CFD Modelling of the Fuel Reactor of a Chemical Loping Combustion Plant to Be Used with Biomethane
Abstract
:1. Introduction
Advancement on CFD Models on Chemical Looping Combustion
- -
- it is capable to model full particle size distribution (PSD) for all the solid species;
- -
- it offers the possibility to model any solid load, from very diluted to packed (i.e., higher than 60% concentration in volume);
- -
- can perform complete Lagrangian calculations for the solids, the mass, momentum, heat transfer and wear;
- -
- it has the possibility to model systems with more than 1E16 particles;
- -
- it can perform calculation of chemical reactions, specifically for each particle it provides highly accurate results, because they are dependent from the composition, temperature and size of each particle.
2. Materials and Methods
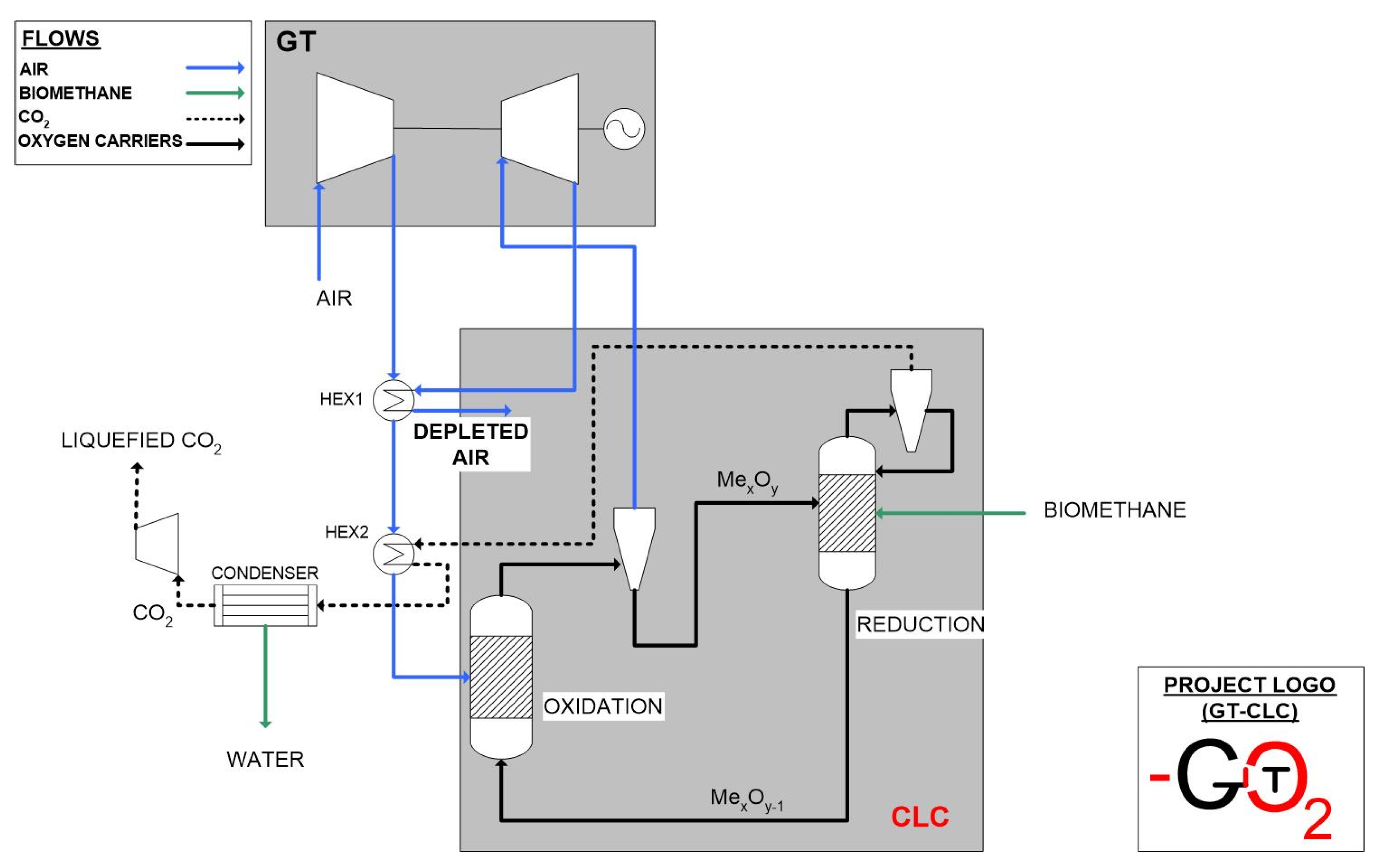
2.1. GTCLC-NEG Plant Description
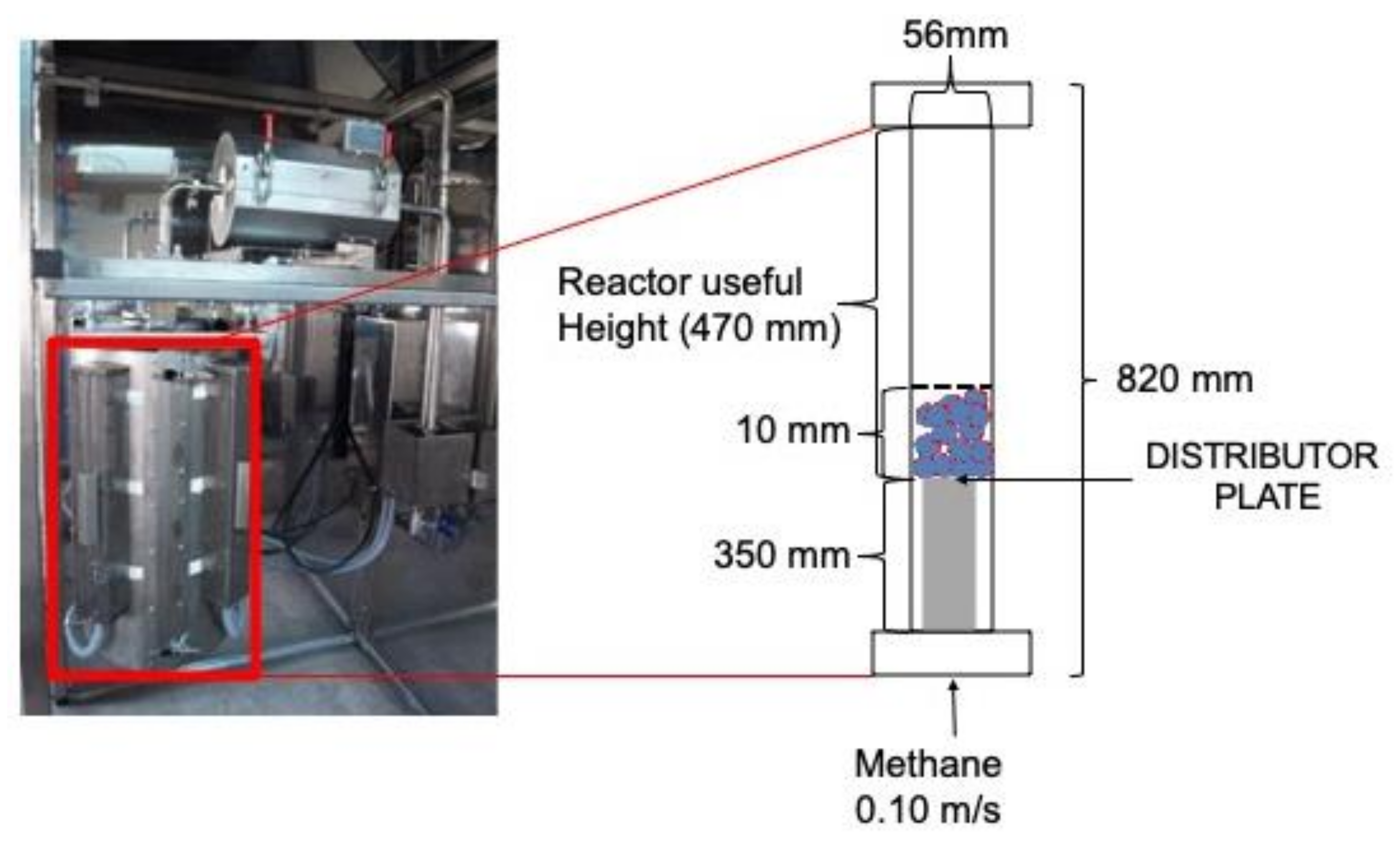
2.2. Batch Reactor Characteristics
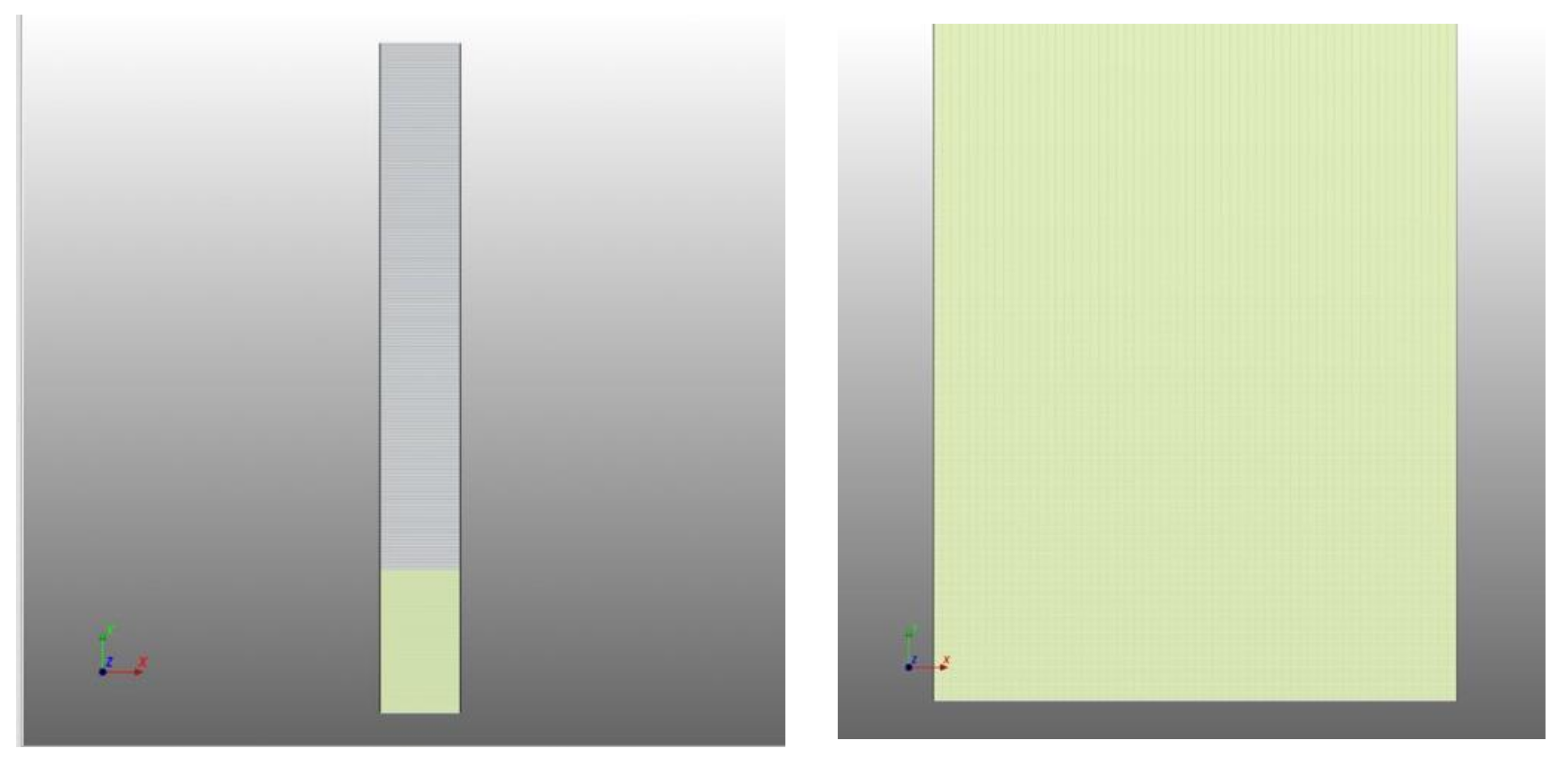
2.3. Mesh Characterization
- -
- x: 7.00E-4 m
- -
- y: 2.61E-03 m.
2.4. The CFD Model
- (a)
- Continuity Equations
- (b)
- Momentum equations
- (a)
- Gas stress tensor
- (b)
- Solid stress tensor
- (c)
- Granular temperature
- (d)
- Inter-phase momentum exchange
- -
- the conservation of internal energy;
- -
- the conservation of granular energy;
- -
- the boundary conditions (with particular attention to the wall heat transfer phenomena).
2.5. The Modeling Platform
2.6. The Modeling Conditions
2.7. The Solver and Convergence Parameters
- -
- residuals: these are the criteria used for convergence for each type of equation, as well as the maximum number of iterations and residuals normalization options;
- -
- discretization: defines temporal, special discretization schemes and relaxation factors for each equation;
- -
- linear solver: defines the linear equation solver, tolerance and maximum number of iterations for each equation;
- -
- preconditioner: defines the preconditioner options for each equation;
- -
- advanced: defines less common parameters, such as the maximum inlet velocity factor, drag and IA theory under-relaxation factors and fourth order interpolation scheme.
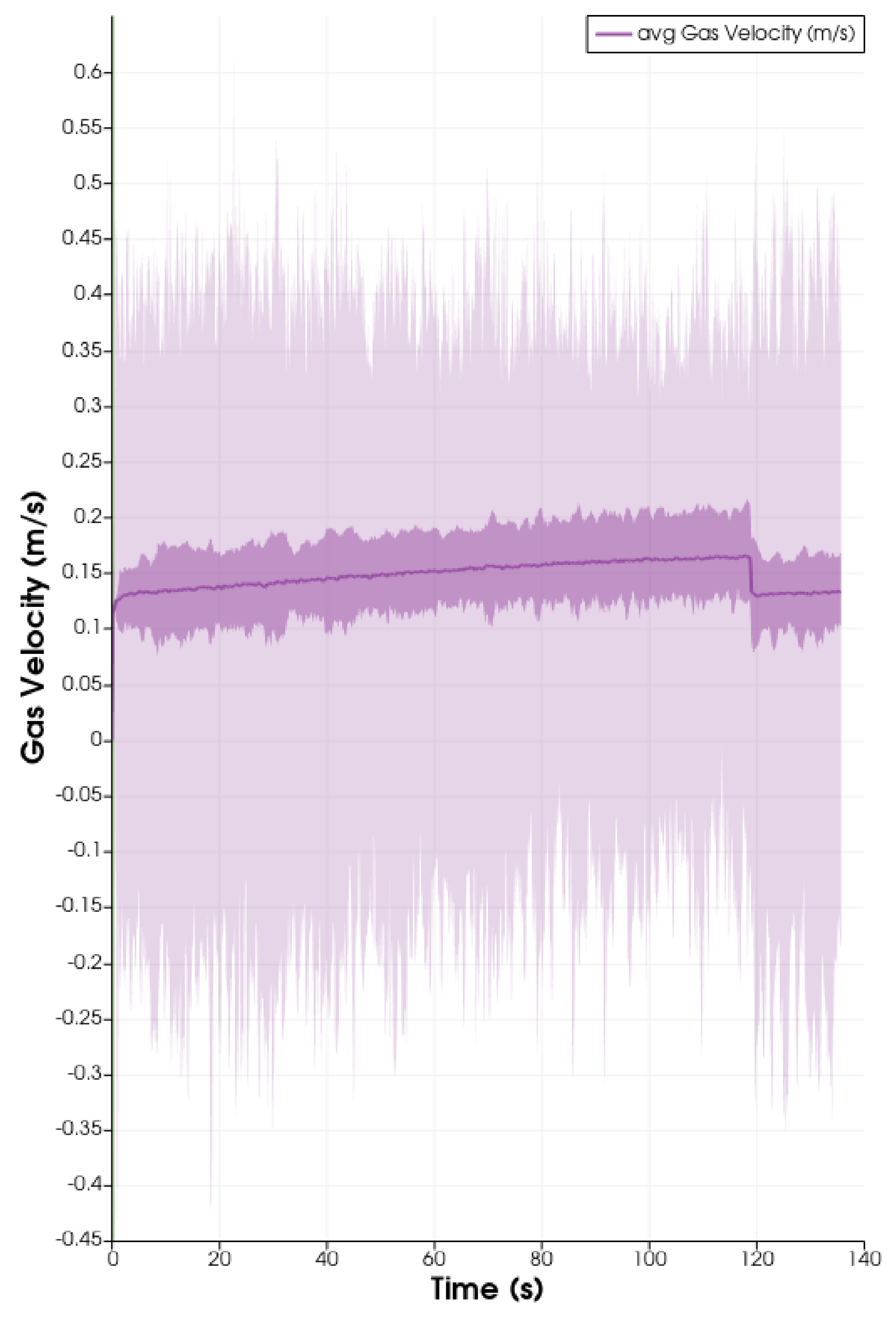
3. Results
4. Discussion
4.1. Further Validation
4.2. Sensitivity Analysis on Mesh Refinement
4.3. Sensitivity Analysis on the Approaches Used to Model Turbulence
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Abbreviations | |
| CLC | Chemical Looping Combustion |
| CFD | Computational Fluid Dynamic |
| GHG | Greenhouse Gases |
| DEM | Descrete Element Model |
| PCLC | Pressurised Chemical Looping Combustion |
| Greek letters | |
| α | Constant; (-) |
| β | Coefficient for the interphase force between the fluid phase and the mth solids phase; kg/m3·s |
| ε | phase volume fraction; (-) |
| η | Function of restitution coefficient; (-) |
| θ | granular temperature; (m2/s2) |
| μ | molecular viscosity; (kg/(m·s)) |
| ρ | microscopic density density; (kg/m3) |
| stress tensor; (Pa) | |
| angle of internal friction; (rad) | |
| Symbols | |
| Bh | bed height; (mm) |
| d | diameter of particles; (m) |
| e | coefficient of restitution for the collisions of solids; (-) |
| D | reactor diameter; (mm) |
| Rate of strain tensor; (s−1) | |
| Identity tensor; (s−1) | |
| Second invariant of the deviator of the strain rate tensor; (s−1) | |
