1. Introduction
Distillation is the most widely used mass transfer operating unit in petroleum refining, petrochemical, and other industrial processes. It is also one of the most energy-intensive unit operations in petrochemicals.
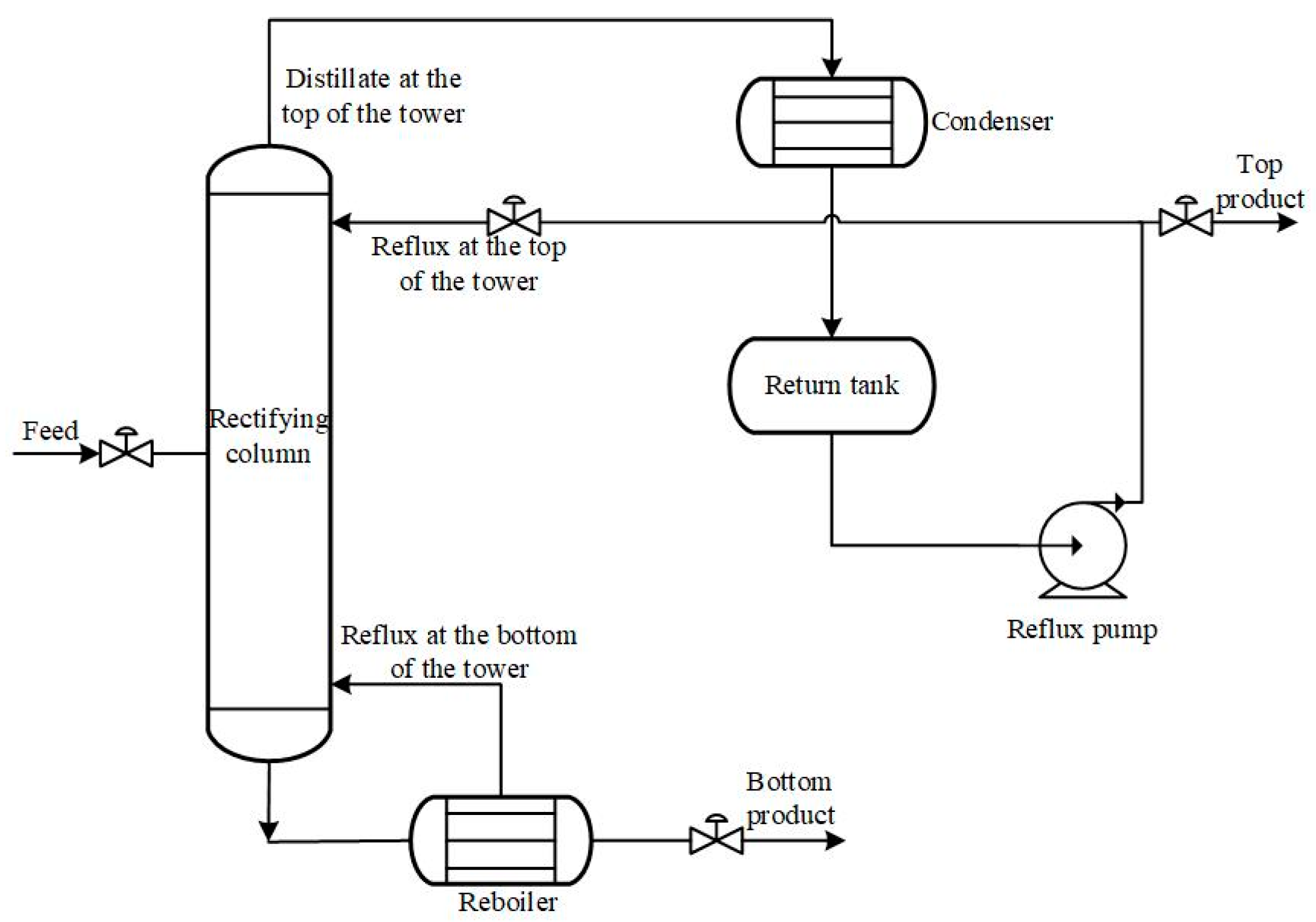
Figure 1 shows the structure of a typical distillation column. The energy consumption of distillation columns accounts for approximately one third of the total energy consumption of chemical plants. Statistics show that approximately 40~50% of the total energy consumption in the petroleum and chemical industry is consumed by the distillation process [
1]. Therefore, improving the efficiency of the distillation process greatly impacts on the energy conservation and emission reduction of the chemical process, which cannot be realized without an efficient modeling and control method [
2,
3].
