects of prebiotics and probiotics on the faecal microbiota and selected immune parameters relevant to the elderly. PLoS ONE 2016, 11, e0162604. [Google Scholar] [CrossRef] [PubMed]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef] [Green Version]
- Gupta, R.S.; Patel, S.; Saini, N.; Chen, S. Robust demarcation of 17 distinct Bacillus species clades, proposed as novel Bacillaceae genera, by phylogenomics and comparative genomic analyses: Description of Robertmurraya kyonggiensis sp. nov. and proposal for an emended genus Bacillus limiting it only to the members of the Subtilis and Cereus clades of species. Int. J. Syst. Evol. Microbiol. 2020, 70, 5753–5798. [Google Scholar] [CrossRef]


| Groups | Time (h) | |||||
|---|---|---|---|---|---|---|
| 0 | 6 | 9 | 12 | 16 | 24 | |
| Glucose 1 | 3.86 ± 0.15 a | 4.69 ± 0.10 b | 5.84 ± 0.20 a | 6.96 ± 0.28 a | 7.23 ± 0.24 a | 7.14 ± 0.14 a |
| XOS 2 | 3.86 ± 0.15 a | 4.53 ± 0.15 b | 5.36 ± 0.43 b | 6.65 ± 0.67 a | 7.05 ± 0.26 ab | 6.96 ± 0.11 ab |
| FOS 2 | 3.86 ± 0.15 a | 4.09 ± 0.17 d | 4.87 ± 0.73 b | 7.03 ± 0.28 a | 7.18 ± 0.12 a | 6.77 ± 0.32 b |
| GOS 2 | 3.86 ± 0.15 a | 4.29 ± 0.30 c | 5.25 ± 0.80 b | 6.10 ± 0.97 a | 7.20 ± 0.39 a | 7.10 ± 0.30 a |
| Pectin 2 | 3.86 ± 0.15 a | 4.95 ± 0.14 a | 5.92 ± 0.12 a | 6.45 ± 0.91 a | 6.83 ± 0.16 b | 6.86 ± 0.16 ab |
| Groups | Day 14 (Before Antibiotic Intervention) | Day 19 (After Salmonella Infection) | Day 25 (Recovery Period) |
|---|---|---|---|
| Normal control | 115.0 ± 20.2 aA | 137.8 ± 23.4 dA | 102.3 ± 23.7 aA |
| Negative control | 124.1 ± 29.3 aC | 52.4 ± 22.1 aA | 94.8 ± 17.6 aB |
| BC | 189.6 ± 27.3 cC | 92.2 ± 6.2 bcA | 145.4 ± 18.1 bB |
| FOS | 170.0 ± 17.9 bcC | 94.9 ± 18.4 cA | 123.3 ± 10.9 abB |
| GOS | 158.6 ± 13.9 bB | 70.0 ± 25.2 abA | 147.5 ± 38.8 bB |
| FB | 223.1 ± 11.5 cdC | 67.0 ± 24.5 aA | 145.6 ± 26.8 bB |
| GB | 232.4 ± 28.0 dC | 121.5 ± 9.0 dA | 182.1 ± 20.0 cB |
| Groups | Inflammation | Edema | Necrosis | Regeneration |
|---|---|---|---|---|
| Normal control | 2.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a |
| Negative control | 4.33 ± 0.82 c | 1.67 ± 1.21 b | 1.83 ± 0.98 d | 2.00 ± 1.67 bc |
| BC | 3.17 ± 0.75 b | 1.00 ± 0.89 ab | 0.33 ± 0.52 ab | 0.33 ± 0.52 ab |
| FOS | 4.33 ± 1.03 c | 1.33 ± 1.03 b | 1.33 ± 1.03 cd | 2.67 ± 2.07 c |
| GOS | 4.00 ± 0.89 bc | 1.33 ± 1.03 b | 1.00 ± 0.89 bcd | 2.33 ± 1.86 c |
| FB | 3.67 ± 0.52 bc | 0.67 ± 0.52 ab | 0.67 ± 0.52 abc | 1.33 ± 1.37 abc |
| GB | 3.00 ± 1.10 b | 0.67 ± 0.82 ab | 0.33 ± 0.82 ab | 0.33 ± 0.82 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, M.-Z.; Sun, T.-C.; Huang, Y.-W.; Wu, Y.-C.; Chen, W.-J.; Chu, H.-F.; Liu, C.-Y.; Chau, C.-F. Bacillus coagulans BACO-17 Alone or in Combination with Galacto-Oligosaccharide Ameliorates Salmonella-Induced Diarrhea and Intestinal Inflammation. Processes 2022, 10, 2123. https://doi.org/10.3390/pr10102123
Wu M-Z, Sun T-C, Huang Y-W, Wu Y-C, Chen W-J, Chu H-F, Liu C-Y, Chau C-F. Bacillus coagulans BACO-17 Alone or in Combination with Galacto-Oligosaccharide Ameliorates Salmonella-Induced Diarrhea and Intestinal Inflammation. Processes. 2022; 10(10):2123. https://doi.org/10.3390/pr10102123
Chicago/Turabian StyleWu, Min-Zi, Tsai-Chien Sun, Yu-Wen Huang, Yi-Ching Wu, Wei-Jen Chen, Hui-Fang Chu, Cheng-Yen Liu, and Chi-Fai Chau. 2022. "Bacillus coagulans BACO-17 Alone or in Combination with Galacto-Oligosaccharide Ameliorates Salmonella-Induced Diarrhea and Intestinal Inflammation" Processes 10, no. 10: 2123. https://doi.org/10.3390/pr10102123