| Igp | momentum transfer from fluid phase to solid phase; (N/m3) |
| g | acceleration due to gravity; (m/s2) |
| g0 | radial distribution at contact |
| h | reactor height; (mm); |
| P | pressure; (Pa) |
| Re | Reynolds number; (-) |
| phase stress tensor; (Pa) | |
| Tr | Radiation temperature; (K) |
| velocity vector; (m/s) | |
| Pedices | |
| g | gas phase; (-) |
| fric | frictional; (-) |
| p | solid phase; (-) |
References
- Cozzi, L.; Gould, T.; Bouckart, S.; Crow, D.; Kim, T.Y.; Mcglade, C. World Energy Outlook 2020; International Energy Agency: Paris, France, 2020; pp. 1–461. [Google Scholar] [CrossRef]
- Chankov, G.; Hinov, N. The energy strategy and energy policy of the european union. Econ. Stud. 2021, 30, 3–31. [Google Scholar]
- Yamagata, Y.; Hanasaki, N.; Ito, A.; Kinoshita, T.; Murakami, D.; Zhou, Q. Estimating water–food–ecosystem trade-offs for the global negative emission scenario (IPCC-RCP2. 6). Sustain. Sci. 2018, 13, 301–313. [Google Scholar] [CrossRef]
- Osman, M. A Pressurized Internally Circulating Reactor (ICR) for Streamlining Development of Chemical Looping Technology. Ph.D. Thesis, Norwegian University of Science and Technology, Trondheim, Norway, 2021. [Google Scholar]
- Abad, A.; Adánez, J.; García-Labiano, F.; De Diego, L.F.; Gayán, P. Modeling of the chemical-looping combustion of methane using a Cu-based oxygen-carrier. Combust. Flame 2010, 157, 602–615. [Google Scholar] [CrossRef] [Green Version]
- Fulchini, F.; Ghadiri, M.; Borissova, A.; Amblard, B.; Bertholin, S.; Cloupet, A.; Yazdanpanah, M. Development of a methodology for predicting particle attrition in a cyclone by CFD-DEM. Powder Technol. 2019, 357, 21–32. [Google Scholar] [CrossRef]
- Li, Z.; Xu, H.; Yang, W. Synergistic effect between CO2 and H2O on biomass chemical looping gasification with hematite as oxygen carrier. In Proceedings of the International Conference on Applied Energy 2019, Västerås, Sweden, 12–15 August 2019; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Chen, X.; Ma, J.; Tian, X.; Wan, J.; Zhao, H. CPFD simulation and optimization of a 50 kWth dual circulating fluidized bed reactor for chemical looping combustion of coal. Int. J. Greenh. Gas Control 2019, 90, 102800. [Google Scholar] [CrossRef]
- Zhou, W.; Zhao, C.; Duan, L.; Qu, C.; Chen, X. Two-dimensional computational fluid dynamics simulation of coal combustion in a circulating fluidized bed combustor. Chem. Eng. J. 2011, 166, 306–314. [Google Scholar] [CrossRef]
- Tabib, M.V.; Johansen, S.T.; Amini, S. A 3D CFD-DEM Methodology for Simulating Industrial Scale Packed Bed Chemical Looping Combustion Reactors. Ind. Eng. Chem. Res. 2013, 52, 12041–12058. [Google Scholar] [CrossRef]
- Banerjee, S.; Agarwal, R. Transient reacting flow simulation of spouted fluidized bed for coal-direct chemical looping combustion with different Fe-based oxygen carriers. Appl. Energy 2015, 160, 552–560. [Google Scholar] [CrossRef]
- Harichandan, A.B.; Shamim, T. CFD analysis of bubble hydrodynamics in a fuel reactor for a hydrogen-fueled chemical looping combustion system. Energy Convers. Manag. 2014, 86, 1010–1022. [Google Scholar] [CrossRef]
- Alobaid, F.; Ohlemüller, P.; Ströhle, J.; Epple, B. Extended Euler–Euler model for the simulation of a 1 MWth chemical–looping pilot plant. Energy 2015, 93, 2395–2405. [Google Scholar] [CrossRef]
