A Simple Strategy for the Simultaneous Determination of Dopamine, Uric Acid, L-Tryptophan and Theophylline Based on a Carbon Nano-Onions Modified Electrode
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Reagents
2.2. Apparatus
2.3. Fabrication of the CNOs
2.4. Fabrication of the CNOs/GCE
3. Results and Discussion
3.1. Surface Characterization of the CNOs
3.2. Electrochemical Reactivity of the CNOs/GCE Electrode
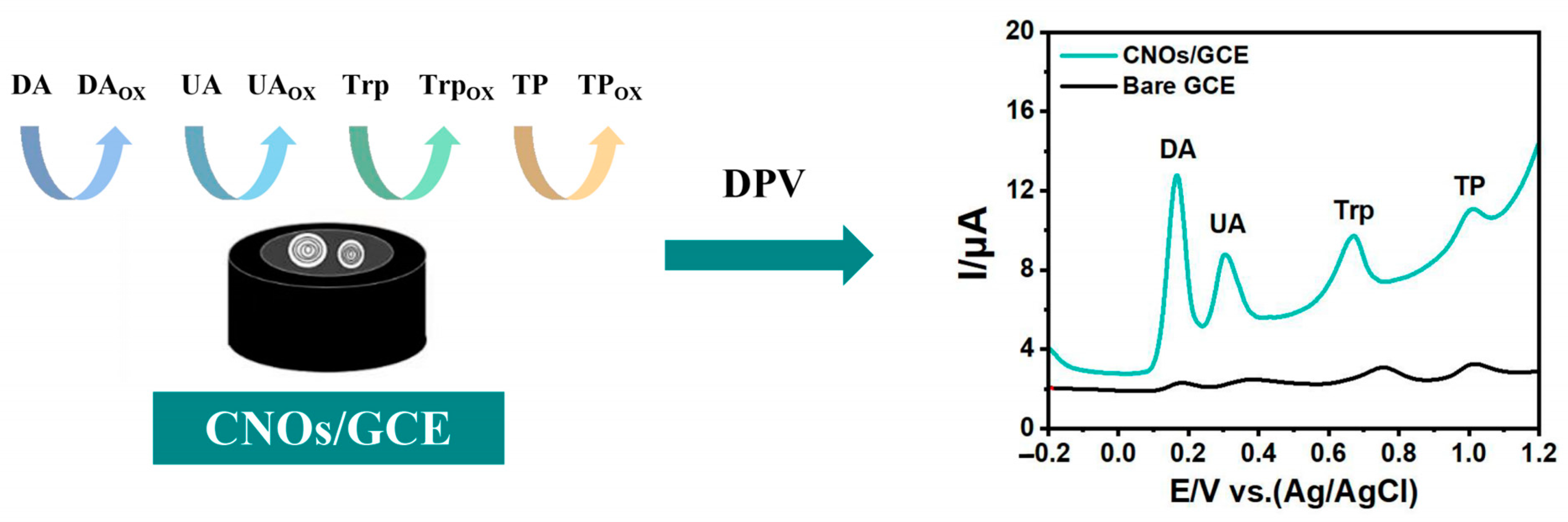
3.3. Electrochemical Performance of CNOs/GCE for DA, UA, Trp and TP
3.4. Optimization of Parameters
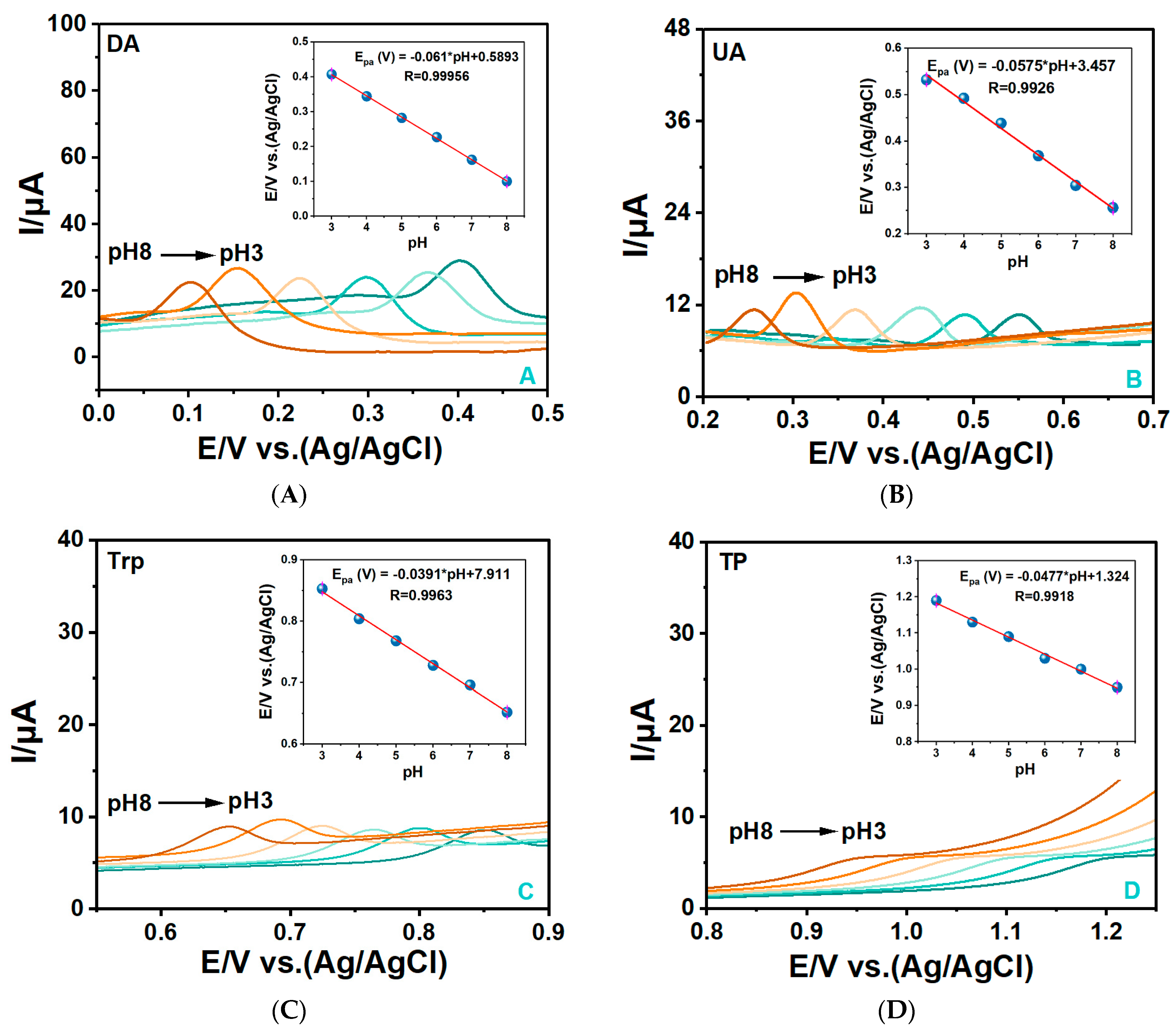
3.4.1. Investigation of the pH Effects
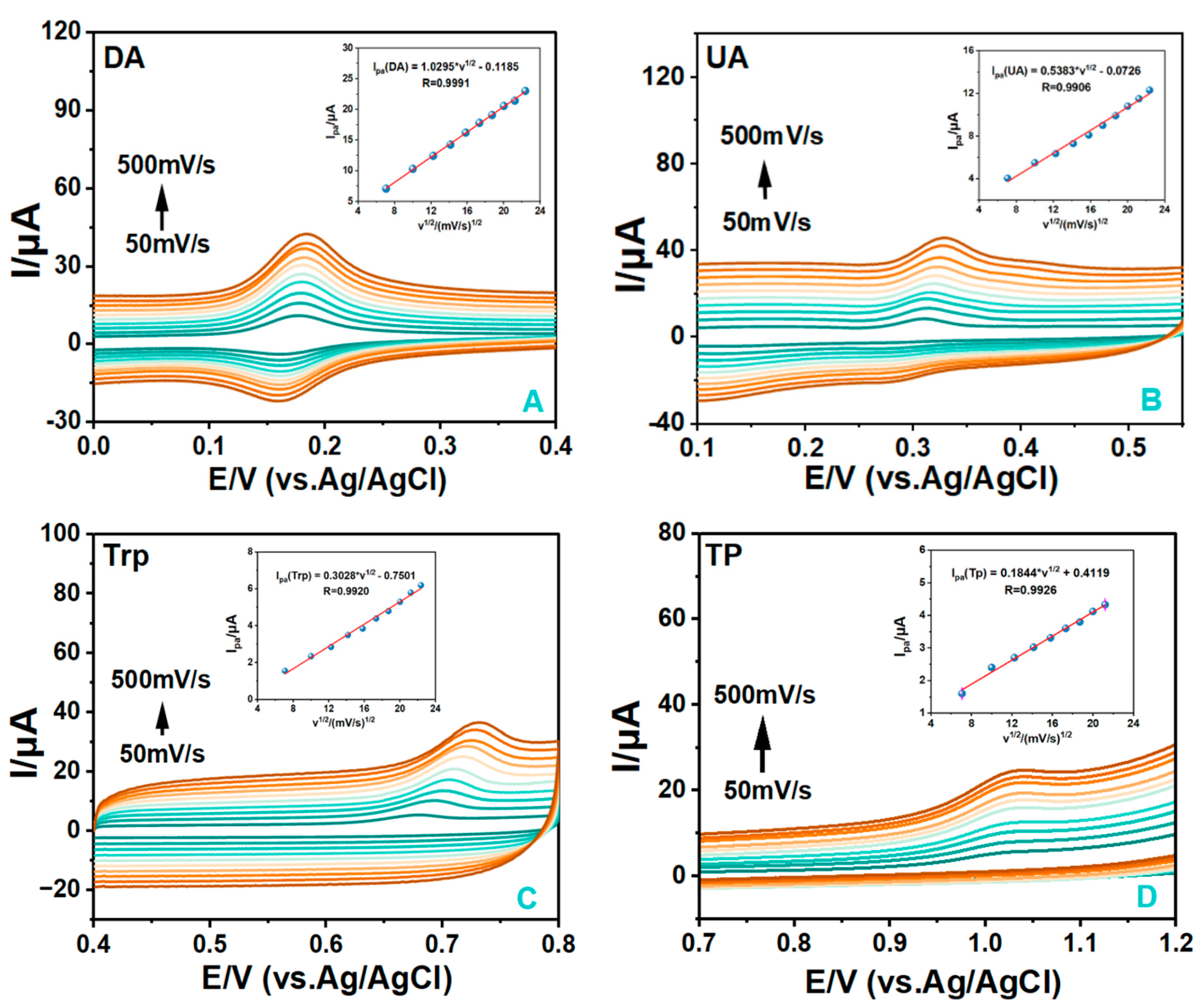
3.4.2. The Effect of Scan Rates
3.4.3. Individual and Simultaneous Determination of DA, UA, Trp and TP
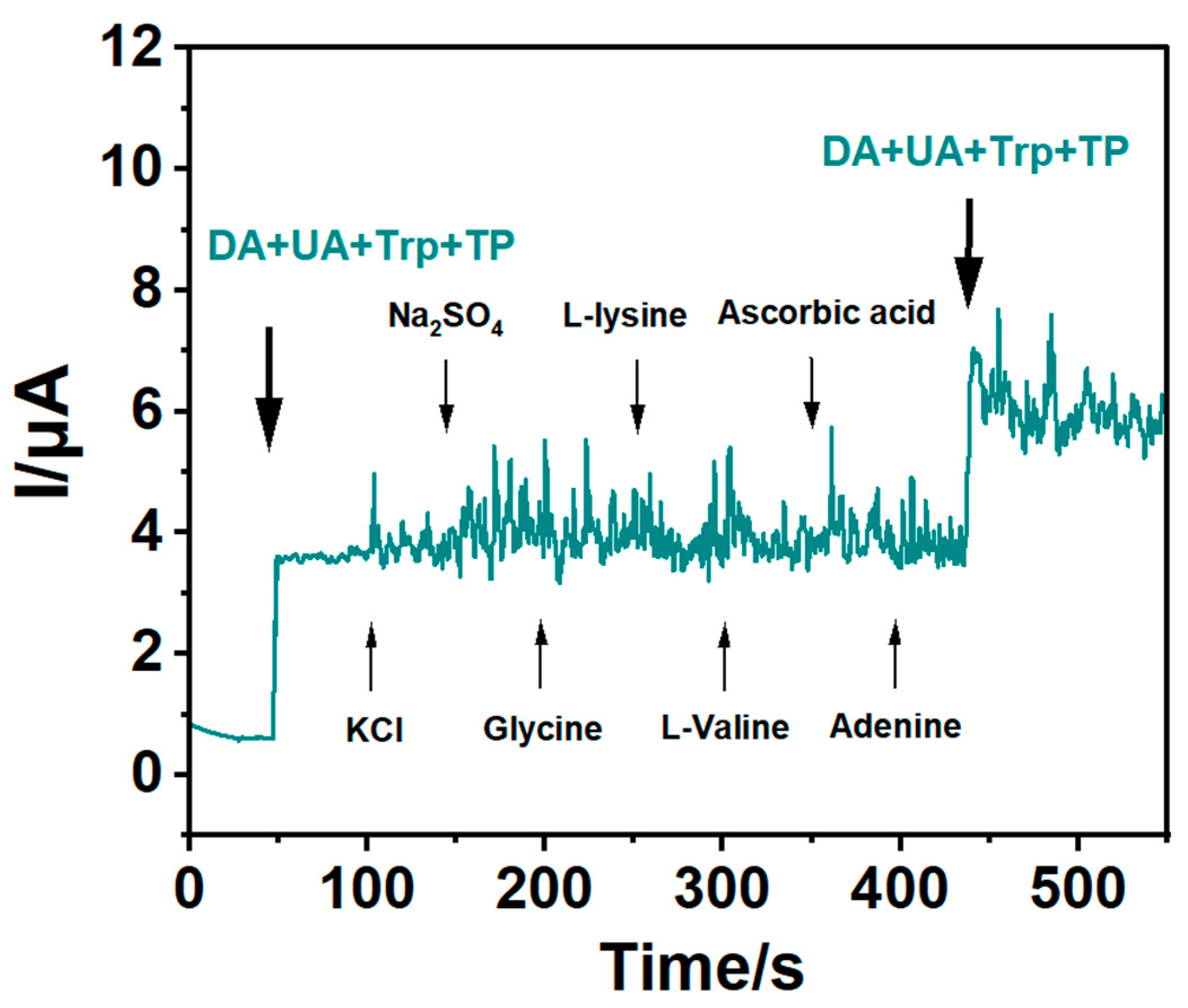
3.5. Interference Studies
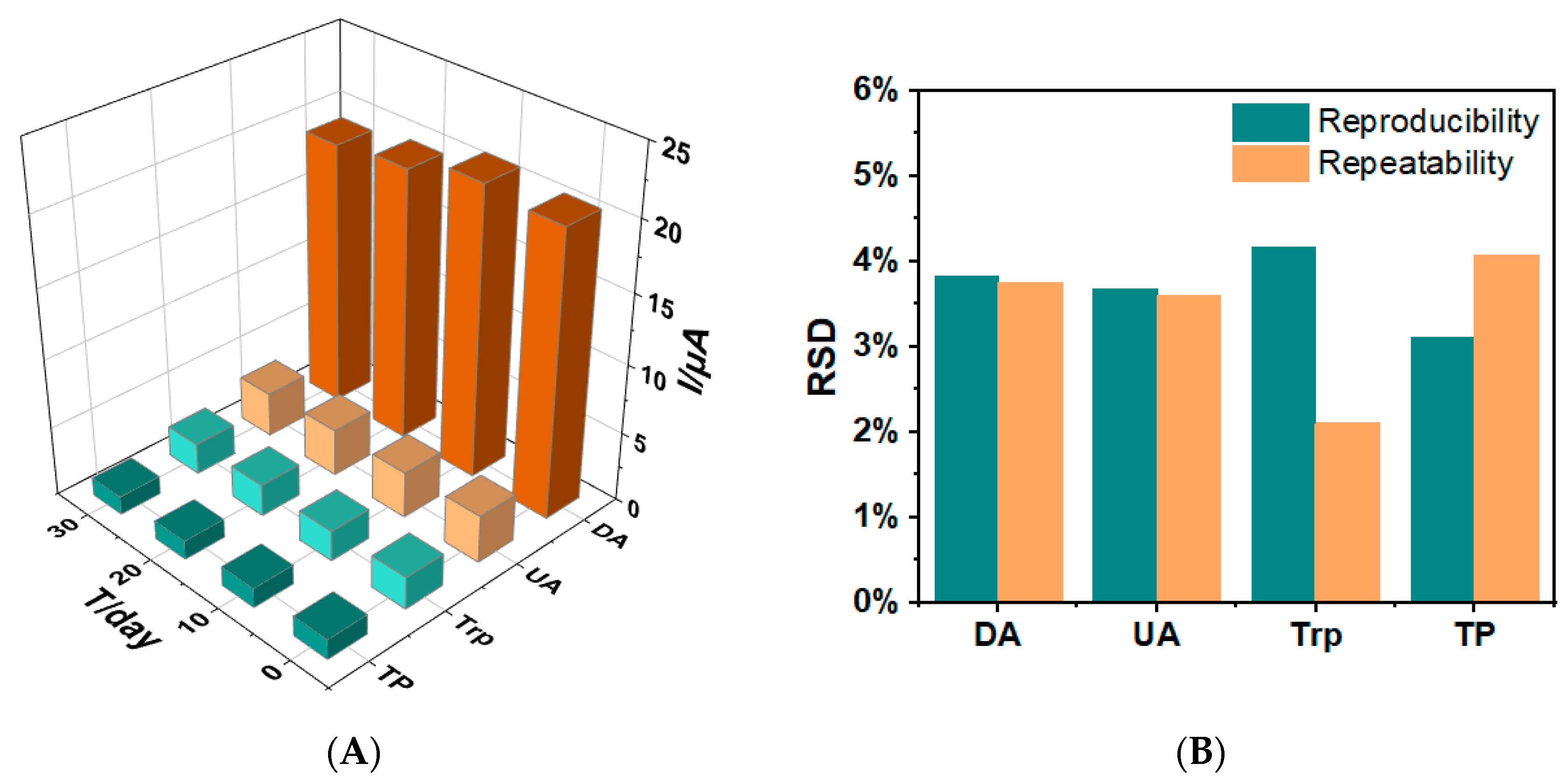
3.6. Reproducibility, Repeatability and Stability Studies
3.7. Real Samples Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Armstrong, M.; Okun, M. Diagnosis and Treatment of Parkinson Disease: A Review. JAMA 2020, 323, 548–560. [Google Scholar] [CrossRef] [PubMed]
- Dhanasekaran, T.; Padmanaban, A.; Gnanamoorthy, G.; Manigandan, R.; Kumar, S.P.; Stephen, A.; Narayanan, V. Recent Advances in Polymer Supporting Layered Double Hydroxides Nanocomposite for Electrochemical Biosensors. Mater. Res. Express 2018, 5, 014011. [Google Scholar] [CrossRef]
- Howes, O.D.; McCutcheon, R.; Owen, M.J.; Murray, R.M. The Role of Genes, Stress, and Dopamine in the Development of Schizophrenia. Biol. Psychiatry 2017, 81, 9–20. [Google Scholar] [CrossRef]
- Dutta, A.; Gautam, R.; Chatterjee, S.; Ariese, F.; Sikdar, S.K.; Umapathy, S. Ascorbate Protects Neurons against Oxidative Stress: A Raman Microspectroscopic Study. ACS Chem. Neurosci. 2015, 6, 1794–1801. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.-L.; Niu, C.; Chen, Q. Structure and component analysis of uratoma around joint. J. Reg. Anat. Oper. Surg. 2019, 28, 6–9. [Google Scholar]
- Shaidarova, L.G.; Chelnokova, I.A.; Il’ina, M.A.; Leksina, Y.A.; Budnikov, H.C. Amperometric Detection of Tryptophane and Pyridoxine on a Dual Screen-Printed Electrode Modified by Gold Nanoparticles in a Flow-Injection System. J. Anal. Chem. 2019, 74, 584–590. [Google Scholar] [CrossRef]