New process equipment design and effective control scheme are two main aspects of energy-saving research in distillation processes [
4]. Many new types of distillation columns have been designed by researchers, such as the divided-wall column [
5,
6], the heat pump-assisted distillation column [
7,
8], the heat integrated distillation column [
9,
10], the pressure-swing distillation column with heat integration [
11,
12], the heat integrated extractive distillation column [
13,
14], and so on. Most of these columns introduce heat exchange sections to largely reuse the internal thermal cycle. Therefore, the energy utilization rate has been largely improved. However, the heat integration part makes the interaction stronger than traditional columns. Therefore, although the new part can largely improve the energy efficiency, it makes the control design more difficult. In addition, the research on energy-saving technology for processing and equipment has shortcomings, such as long cycles, large investment, and slow replacement, so it is difficult to put into commercial production in a short time [
15]. To realize energy saving rapidly in a distillation process, it is necessary to strengthen the operation control. A highly effective control scheme usually relies on a nonlinear dynamic model, which must be both accurate and simplified enough.
The mechanism model is accurate enough to characterize the dynamic properties of the distillation process, and it is usually represented by differential and algebraic equations. However, a series of nonlinear differential equations with high dimension and rigidity must be solved in the modeling process of a distillation column. The size of the differential equations increases with the number of trays in the distillation column, which increases the difficulty of the online solution to the mechanism model. Therefore, it is difficult to utilize online in a control scheme for the mechanism model [
16].
Many approximate linear models were investigated in distillation control design for the problem above, such as the ARX model [
17], the CARIMA model [
18], the internal transfer function model [
19], the impulse response model [
20], and so on. Manipulated variables can be easily solved out by these linear models in an online control loop. However, this oversimplified model would certainly bring in model mismatch, making the control schemes effective only in a limited operating range [
21]. Once a significant disturbance is involved, the control performance will probably deteriorate. In addition, PID is the most commonly used control method for distillation columns, independent of the process model. However, PID is a linear method and, therefore, cannot process serious nonlinear problems perfectly [
22], which widely exist in distillation columns.
Many researchers aim to establish an appropriate nonlinear simplified model to overcome the disadvantages of both the mechanism model and the linearization method. Among these studies, a nonlinear wave theory has provided a promising way to solve the problems above. The nonlinear wave theory was inspired by the chromatograph study by Hwang and his coworkers [
23], who found that the curve of the concentration on each tray kept a relatively stable shape from the results of both simulation and experimental research. Therefore, the concentration distribution along the entire column could be regarded as a whole, which would essentially decrease the number of state variables to characterize the distillation process. Moreover, these variables could be used to establish a simplified nonlinear dynamic model [
24]. In particular, this simplified nonlinear modeling method shows a decisive advantage in modeling complex chemical processes with self-organizing properties. Based on the above complex nonlinear modeling research, the concentration distribution curve and its movement characteristics in the distillation column are gradually excavated. However, some problems limit the application of this approach. First, the assumption of the stable shape of the concentration curve was established from the simulation and experiment by data analysis [
25]. Second, some unrealistic assumptions were adopted to reduce the application difficulty of the theory. For example, the movement of the concentration curve was regarded as keeping a constant rate, which would not be applicable in most distillation processes. In brief, the nonlinear wave theory provides a promising solution, and some detailed assumptions can be refined to suit actual situations.