- Kruggel-Emden, H.; Rickelt, S.; Stepanek, F.; Munjiza, A. Development and testing of an interconnected multiphase CFD-model for chemical looping combustion. Chem. Eng. Sci. 2010, 65, 4732–4745. [Google Scholar] [CrossRef]
- Breault, R.W.; Weber, J.; Straub, D.; Bayham, S. Computational Fluid Dynamics Modeling of the Fuel Reactor in NETL’s 50 kWth Chemical Looping Facility. J. Energy Resour. Technol. 2017, 139, 042211. [Google Scholar] [CrossRef]
- Li, S.; Shen, Y. Numerical study of gas-solid flow behaviors in the air reactor of coal-direct chemical looping combustion with Geldart D particles. Powder Technol. 2020, 361, 74–86. [Google Scholar] [CrossRef]
- Porrazzo, R.; White, G.; Ocone, R. Fuel reactor modelling for chemical looping combustion: From micro-scale to macro-scale. Fuel 2016, 175, 87–98. [Google Scholar] [CrossRef] [Green Version]
- Parker, J.M. CFD model for the simulation of chemical looping combustion. Powder Technol. 2014, 265, 47–53. [Google Scholar] [CrossRef]
- Menon, K.G.; Patnaikuni, V.S. CFD simulation of fuel reactor for chemical looping combustion of Indian coal. Fuel 2017, 203, 90–101. [Google Scholar] [CrossRef]
- Chen, L.; Yang, X.; Li, G.; Li, X.; Snape, C. Prediction of bubble fluidisation during chemical looping combustion using CFD simulation. Comput. Chem. Eng. 2017, 99, 82–95. [Google Scholar] [CrossRef]
- Peng, Z.; Doroodchi, E.; Alghamdi, Y.A.; Shah, K.; Luo, C.; Moghtaderi, B. CFD–DEM simulation of solid circulation rate in the cold flow model of chemical looping systems. Chem. Eng. Res. Des. 2015, 95, 262–280. [Google Scholar] [CrossRef]
- Shuai, W.; Yunchao, Y.; Huilin, L.; Jiaxing, W.; Pengfei, X.; Guodong, L. Hydrodynamic simulation of fuel-reactor in chemical looping combustion process. Chem. Eng. Res. Des. 2011, 89, 1501–1510. [Google Scholar] [CrossRef]
- Lin, J.; Luo, K.; Sun, L.; Wang, S.; Hu, C.; Fan, J. Numerical Investigation of a Syngas-Fueled Chemical Looping Combustion System. Energy Fuels 2020, 34, 12800–12809. [Google Scholar] [CrossRef]
- Reinking, Z.; Whitty, K.J.; Lighty, J.S. A simulation-based parametric study of CLOU chemical looping reactor performance. Fuel Process. Technol. 2021, 215, 106755. [Google Scholar] [CrossRef]
- Hamidouche, Z.; Masi, E.; Fede, P.; Simonin, O.; Mayer, K.; Penthor, S. Unsteady three-dimensional theoretical model and numerical simulation of a 120-kW chemical looping combustion pilot plant. Chem. Eng. Sci. 2019, 193, 102–119. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, I.; De Lasa, H. CO2 Capture Using Chemical Looping Combustion from a Biomass-Derived Syngas Feedstock: Simulation of a Riser–Downer Scaled-Up Unit. Ind. Eng. Chem. Res. 2020, 59, 6900–6913. [Google Scholar] [CrossRef]
- Seo, M.W.; Nguyen, T.D.; Lim, Y.I.; Kim, S.D.; Park, S.; Song, B.H.; Kim, Y.J. Solid circulation and loop-seal characteristics of a dual circulating fluidized bed: Experiments and CFD simulation. Chem. Eng. J. 2011, 168, 803–811. [Google Scholar] [CrossRef]
- Van der Watt, J.G.; Laudal, D.; Krishnamoorthy, G.; Feilen, H.; Mann, M.; Shallbetter, R.; Nelson, T.; Srinivasachar, S. Development of a Spouted Bed Reactor for Chemical Looping Combustion. J. Energy Resour. Technol. 2018, 140, 112002. [Google Scholar] [CrossRef]
- NETL. MFIX Open Source Multiphase Flow Modeling for Real-World Applications; National Energy Technology Laboratory: Pittsburgh, PA, USA, 2016.