- Tajik, S.; Taher, M.A.; Beitollahi, H. Application of a New Ferrocene-Derivative Modified-Graphene Paste Electrode for Simultaneous Determination of Isoproterenol, Acetaminophen and Theophylline. Sens. Actuators B Chem. 2014, 197, 228–236. [Google Scholar] [CrossRef]
- Luo, X.; Chen, L.; Yang, J.; Li, S.; Li, M.; Mo, Q.; Li, Y.; Li, X. Electrochemically Simultaneous Detection of Ascorbic Acid, Sulfite and Oxalic Acid on Pt-Pd Nanoparticles/Chitosan/Nitrogen Doped Graphene Modified Glassy Carbon Electrode: A Method for Drug Quality Control. Microchem. J. 2021, 169, 106623. [Google Scholar] [CrossRef]
- Bettazzi, F.; Ingrosso, C.; Sfragano, P.S.; Pifferi, V.; Falciola, L.; Curri, M.L.; Palchetti, I. Gold Nanoparticles Modified Graphene Platforms for Highly Sensitive Electrochemical Detection of Vitamin C in Infant Food and Formulae. Food Chem. 2021, 344, 128692. [Google Scholar] [CrossRef]
- Huang, H.; Yue, Y.; Chen, Z.; Chen, Y.; Wu, S.; Liao, J.; Liu, S.; Wen, H. Electrochemical Sensor Based on a Nanocomposite Prepared from TmPO4 and Graphene Oxide for Simultaneous Voltammetric Detection of Ascorbic Acid, Dopamine and Uric Acid. Microchim. Acta 2019, 186, 189. [Google Scholar] [CrossRef]
- Taei, M.; Jamshidi, M.S. A Voltammetric Sensor for Simultaneous Determination of Ascorbic Acid, Noradrenaline, Acetaminophen and Tryptophan. Microchem. J. 2017, 130, 108–115. [Google Scholar] [CrossRef]
- Shi, L.; Wu, N.; Liu, W.; Yang, G.; Wang, Z. Electrochemical Determination of Ascorbic Acid, Dopamine and Uric Acid at Polyaniline-Graphene Nanocomposites Modified Electrode. Int. J. Electrochem. Sci. 2021, 16, 210556. [Google Scholar] [CrossRef]
- Peng, X.; Xie, Y.; Du, Y.; Song, Y.; Chen, S. Simultaneous Detection of Ascorbic Acid, Dopamine and Uric Acid Based on Vertical N-Doped Carbon Nanosheets/Three-Dimensional Porous Carbon. J. Electroanal. Chem. 2022, 904, 115850. [Google Scholar] [CrossRef]
- Jiang, Y.; Xiao, X.; Li, C.; Luo, Y.; Chen, S.; Shi, G.; Han, K.; Gu, H. Facile Ratiometric Electrochemical Sensor for In Vivo/Online Repetitive Measurements of Cerebral Ascorbic Acid in Brain Microdiaysate. Anal. Chem. 2020, 92, 3981–3989. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Ding, D.; Wang, J.; Lin, X.; Diao, G. Three-Dimensional Nitrogen-Doped Graphene-Based Metal-Free Electrochemical Sensors for Simultaneous Determination of Ascorbic Acid, Dopamine, Uric Acid, and Acetaminophen. Analyst 2021, 146, 964–970. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, S. Electrochemical Sensors Based on Nitrogen-Doped Reduced Graphene Oxide for the Simultaneous Detection of Ascorbic Acid, Dopamine and Uric Acid. J. Alloys Compd. 2020, 842, 155873. [Google Scholar] [CrossRef]
- Yang, L.; Huang, N.; Lu, Q.; Liu, M.; Li, H.; Zhang, Y.; Yao, S. A Quadruplet Electrochemical Platform for Ultrasensitive and Simultaneous Detection of Ascorbic Acid, Dopamine, Uric Acid and Acetaminophen Based on a Ferrocene Derivative Functional Au NPs/Carbon Dots Nanocomposite and Graphene. Anal. Chim. Acta 2016, 903, 69–80. [Google Scholar] [CrossRef]
- Chen, J.; He, P.; Bai, H.; He, S.; Zhang, T.; Zhang, X.; Dong, F. Poly(β-Cyclodextrin)/Carbon Quantum Dots Modified Glassy Carbon Electrode: Preparation, Characterization and Simultaneous Electrochemical Determination of Dopamine, Uric Acid and Tryptophan. Sens. Actuators B Chem. 2017, 252, 9–16. [Google Scholar] [CrossRef]
- Sun, L.; Li, H.; Li, M.; Li, P.; Li, C.; Yang, B. Simultaneous Determination of Small Biomolecules and Nitrite Using an Au/TiO2 /Carbon Nanotube Composite-Modified Electrode. J. Electrochem. Soc. 2016, 163, B567–B572. [Google Scholar] [CrossRef]
- Priyatharshni, S.; Tamilselvan, A.; Viswanathan, C.; Ponpandian, N. LaCoO3 Nanostructures Modified Glassy Carbon Electrode for Simultaneous Electrochemical Detection of Dopamine, Ascorbic Acid and Uric Acid. J. Electrochem. Soc. 2017, 164, B152. [Google Scholar] [CrossRef]
- Wang, Z.; An, R.; Dai, Y.; Luo, H. A Simple Strategy for the Simultaneous Determination of Dopamine, Uric Acid, L-Tryptophan and Theophylline Based on a Carbon Dots Modified Electrode. Int. J. Electrochem. Sci. 2021, 16, 210450. [Google Scholar] [CrossRef]
- Ferlazzo, A.; Espro, C.; Iannazzo, D.; Neri, G. Determination of Phenylalanine by a Novel Enzymatic PHD/SPE Biosensor. IEEE Trans. Instrum. Meas. 2023, 72, 1–8. [Google Scholar] [CrossRef]
- Gan, A.W.; Seah, G.E.K.K.; Kwek, L.H.; Goh, S.S. Ion Transfer Voltammetry of Amino Acids with an All-solid-state Ion-selective Electrode for Non-destructive Phenylalanine Sensing. Electroanalysis 2023, 35, e202200501. [Google Scholar] [CrossRef]