In this paper, a nonlinear dynamic model is established according to the equilibrium theory in the distillation column. Based on the equilibrium relationships in the distillation separation process, we can derive that the concentration distribution curve has a fixed descriptive function form, consistent with the simulation and experiment by Hwang. Therefore, the idea of taking the concentration distribution curve as a whole has been supported by the separation mechanism. In addition, the movement of the concentration distribution curve is re-described without the assumption of a constant moving rate, which would be more practical than the traditional nonlinear wave theory. Based on the concentration curve describing function and the moving rate description, a nonlinear dynamic model can be established. The proposed model can not only accurately describe the characteristics of the system, but also greatly simplify the structure compared with the mechanism model. Finally, a generic model control (GMC) scheme based on the proposed nonlinear model is designed for the distillation column. In the case study, the GMC controller performs better than a traditional PID control method. GMC shows good performance in both servo and regulation control. Experimental results demonstrate the effectiveness of the proposed nonlinear distillation column model and the model-based control scheme.
2. Modeling of the Distillation Column Based on the Equilibrium Theory
In the nonlinear wave theory mentioned above, the concentration distribution in the distillation column can be regarded as a whole. When the operating condition changes, the concentration curve moves along the column and behaves like wave profile propagation. For example,
Figure 2 shows the movement of the concentration distribution curve along the distillation column when the feed composition
Zf increases by 5% in a distillation column with 10 trays. After the feed composition changes, the original profile 1 moves to the right in the column and finally stops at the position of profile 2, which represents a new steady state. The profile shape of the curve keeps a relatively stable structure during the process. The moving process of the concentration distribution curve represents the entire system’s dynamic response process after the change of operating conditions.
Essentially, the motion of the concentration distribution curve is a symptom of the mass transfer in the separation process.
Figure 3 is a schematic diagram of the balanced relationship of a single tray. A basic assumption is adopted in the tray model that the gas and liquid phases are uniformly mixed and in thermodynamic equilibrium. The droplets in the gas phase, the bubbles in the liquid phase, and the liquid phase flowing down through the main section are ignored. The dynamics in the downpipe are not considered.
If there are enough trays in a plate distillation column, it can be assumed that the structure of the plate distillation column is similar to that of a packed column [
26]. Therefore, the mass transfer partial differential equation in a minimal unit volume in the distillation process can be regarded as follows:
where
x and
y are liquid and gas phase concentrations, respectively.
DT is the dimensionless mass transfer coefficient,
L is the liquid molar flow rate,
V is the gas molar flow rate,
describes the gas-liquid equilibrium relationship, and
u is the dimensionless space coordinate variable.
Suppose that the process is in a quasi-steady state where
∂x/∂t = 0. Then, transform the original coordinate system into a
coordinate system with a velocity of
v to better explain the idea of the concentration curve and its motion as a whole. Let the coordinates transform as follows:
Substituting Equations (3) and (4) into Equations (1) and (2), we can get:
where
is the origin of the new coordinate of
.
According to Equations (5) and (6), the following equation can be obtained:
where
C1 and
C2 are the constant terms generated by the indefinite integration of Equations (5) and (6). To simplify the derivation, a second-order polynomial
y*(x) =
ax2 +
bx +
c is used to approximate the gas-liquid equilibrium relation
y*(
x). The integrand in Equation (7) can be expressed in a concise form:
where
,
, and
are equivalent parameters generated by factorization. Therefore, the analytical solution of the concentration distribution can be integrated by plugging Equation (8) into Equation (7):
where
is a constant term generated by the integration.