- Alobaid, F.; Almohammed, N.; Farid, M.M.; May, J.; Rößger, P.; Richter, A.; Epple, B. Progress in CFD Simulations of Fluidized Beds for Chemical and Energy Process Engineering. Prog. Energy Combust. Sci. 2021, 100930. [Google Scholar] [CrossRef]
- Son, S.R.; Kim, S.D. Chemical-Looping Combustion with NiO and Fe2O3 in a Thermobalance and Circulating Fluidized Bed Reactor with Double Loops. Ind. Eng. Chem. Res. 2006, 45, 2689–2696. [Google Scholar] [CrossRef]
- Abad, A.; Adanez, J.; García-Labiano, F.; De Diego, L.F.; Gayan, P.; Celaya, J. Mapping of the range of operational conditions for Cu-, Fe-, and Ni-based oxygen carriers in chemical-looping combustion. Chem. Eng. Sci. 2007, 62, 533–549. [Google Scholar] [CrossRef] [Green Version]
- Adánez, J.; Gayán, P.; Celaya, J.; De Diego, L.F.; García-Labiano, F.; Abad, A. Chemical Looping Combustion in a 10 kWth Prototype Using a CuO/Al2O3 Oxygen Carrier: Effect of Operating Conditions on Methane Combustion. Ind. Eng. Chem. Res. 2006, 45, 6075–6080. [Google Scholar] [CrossRef]
- Luis, F.; Garci, F.; Gayán, P.; Celaya, J.; Palacios, J.M.; Adánez, J. Operation of a 10 kWth chemical-looping combustor during 200 h with a CuO–Al2O3 oxygen carrier. Fuel 2007, 86, 1036–1045. [Google Scholar]
- García-Labiano, F.; De Diego, L.F.; Adánez, J.; Abad, A.A.; Gayán, P. Reduction and Oxidation Kinetics of a Copper-Based Oxygen Carrier Prepared by Impregnation for Chemical-Looping Combustion. Ind. Eng. Chem. Res. 2004, 43, 8168–8177. [Google Scholar] [CrossRef]
- Zerobin, F.; Penthor, S.; Bertsch, O.; Pröll, T. Fluidized bed reactor design study for pressurized chemical looping combustion of natural gas. Powder Technol. 2017, 316, 569–577. [Google Scholar] [CrossRef]
- Wolf, J.; Anheden, M.; Yan, J. Comparison of nickel- and iron-based oxygen carriers in chemical looping combustion for CO2 capture in power generation. Fuel 2005, 84, 993–1006. [Google Scholar] [CrossRef]
- Consonni, S.; Lozza, G.; Pelliccia, G.; Rossini, S.; Saviano, F. Chemical-Looping Combustion for Combined Cycles with CO2 Capture. J. Eng. Gas Turbines Power 2006, 128, 525–534. [Google Scholar] [CrossRef]
- Li, T.; Dietiker, J.-F.; Shahnam, M. MFIX simulation of NETL/PSRI challenge problem of circulating fluidized bed. Chem. Eng. Sci. 2012, 84, 746–760. [Google Scholar] [CrossRef]
- Gidaspow, D. Multiphase Flow and Fluidization: Continuum and Kinetic Theory Descriptions; Academic Press: Cambridge, MA, USA, 1994. [Google Scholar]
- Lun, C.K.K.; Savage, S.B.; Jeffrey, D.J.; Chepurniy, N. Kinetic theories for granular flow: Inelastic particles in Couette flow and slightly inelastic particles in a general flowfield. J. Fluid Mech. 1984, 140, 223–256. [Google Scholar] [CrossRef]
- Wen, C.Y. Mechanics of fluidization. Chem. Eng. Prog. Symp. Ser. 1966, 62, 100–111. [Google Scholar]
- Ergun, S. Fluid flow through packed columns. Chem. Eng. Prog. 1952, 48, 89–94. [Google Scholar]
- Benyahia, S.; Syamlal, M.; O’brien, T. Summary of MFIX Equations 2012. Available online: https://mfix.netl.doe.gov/doc/mfix-archive/mfix_current_documentation/MFIXEquations2012-1.pdf (accessed on 29 December 2021).