- Ferlazzo, A.; Espro, C.; Iannazzo, D.; Bonavita, A.; Neri, G. Yttria-Zirconia Electrochemical Sensor for the Detection of Tyrosine. Mater. Today Commun. 2023, 35, 106036. [Google Scholar] [CrossRef]
- Xu, Z.; Qiao, X.; Tao, R.; Li, Y.; Zhao, S.; Cai, Y.; Luo, X. A Wearable Sensor Based on Multifunctional Conductive Hydrogel for Simultaneous Accurate PH and Tyrosine Monitoring in Sweat. Biosens. Bioelectron. 2023, 234, 115360. [Google Scholar] [CrossRef]
- Zhang, L.; Li, C.; Li, F.; Li, S.; Ma, H.; Gu, F. A Sensing Platform Based on Cu-MOF Encapsulated Dawson-Type Polyoxometalate Crystal Material for Electrochemical Detection of Xanthine. Microchim. Acta 2023, 190, 24. [Google Scholar] [CrossRef]
- Kodakat, K.; Girish Kumar, K. Fabrication of a Selective and Sensitive Electro-Synthesized Molecularly Imprinted Polymer-Based Electrochemical Sensor for the Determination of Xanthine. J. Appl. Electrochem. 2023. [Google Scholar] [CrossRef]
- Xu, Q.-Q.; Luo, L.; Liu, Z.-G.; Guo, Z.; Huang, X.-J. Highly Sensitive and Selective Serotonin (5-HT) Electrochemical Sensor Based on Ultrafine Fe3O4 Nanoparticles Anchored on Carbon Spheres. Biosens. Bioelectron. 2023, 222, 114990. [Google Scholar] [CrossRef]
- Han, J.; Stine, J.M.; Chapin, A.A.; Ghodssi, R. A Portable Electrochemical Sensing Platform for Serotonin Detection Based on Surface-Modified Carbon Fiber Microelectrodes. Anal. Methods 2023, 15, 1096–1104. [Google Scholar] [CrossRef]
- Mykhailiv, O.; Zubyk, H.; Plonska-Brzezinska, M.E. Carbon Nano-Onions: Unique Carbon Nanostructures with Fascinating Properties and Their Potential Applications. Inorganica Chim. Acta 2017, 468, 49–66. [Google Scholar] [CrossRef]
- Sun, W.; Zhang, X.; Jia, H.; Zhu, Y.; Guo, Y.; Gao, G.; Li, Y.; Wu, F. Water-Dispersible Candle Soot–Derived Carbon Nano-Onion Clusters for Imaging-Guided Photothermal Cancer Therapy. Small 2019, 15, 1804575. [Google Scholar] [CrossRef] [PubMed]
- Bobrowska, D.M.; Castro, E.; Echegoyen, L.; Plonska-Brzezinska, M.E. Carbon Nano-Onion and Zinc Oxide Composites as an Electron Transport Layer in Inverted Organic Solar Cells. ChemNanoMat 2020, 6, 248–257. [Google Scholar] [CrossRef]