According to Equation (9), the concentration distribution of each tray has a fixed descriptive function form. The parameters (
,
,
and
) in the formula would certainly vary with time during a dynamic process, but the formula structure does not change. Now, we transform the system from the new coordinate to the old one. Letting
,
β =
q3(
q2 −
q1), we can obtain the description function of the concentration distribution:
In the process of distillation separation, the middle part of the profile shape in the dynamic process is basically in a quasi-steady state, and
q4 changes little over time. Therefore, the moving velocity of the distribution curve can be represented by the velocity of the position
S (
S is called the inflection point of the concentration distribution curve):
The distribution parameters,
q1,
q2, and
β, have practical physical significance in the distribution description function.
q1 and
q2 are the maximum and minimum approximations of the distribution description function, and
β represents the slope of the inflection point. Therefore, the concentration distribution description function of the rectifying and stripping sections in a plate column can be obtained as follows:
where
is the estimated concentration value on the
jth tray.
Xr_max,
Xr_min,
kr,
Xs_max,
Xs_min, and
ks are the concentration distribution parameters.
f is the feed tray, and
n is the total number of trays. Note that the functions of the rectifying and stripping sections are described separately since the feed may influence the continuity of the concentration curve.
The parameters of the concentration distribution have physical significance, as mentioned above.
Xr_max and
Xs_max are the maximum approximate concentrations of the rectifying and stripping sections, respectively.
Xr_min and
Xs_min are the minimum approximate concentrations of the rectifying section and the stripping section, respectively.
kr and
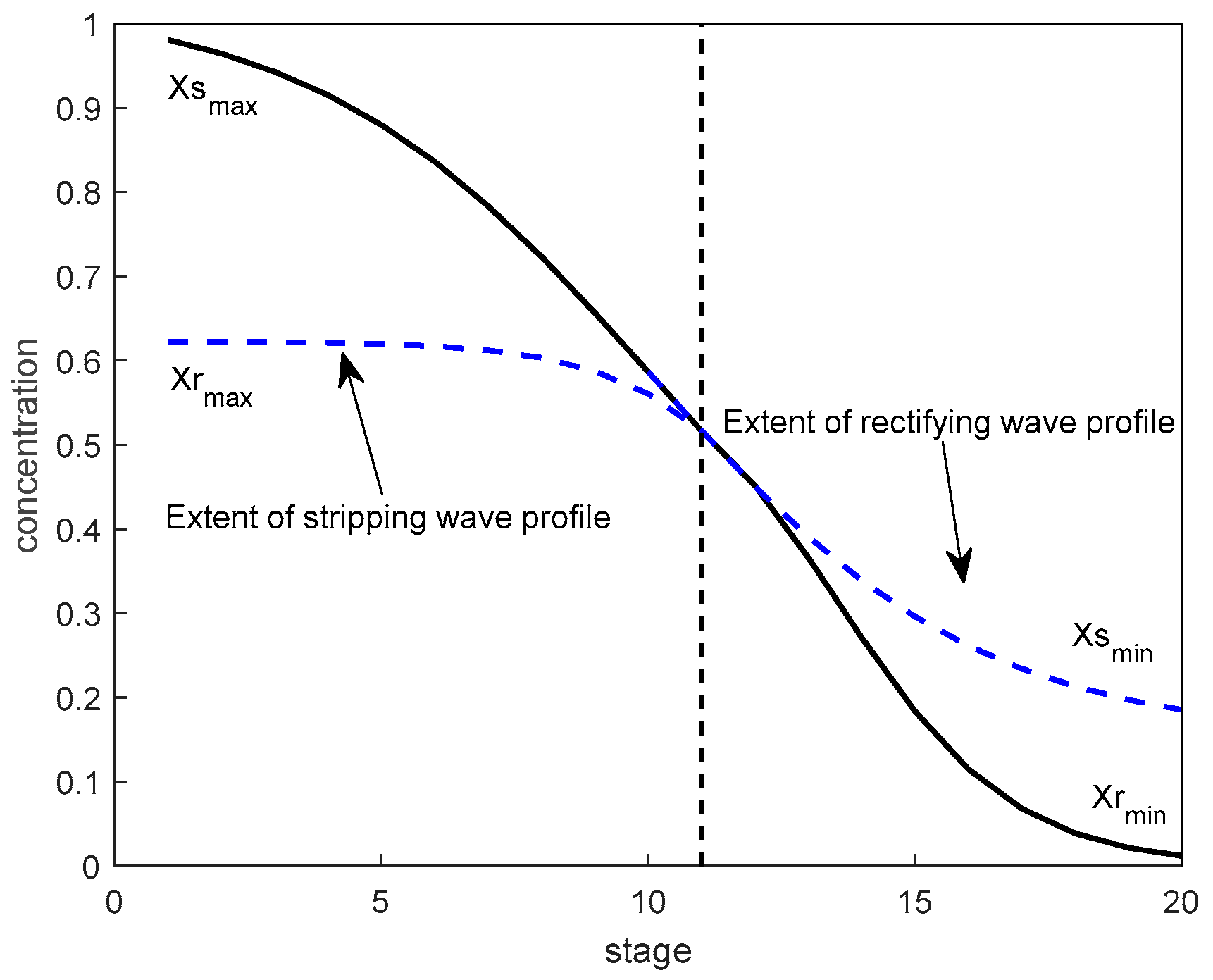
ks represent the slope at the inflection point. For an intuitive representation, Case 2 in
Section 3 is taken as an example here to describe the concentration distribution in the initial steady state and its expansion curve in the rectifying section and the stripping section, as shown in
Figure 4.
Figure 5 shows the estimated errors of liquid component concentrations predicted by Equations (12) and (13). The parameters are determined by a simple least squares method. The largest prediction error is approximately 1 × 10
−3, mainly around the feed tray with a strong interference factor. The results show that the solution by Equations (12) and (13) can be used to describe the shape of the distribution curve with relatively high precision.
By differentiating both sides of Equations (12) and (13), the relation between the component concentration and the moving velocity of the distribution curve (the velocity of the inflection points) can be obtained:
where we ignore the variation with time of the parameters since they change very slow during a dynamic process.
The material conservation relationships of each tray of the distillation column are shown as follows:
where
M1,
M, and
MB are the liquid holdings of the top of the column, each tray, and the bottom of the column, respectively,
F is the feed flow rate, and
Zf is the feed composition. The liquid flow rate is
L and the gas flow rate is
V.
D and
W are the product quantities of the top and bottom of the column, respectively, and
Y is the concentration of the light component in the gas phase.
The gas-liquid molar flow rate is calculated according to mass conservation as follows:
where
V1 is the gas molar flow rate in the stripping section,
L1 is the liquid molar flow rate in the stripping section, and
q is feed thermal condition.
The calculation formula of the product quantity at the top and bottom of the column is:
where
R is the rflux ratio.
The concentration of the light component in the gas phase can be calculated by the gas-liquid equilibrium relationship in an ideal mixture separation system:
where, α is the relative volatility.
We plug Equations (14) and (15) into Equations (16)–(20), and the trays in the rectifying section and the stripping section are summed up, respectively. Then, the moving velocity formula of the concentration distribution curve of the distillation column can be obtained through an algebraic transformation as follows:
In conclusion, the nonlinear dynamic model of a distillation column can be established based on the equilibrium theory, including the moving velocity of the concentration distribution curve (Equations (27) and (28)), the description functions of the concentration distribution curve (Equations (12) and (13)), the gas-liquid equilibrium (Equation (26)), the gas-liquid molar flow calculation (Equations (21)–(23)), and the product quantity at the top and bottom trays of the distillation column (Equations (24) and (25)). The simulation is carried out in Matlab 2019b. In the simulation solving process, the initial values of critical variables should be provided first, such as the position of the inflection point and the profile parameters. Then, based on the algebraic and differential equations listed above, a basic Euler method can be applied to solve this DAE problem.
The proposed nonlinear dynamic model removes the material conservation differential equations compared with the mechanism model. Two differential variables are added, namely the velocities of the inflection points of the rectifying section and the stripping section. The specific comparison is shown in
Table 1. Reducing the number of differential equations and variables simplifies the structure of the nonlinear model, making online solution and control design more convenient.