- Syamlal, M. MFIX documentation numerical technique. MFIX Doc. Numer. Tech. 1998, 5824, 80. [Google Scholar] [CrossRef] [Green Version]
- Syamlal, M.; Rogers, W.; O’brien, T.J. MFIX documentation theory guide. MFIX Doc. Theory Guide 1993. [Google Scholar] [CrossRef] [Green Version]
- Cabello, A.; Abad, A.; García-Labiano, F.; Gayán, P.; De Diego, L.F.; Adánez, J. Kinetic determination of a highly reactive impregnated Fe2O3/Al2O3 oxygen carrier for use in gas-fueled chemical looping combustion. Chem. Eng. J. 2014, 258, 265–280. [Google Scholar] [CrossRef] [Green Version]
- Mora, J.C.; Nederstigt, Y.C.M.; Hill, J.M.; Ponnurangam, S. Promoting Effect of Supports with Oxygen Vacancies as Extrinsic Defects on the Reduction of Iron Oxide. J. Phys. Chem. C 2021, 125, 14299–14310. [Google Scholar] [CrossRef]
- Abad, A.; Adánez, J.; Cuadrat, A.; García-Labiano, F.; Gayán, P.; De Diego, L.F. Kinetics of redox reactions of ilmenite for chemical-looping combustion. Chem. Eng. Sci. 2011, 66, 689–702. [Google Scholar] [CrossRef] [Green Version]
- Abanades, J.C.; Abdel-Ghani, M.; Abdelkarim, A.; Abe, E.; Abe, Y.; Abouzeid, A.Z. Cumulative Author Index of Volumes 50–75. Powder Technol. 1993, 75, 209–216. [Google Scholar]
- Leonard, B.P.; Mokhtari, S. Beyond first-order upwinding: The ultra-sharp alternative for non-oscillatory steady-state simulation of convection. Int. J. Numer. Methods Eng. 1990, 30, 729–766. [Google Scholar] [CrossRef]
- Patankar, S.V. Numerical Heat Transfer and Fluid Flow; Hemisphere Publishing: New York, NY, USA, 1980; ISBN 9781315275130. [Google Scholar]
- Spalding, D.B. Numerical computation of multi-phase fluid flow and heat transfer. In Von Karman Institute for Fluid Dynamics Numerical Computation of Multi-Phase Flows; Imperial College: London, UK, 1981; pp. 161–191. [Google Scholar]
- Harlow, F.H.; Amsden, A.A. Numerical calculation of multiphase fluid flow. J. Comput. Phys. 1975, 17, 19–52. [Google Scholar] [CrossRef]