- Yan, X.; Duan, P.; Zhang, F.; Li, H.; Zhang, H.; Zhao, M.; Zhang, X.; Xu, B.; Pennycook, S.J.; Guo, J. Stable Single-Atom Platinum Catalyst Trapped in Carbon Onion Graphitic Shells for Improved Chemoselective Hydrogenation of Nitroarenes. Carbon 2019, 143, 378–384. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y.; Kim, D.Y. Electrochemical Sensing Performance of Nanodiamond-Derived Carbon Nano-Onions: Comparison with Multiwalled Carbon Nanotubes, Graphite Nanoflakes, and Glassy Carbon. Carbon 2016, 98, 74–82. [Google Scholar] [CrossRef]
- Borgohain, R.; Yang, J.; Selegue, J.P.; Kim, D.Y. Controlled Synthesis, Efficient Purification, and Electrochemical Characterization of Arc-Discharge Carbon Nano-Onions. Carbon 2014, 66, 272–284. [Google Scholar] [CrossRef]
- Ozoemena, O.C.; Shai, L.J.; Maphumulo, T.; Ozoemena, K.I. Electrochemical Sensing of Dopamine Using Onion-like Carbons and Their Carbon Nanofiber Composites. Electrocatalysis 2019, 10, 381–391. [Google Scholar] [CrossRef]
- Breczko, J.; Plonska-Brzezinska, M.E.; Echegoyen, L. Electrochemical Oxidation and Determination of Dopamine in the Presence of Uric and Ascorbic Acids Using a Carbon Nano-Onion and Poly(Diallyldimethylammonium Chloride) Composite. Electrochim. Acta 2012, 72, 61–67. [Google Scholar] [CrossRef]
- Bian, Y.; Liu, L.; Liu, D.; Zhu, Z.; Shao, Y.; Li, M. Electrochemical Synthesis of Carbon Nano Onions. Inorg. Chem. Front. 2020, 7, 4404–4411. [Google Scholar] [CrossRef]
- Choucair, M.; Stride, J.A. The Gram-Scale Synthesis of Carbon Onions. Carbon 2012, 50, 1109–1115. [Google Scholar] [CrossRef]
- Kroto, H.W. Carbon Onions Introduce New Flavour to Fullerene Studies. Nature 1992, 359, 670–671. [Google Scholar] [CrossRef]
- Tang, H.; Yuan, X.; Yu, P.; Hu, Q.; Wang, M.; Yao, Y.; Wu, L.; Zou, Q.; Ke, Y.; Zhao, Y.; et al. Revealing the Formation Mechanism of Ultrahard Nanotwinned Diamond from Onion Carbon. Carbon 2018, 129, 159–167. [Google Scholar] [CrossRef]
- Dickinson, E.J.F.; Streeter, I.; Compton, R.G. Theory of Chronoamperometry at Cylindrical Microelectrodes and Their Arrays. J. Phys. Chem. C 2008, 112, 11637–11644. [Google Scholar] [CrossRef]
- Sun, Y.-P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.A.S.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H.; et al. Quantum-Sized Carbon Dots for Bright and Colorful Photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef] [PubMed]
- Noor, A.M.; Shahid, M.M.; Rameshkumar, P.; Huang, N.M. A Glassy Carbon Electrode Modified with Graphene Oxide and Silver Nanoparticles for Amperometric Determination of Hydrogen Peroxide. Microchim. Acta 2016, 183, 911–916. [Google Scholar] [CrossRef]
- Bolzán, A.E. Electrodeposition of Copper on Glassy Carbon Electrodes in the Presence of Picolinic Acid. Electrochim. Acta 2013, 113, 706–718. [Google Scholar] [CrossRef]
- Jiang, G.; Jiang, T.; Zhou, H.; Yao, J.; Kong, X. Preparation of N-Doped Carbon Quantum Dots for Highly Sensitive Detection of Dopamine by an Electrochemical Method. RSC Adv. 2015, 5, 9064–9068. [Google Scholar] [CrossRef]
- Arroquia, A.; Acosta, I.; Armada, M.P.G. Self-Assembled Gold Decorated Polydopamine Nanospheres as Electrochemical Sensor for Simultaneous Determination of Ascorbic Acid, Dopamine, Uric Acid and Tryptophan. Mater. Sci. Eng. C 2020, 109, 110602. [Google Scholar] [CrossRef]
- Yao, W.; Guo, H.; Liu, H.; Li, Q.; Wu, N.; Li, L.; Wang, M.; Fan, T.; Yang, W. Highly Electrochemical Performance of Ni-ZIF-8/ N S-CNTs/CS Composite for Simultaneous Determination of Dopamine, Uric Acid and L-Tryptophan. Microchem. J. 2020, 152, 104357. [Google Scholar] [CrossRef]
- Yang, Y.J.; Guo, L.; Zhang, W. The Electropolymerization of CTAB on Glassy Carbon Electrode for Simultaneous Determination of Dopamine, Uric Acid, Tryptophan and Theophylline. J. Electroanal. Chem. 2016, 768, 102–109. [Google Scholar] [CrossRef]
- Jesny, S.; Girish Kumar, K. Non-Enzymatic Electrochemical Sensor for the Simultaneous Determination of Xanthine, Its Methyl Derivatives Theophylline and Caffeine as Well as Its Metabolite Uric Acid. Electroanalysis 2017, 29, 1828–1837. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, H.; Guo, L. Simultaneous Determination of Theophylline and Caffeine by Large Mesoporous Carbon/Nafion Modified Electrode. J. Electroanal. Chem. 2013, 706, 7–12. [Google Scholar] [CrossRef]
- Guo, H.; Wang, M.; Zhao, L.; Youliwasi, N.; Liu, C. The Effect of Co and N of Porous Carbon-Based Materials Fabricated via Sacrificial Templates MOFs on Improving DA and UA Electrochemical Detection. Microporous Mesoporous Mater. 2018, 263, 21–27. [Google Scholar] [CrossRef]