- Rivard, W.; Torrey, M. K-FIX: A Computer Program for Transient, Two-Dimensional, Two-Fluid Flow; Los Alamos Scientific Lab: Los Alamos, NM, USA, 1976. [Google Scholar]
- Kotteda, V.M.K.; Kumar, V.; Spotz, W. Performance of preconditioned iterative solvers in MFiX–Trilinos for fluidized beds. J. Supercomput. 2018, 74, 4104–4126. [Google Scholar] [CrossRef]
- Van der Vorst, H.A. Bi-CGSTAB: A fast and smoothly converging variant of Bi-CG for the solution of nonsymmetric linear systems. SIAM J. Sci. Stat. Comput. 1992, 13, 631–644. [Google Scholar] [CrossRef]
- Abad, A.; Adanez, J.; De Diego, L.F.; Gayan, P.; García-Labiano, F.; Lyngfelt, A. Fuel reactor model validation: Assessment of the key parameters affecting the chemical-looping combustion of coal. Int. J. Greenh. Gas Control 2013, 19, 541–551. [Google Scholar] [CrossRef] [Green Version]
- Abad, A.; Gayán, P.; De Diego, L.F.; García-Labiano, F.; Adánez, J. Fuel reactor modelling in chemical-looping combustion of coal: 1. Model formulation. Chem. Eng. Sci. 2013, 87, 277–293. [Google Scholar] [CrossRef] [Green Version]
- Abad, A.; Mattisson, T.; Lyngfelt, A.; Johansson, M. The use of iron oxide as oxygen carrier in a chemical-looping reactor. Fuel 2007, 86, 1021–1035. [Google Scholar] [CrossRef]
- Peng, Z.; Doroodchi, E.; Alghamdi, Y.; Moghtaderi, B. Mixing and segregation of solid mixtures in bubbling fluidized beds under conditions pertinent to the fuel reactor of a chemical looping system. Powder Technol. 2013, 235, 823–837. [Google Scholar] [CrossRef]
- Pans, M.A.; Gayán, P.; Abad, A.; García-Labiano, F.; De Diego, L.F.; Adánez, J. Use of chemically and physically mixed iron and nickel oxides as oxygen carriers for gas combustion in a CLC process. Fuel Process. Technol. 2013, 115, 152–163. [Google Scholar] [CrossRef] [Green Version]




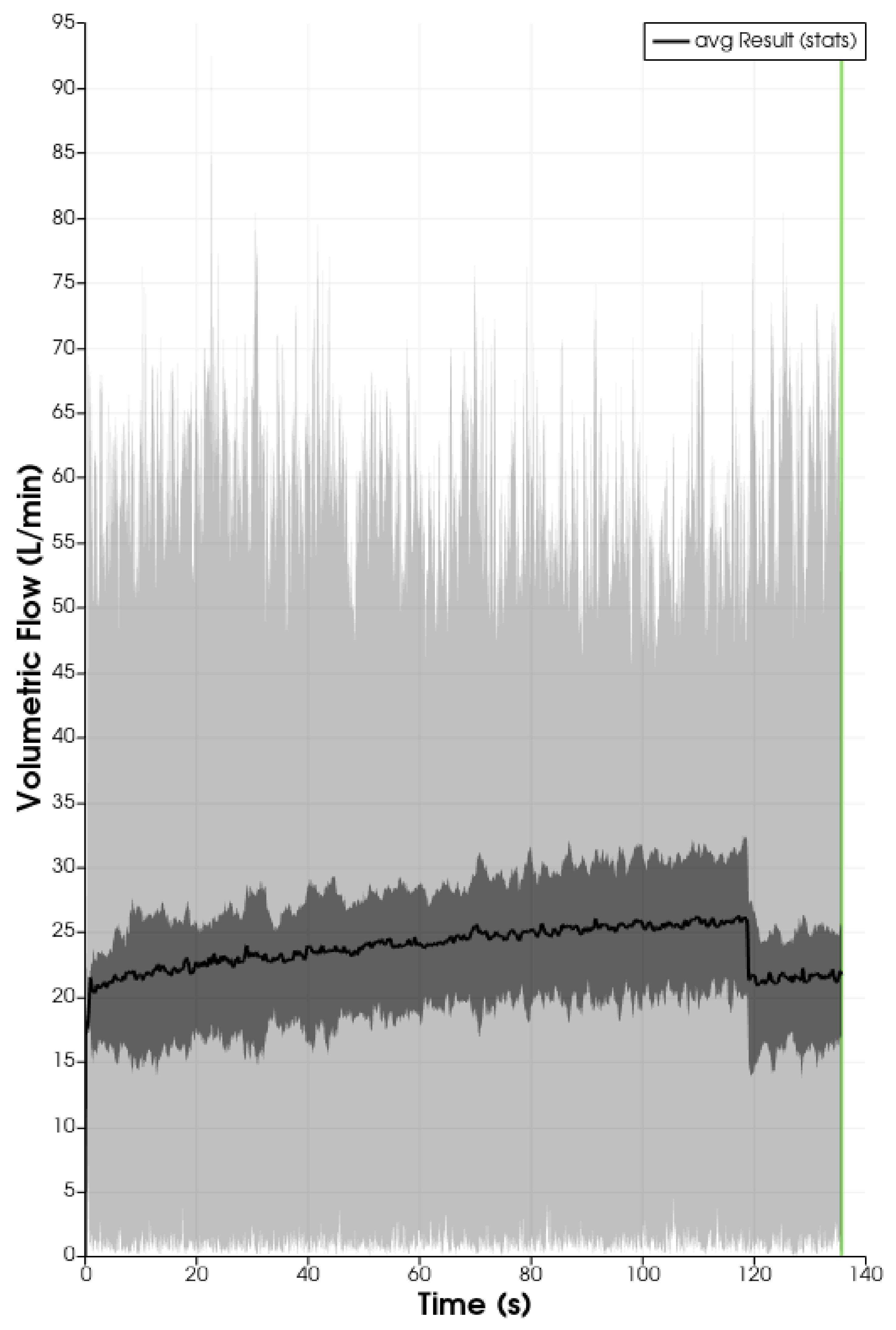

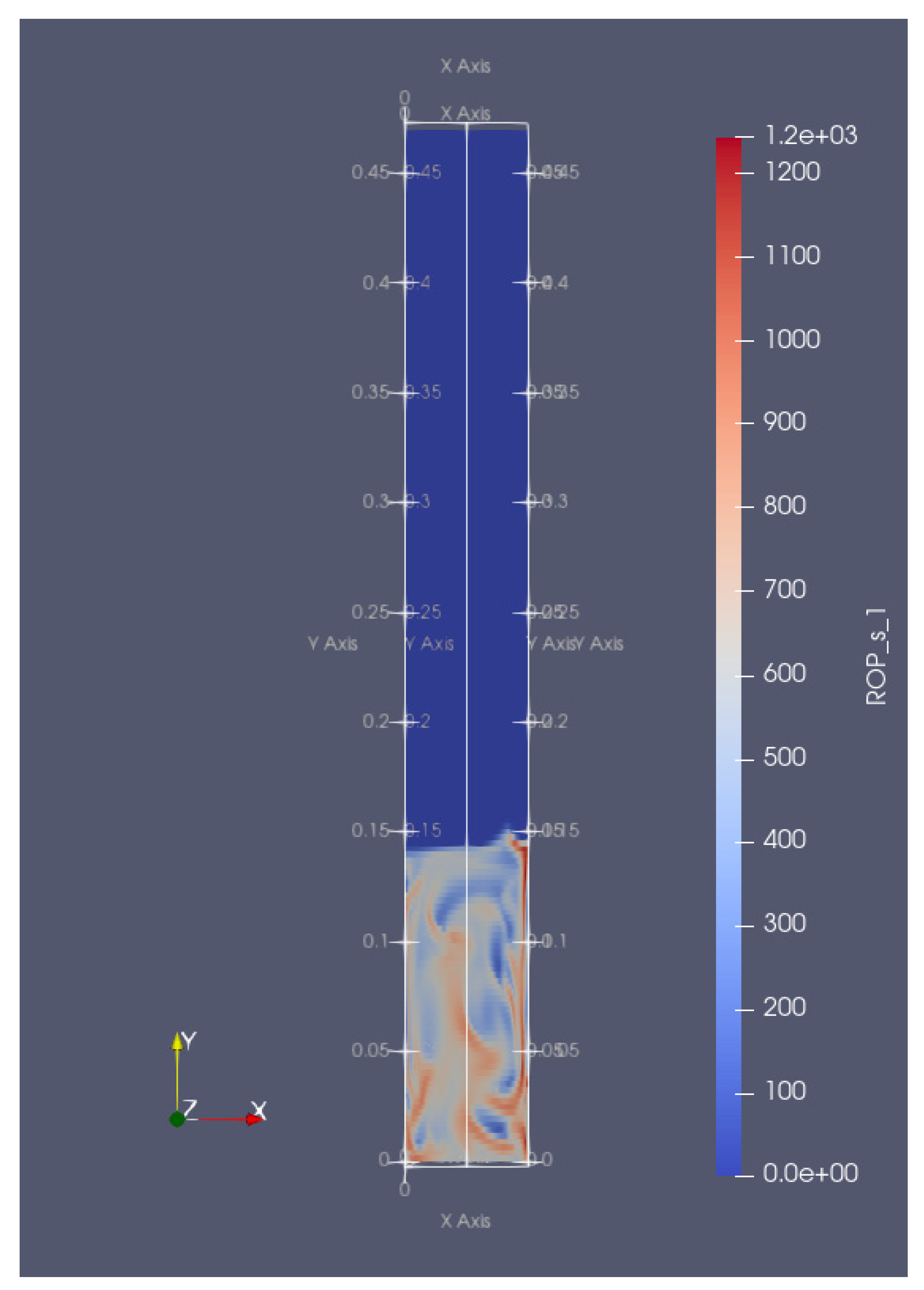
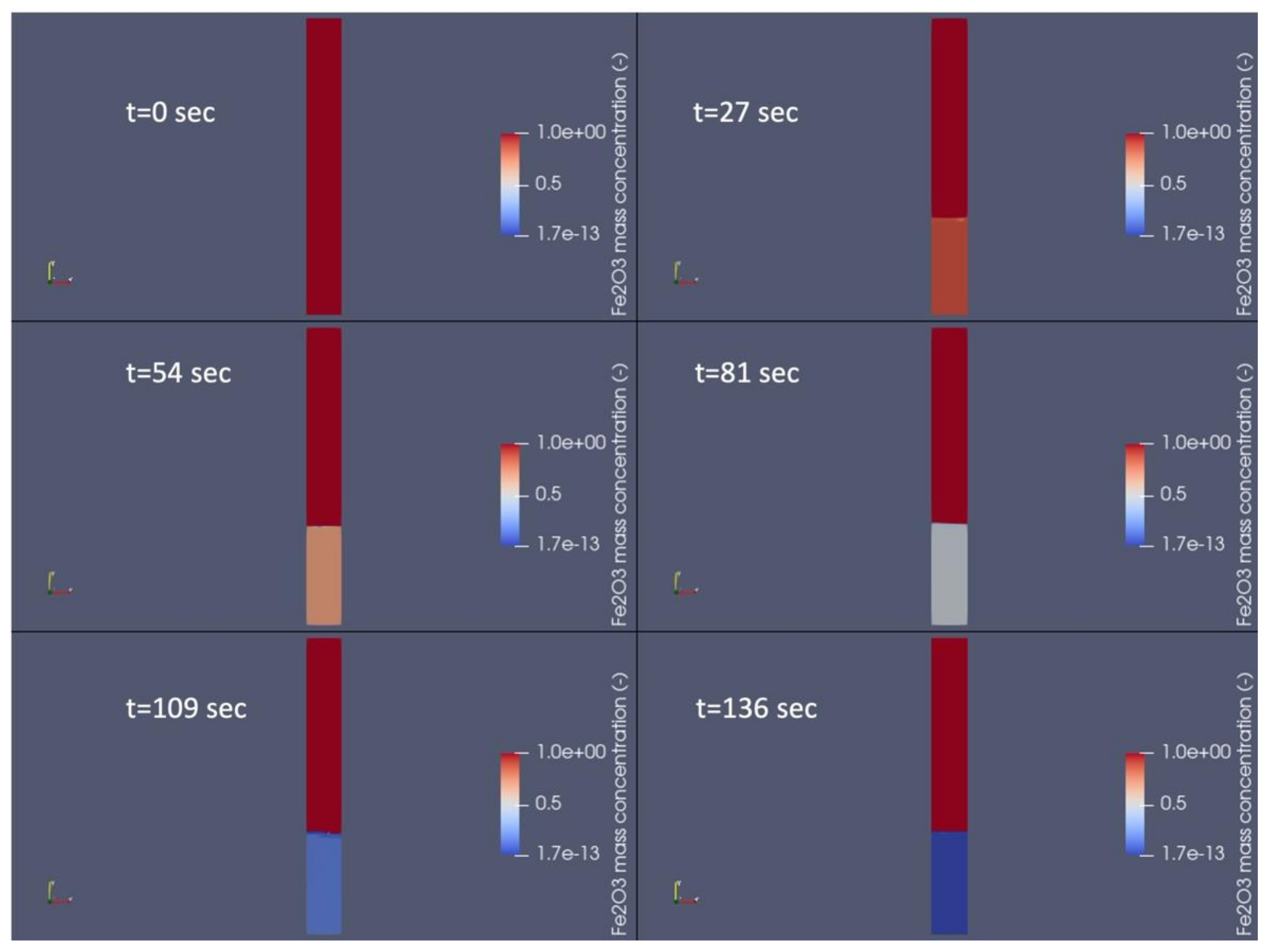
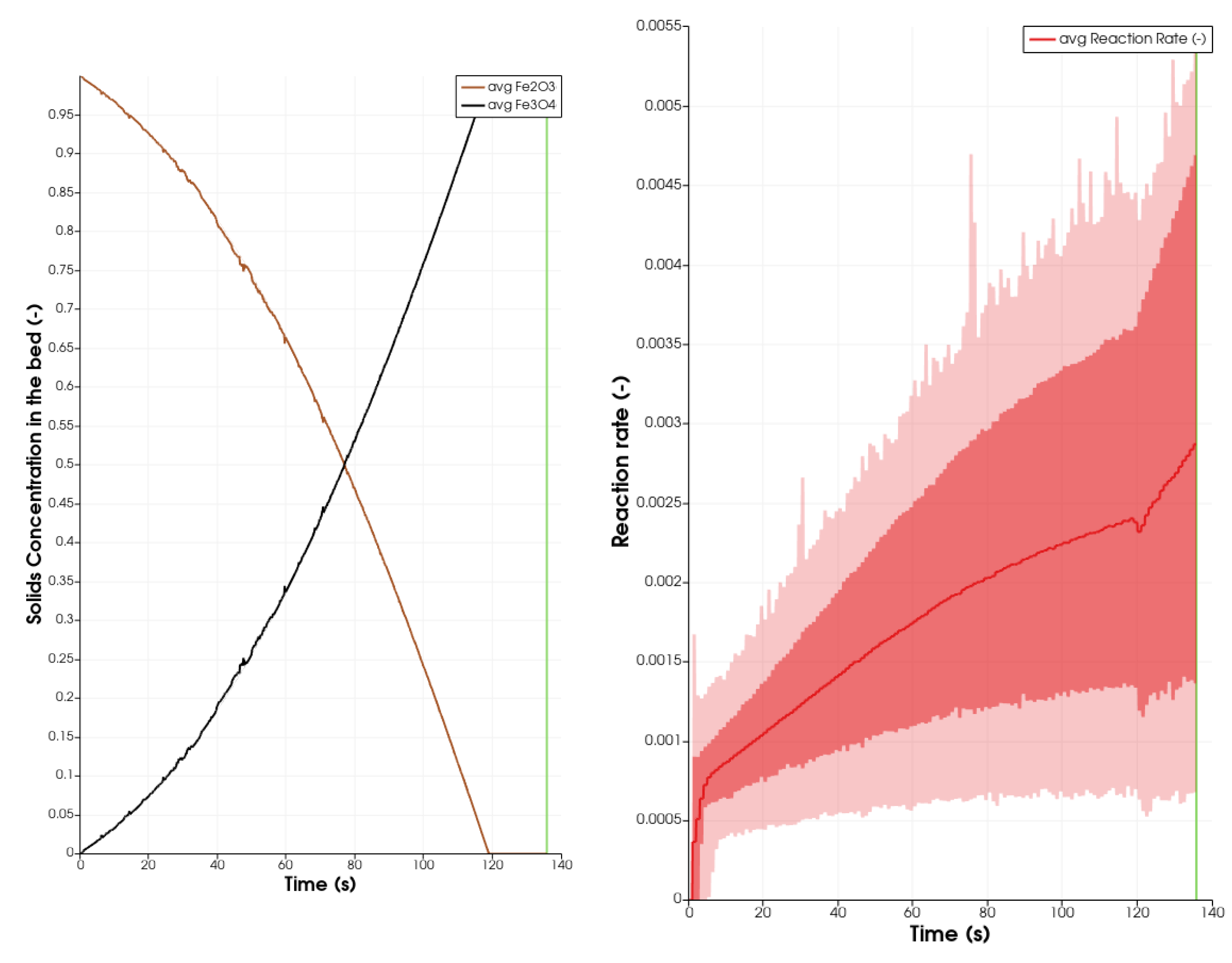
 is taken from [60]
is taken from [60]  is taken from [60]
is taken from [60]  is taken from [60].