- Ghanbari, M.H.; Norouzi, Z.; Etzold, B.J.M. Increasing Sensitivity and Selectivity for Electrochemical Sensing of Uric Acid and Theophylline in Real Blood Serum through Multinary Nanocomposites. Microchem. J. 2023, 191, 108836. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, W.; Meng, F.; Yang, M.; Pan, Y.; Miao, P. Hand-in-Hand RNA Nanowire-Based Aptasensor for the Detection of Theophylline. Biosens. Bioelectron. 2018, 101, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Setoudeh, N.; Jahani, S.; Kazemipour, M.; Foroughi, M.M.; Hassani Nadiki, H. Zeolitic Imidazolate Frameworks and Cobalt-Tannic Acid Nanocomposite Modified Carbon Paste Electrode for Simultaneous Determination of Dopamine, Uric Acid, Acetaminophen and Tryptophan: Investigation of Kinetic Parameters of Surface Electrode and Its Analytical Performance. J. Electroanal. Chem. 2020, 863, 114045. [Google Scholar] [CrossRef]
- Zhu, D.; Bai, X.; Zhang, P.; Li, X. An Electrochemical Sensor Based on Boron/Nitrogen Co-Doped Honeycomb-like Porous Carbon Encapsulation Molybdenum Trioxides for the Simultaneous Detection of Xanthine, Uric Acid and Dopamine. Colloids Surf. A Physicochem. Eng. Asp. 2022, 655, 130304. [Google Scholar] [CrossRef]
- Mounesh; Malathesh, P.; Praveen Kumara, N.Y.; Jilani, B.S.; Mruthyunjayachari, C.D.; Venugopala Reddy, K.R. Synthesis and Characterization of Tetra-Ganciclovir Cobalt (II) Phthalocyanine for Electroanalytical Applications of AA/DA/UA. Heliyon 2019, 5, e01946. [Google Scholar] [CrossRef]
- Mohiuddin, A.K.; Yasmin, S.; Jeon, S. CoxNi1−x Double Hydroxide Decorated Graphene NPs for Simultaneous Determination of Dopamine and Uric Acid. Sens. Actuators A Phys. 2023, 355, 114314. [Google Scholar] [CrossRef]





| Biosensors | Linear Range (μM) | Detection Limit (μM) | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|
| DA | UA | Trp | TP | DA | UA | Trp | TP | ||
| (Au-PDNs)/GCE | 1–160 160–350 | 1–120 120–350 | 1.0–160 160–280 | \ | 0.0001 | 0.0001 | 0.0001 | \ | [47] |
| Ni-ZIF-8/N S-CNTs/CS/GCE | 8–500 | 1–600 | 1–600 | \ | 0.93 | 0.41 | 0.69 | \ | [48] |
| poly(CTAB)/GCE | 0.5–1000 | 1–1000 | 1–1000 | 0.5–1000 | 0.11 | 0.33 | 0.44 | 0.11 | [49] |
| pPABSA/GCE | \ | \ | \ | 0.9–100 | \ | \ | \ | 7.02 | [50] |
| β-CD/CQDs/GCE | 4–220 | 0.3–200 | 5–270 | \ | 0.14 | 0.01 | 0.16 | \ | [18] |
| LMC/Nafion/GCE | \ | \ | \ | 0.8–180.0 | \ | \ | \ | 0.37 | [51] |
| CDs/GCE | 0.5–50 | 3–28.5 28.5–75 | 1–65 | 10–200 | 0.007 | 0.011 | 0.11 | 0.33 | [21] |
| Nitrogen-doped graphene/GCE | 0.5–170 | 0.1–20 | \ | \ | 0.25 | 0.045 | \ | \ | [13] |
| NCCNPs/GCE | 0.002–0.0695 | 0.005–0.192 | \ | \ | 0.34 | 0.98 | \ | \ | [52] |
| 1,4-BBFT/ Carbon paste | \ | \ | \ | 0.06–700 | \ | \ | \ | 0.012 | [7] |
| PAMT/AuNPs/TiO2@CuO-B/RGO/GCE | \ | 0.0005–10 | \ | 0.001–10 | \ | 0.00018 | \ | 0.00036 | [53] |
| Hand-in-hand RNA nanowire | \ | \ | \ | 0.5–70 | \ | \ | \ | 0.05 | [54] |
| ZIF-8@CoTA/CPE | 0.02–0.44 | 0.02–0.44 | 0.02–0.44 | \ | 0.0012 | 0.0067 | 0.0051 | \ | [55] |
| MoO3@B/N-PC | 0.079–104.1 | 0.086–102.5 | \ | \ | 0.069 | 0.078 | \ | \ | [56] |
| CoTGPc/GCE | 2–10 | 2–10 | \ | \ | 0.03 | 0.1 | \ | \ | [57] |
| CoxNi1−x(OH)2/G | 0.1–750 | 0.25–925 | \ | \ | 0.043 | 0.097 | \ | \ | [58] |
| CNOs/GCE | 0.01–38.16 | 0.06–68.16 | 1.00–108.25 | 5.16–108.25 | 0.0039 | 0.0087 | 0.18 | 0.35 | This work |
| Sample | Serum (μM) | Added (μM) | Founded (μM) | Recovery (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DA | UA | Trp | TP | DA | UA | Trp | TP | DA | UA | Trp | TP | DA | UA | Trp | TP | |
| 1 | 0 | 10.93 | 2.59 | 0 | 3.5 | 20 | 6 | 35 | 3.31 | 29.7 | 8.05 | 33.61 | 94.57 | 96.02 | 93.71 | 96.03 |
| 2 | 0 | 12.81 | 3.24 | 0 | 4.5 | 25 | 12 | 55 | 4.27 | 35.81 | 14.52 | 54.98 | 94.89 | 94.71 | 95.28 | 99.96 |
| 3 | 0 | 10.66 | 1.13 | 0 | 5.5 | 30 | 18 | 75 | 5.33 | 38.74 | 18.47 | 74.19 | 96.91 | 95.28 | 96.55 | 98.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, R.; Kuang, W.; Li, Z.; Mu, T.; Luo, H. A Simple Strategy for the Simultaneous Determination of Dopamine, Uric Acid, L-Tryptophan and Theophylline Based on a Carbon Nano-Onions Modified Electrode. Processes 2023, 11, 2547. https://doi.org/10.3390/pr11092547
An R, Kuang W, Li Z, Mu T, Luo H. A Simple Strategy for the Simultaneous Determination of Dopamine, Uric Acid, L-Tryptophan and Theophylline Based on a Carbon Nano-Onions Modified Electrode. Processes. 2023; 11(9):2547. https://doi.org/10.3390/pr11092547
Chicago/Turabian StyleAn, Rui, Wenzhu Kuang, Zijian Li, Tiancheng Mu, and Hongxia Luo. 2023. "A Simple Strategy for the Simultaneous Determination of Dopamine, Uric Acid, L-Tryptophan and Theophylline Based on a Carbon Nano-Onions Modified Electrode" Processes 11, no. 9: 2547. https://doi.org/10.3390/pr11092547