is taken from [60].


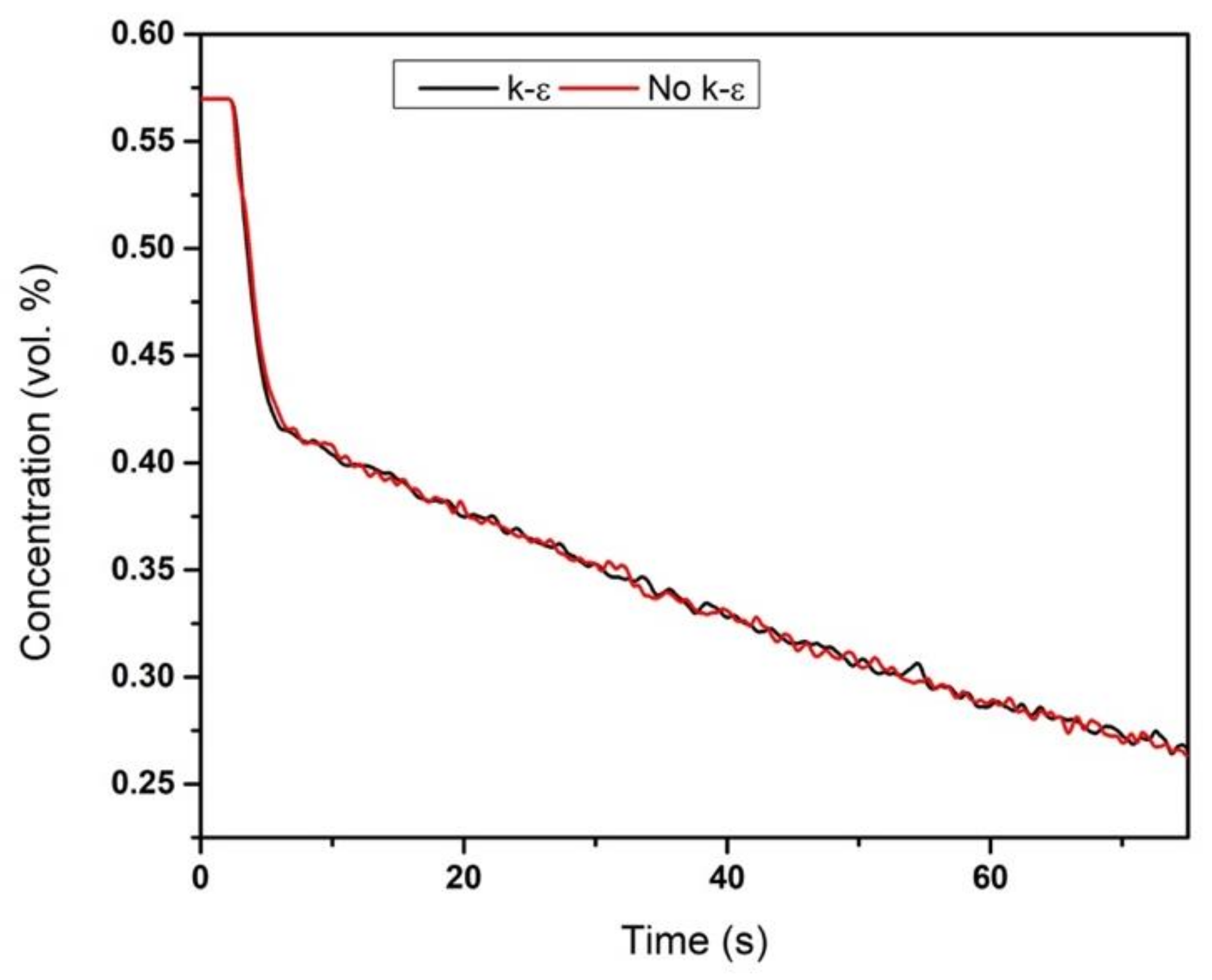
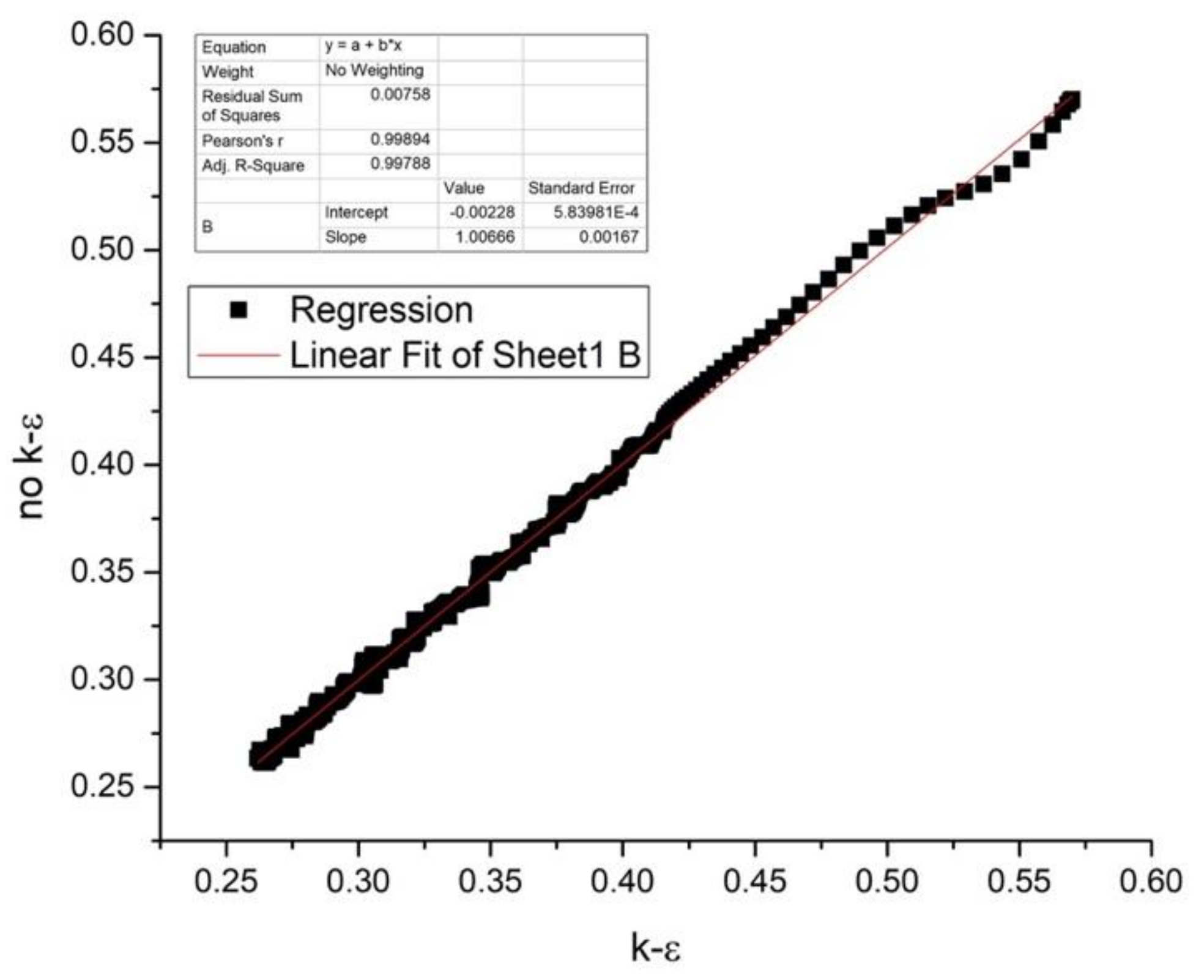
| Group | Source | Software | DEM |
|---|---|---|---|
| Leeds Uni, IFP Energies Nouvelles and Total | [6] | ANSYS FLUENT and EDEM | Yes |
| Singapore NUS | [7] | N.R. | No |
| HUST, China | [8] | CPFD | No |
| Nanjing | [9] | ANSYS FLUENT | No |
| SINTEF | [10] | PFC3D | Yes |
| Washington University | [11] | ANSYS FLUENT | Yes |
| Masdar Institute of Science and Technology | [12] | ANSYS FLUENT | No |
| TU Darmstadt | [13] | ANSYS FLUENT | No |
| Imperial College | [14] | ANSYS FLUENT | No |
| NETL | [15] | Barracuda | No |
| University of New South Wales | [16] | ANSYS FLUENT | No |
| Harriot Watt University | [17] | MFIX | No |
| CPFD Software | [18] | Barracuda-VRTM | No |
| Indian Institute of Technology | [19] | ANSYS FLUENT | No |
| The University of Nottingham | [20] | ANSYS FLUENT | No |
| The University of Newcastle (Australia) | [21] | ANSYS FLUENT | Yes |
| Harbin Institute of Technology | [22] | K-FIX | No |
| Zhejiang University | [23] | MFIX | No |
| University of Utah | [24] | Barracuda-VRTM | No |
| IMFT Tolouse, TU Wien | [25] | NEPTUNE_CFD | No |
| The University of Western Ontario | [26] | Barracuda-VRTM | No |
| KAIST | [27] | ANSYS FLUENT | No |
| University of North Dakota | [28] | MFIX | No |
| Mesh< | Mesh> | |
|---|---|---|
| Scalar Standard Cells | 14,000 | 30,000 |
| Aspect ratio of scalar standard cells | 1 | 1 |
| Parameter | Value | Unit of Measure |
|---|---|---|
| Oxygen carrier | Fe2O3 | - |
| Promoting Support | Al2O3 | - |
| Total Fe2O3 content 1 | 20 | wt% |
| Average Particle size | 200–400 | μm |
| Particle density | 3950 | kgm−3 |
| Porosity | 50.5 | % |
| BET | 39.1 | m2/g |
| Order of reaction | 0.25 | - |
| Pre-exponential factor kinetics | 4.34 × 101 | m3nmol−ns−1 |
| Activation energy kinetics | 66 | kJ/mol |
| Oder of diffusion | - | 0 |
| Pre-exponential factor diffusion | 9.80 × 1030 | m3nmol−ns−1 |
| Activation energy diffusion | 672 | kJ/mol |
| Parameter | Value | Unit of Measure |
|---|---|---|
| Temperature | 950 | °C |
| P | 1 | atm |
| D | 56 | mm |
| h | 470 | mm |
| u0 | 0.10 | m/s |
| ε | 0.5 | - |
| Bh (bed height) | 10 | mm |
| Wall thermal behavior | Adiabatic | - |
| Grid size | 14,000 | cells |
| Solver | Two-fluid model (MFIX-TFM) | - |
| Drag model | Syamlal-O’Brien | - |
| C1 | 0.8 | - |
| D1 | 2.65 | - |
| Momentum formulation | Model A, See Abanades et al. 1993 [49] | - |
| UDF | User Defined Function | - |
| Thermal conductivity of solids | Bauer and Schlünder | - |
| Diffusivity | Dilute mixture approximation (air) | - |
| Pressure outlet | “Pressure Outflow” | 1.0132e + 05 Pa |
| Temporal Discretization | Implicit Euler | - |
|---|---|---|
| Spatial discretization | ||
| Scheme | Relaxation factor | |
| Gas pressure | First-order upwind | 0.8 |
| Volume fraction | First-order upwind | 0.5 |
| U-momentum | First-order upwind | 0.5 |
| V-momentum | First-order upwind | 0.5 |
| W-momentum | First-order upwind | 0.5 |
| Energy | First-order upwind | 1.0 |
| Mass Fraction | First-order upwind | 1.0 |
| Granular Energy | First-order upwind | 0.5 |
| Scalar/k-ε | First-order upwind | 0.8 |
| DES diffusion | First-order upwind | 1.0 |
| Solver | Iterations | Tolerance | |
|---|---|---|---|
| Gas pressure | BICGSTAB | 20 | 0.0001 |
| Volume fraction | BICGSTAB | 20 | 0.0001 |
| U-momentum | BICGSTAB | 5 | 0.0001 |
| V-momentum | BICGSTAB | 5 | 0.0001 |
| W-momentum | BICGSTAB | 5 | 0.0001 |
| Energy | BICGSTAB | 15 | 0.0001 |
| Mass fraction | BICGSTAB | 15 | 0.0001 |
| Granular energy | BICGSTAB | 15 | 0.0001 |
| Scalar/κ-ε | BICGSTAB | 15 | 0.0001 |
| DES diffusion | BICGSTAB | 10 | 0.0001 |
| Preconditioner | Sweep | |
|---|---|---|
| Gas pressure | Line relaxation | Red-black sweep |
| Volume fraction | Line relaxation | Red-black sweep |
| U-momentum | Line relaxation | Red-black sweep |
| V-momentum | Line relaxation | Red-black sweep |
| W-momentum | Line relaxation | Red-black sweep |
| Energy | Line relaxation | Red-black sweep |
| Mass fraction | Line relaxation | Red-black sweep |
| Granular energy | Line relaxation | Red-black sweep |
| Scalar/κ-ε | Line relaxation | Red-black sweep |
| DES diffusion | Line relaxation | Red-black sweep |
| Maximum Inlet-Velocity Factor | 1 |
|---|---|
| Dilute threshold | 0.0001 |
| Minimum tracked solids volume factor | 1E-08 |
| Skip continuing residuals if volume fraction below | 1E-07 |
| Drag under-relaxation factor | 1.0 |
| IA theory conductivity under-relaxation factor | 1.0 |
| Parameter | Value |
|---|---|
| Maximum iterations | 50 |
| Fluid normalization | 0.0 |
| Fluid pressure correction scale factor | 10.0 |
| Solids normalization | - |
| Solids volume fraction correction scale factor | 10.0 |
| Maximum residual at convergence Continuity + momentum Energy Species Granular energy Scalar κ-ε | 0.001 0.0001 0.0001 0.0001 |
| Maximum residual for divergence | 10,000.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartocci, P.; Abad, A.; Cabello, A.; de las Obras Loscertales, M.; Lu, W.; Yang, H.; Fantozzi, F. CFD Modelling of the Fuel Reactor of a Chemical Loping Combustion Plant to Be Used with Biomethane. Processes 2022, 10, 588. https://doi.org/10.3390/pr10030588
Bartocci P, Abad A, Cabello A, de las Obras Loscertales M, Lu W, Yang H, Fantozzi F. CFD Modelling of the Fuel Reactor of a Chemical Loping Combustion Plant to Be Used with Biomethane. Processes. 2022; 10(3):588. https://doi.org/10.3390/pr10030588
Chicago/Turabian StyleBartocci, Pietro, Alberto Abad, Arturo Cabello, Margarita de las Obras Loscertales, Wang Lu, Haiping Yang, and Francesco Fantozzi. 2022. "CFD Modelling of the Fuel Reactor of a Chemical Loping Combustion Plant to Be Used with Biomethane" Processes 10, no. 3: 588. https://doi.org/10.3390/pr10030588







