Synthesis of Silver Nanoparticles: From Conventional to ‘Modern’ Methods—A Review
Abstract
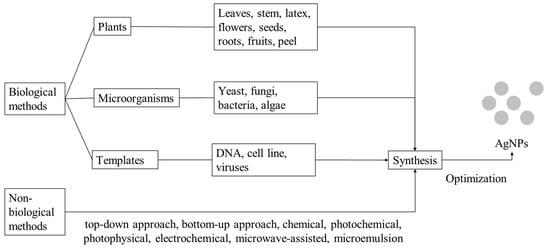
:1. Introduction
2. Synthesis of Silver Nanoparticles
2.1. Physical Methods
2.2. Chemical Methods
2.2.1. Chemical Reduction
2.2.2. Microemulsion Techniques
2.2.3. Photochemical Method
2.2.4. Polymers and Polysaccharides
2.2.5. Electrochemical Synthetic Method
2.2.6. Microwave-Assisted Synthesis
2.3. Green Chemistry Approach for the Synthesis of AgNPs
2.3.1. Plants
2.3.2. Microorganisms
Bacteria
Algae and Fungi
3. Factors Affecting Silver Nanoparticle Synthesis and Their Stability
3.1. Temperature
3.2. pH
3.3. Time
3.4. Pressure
3.5. AgNO3 Concentration
3.6. Other Factors
4. Recent Advanced Synthesis Methods and Future Challenges
4.1. Recent Advanced Synthesis Methods
4.2. Future Challenges
5. Conclusions and Outlooks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NPs: | nanoparticles |
| AgNPs: | silver nanoparticles |
| AMR: | antimicrobial resistance |
| SERS: | surface-enhanced Roman spectroscopy |
| PVA: | polyvinyl alcohol |
| BSA: | bolvine |
| PVP: | polyvinylpyrrolidone |
| PEG: | polyethylene glycol |
| SDS: | sodium dodecyl sulfate |
| TMC: | tobacco mosaic virus |
References
- Natsuki, J. A Review of Silver Nanoparticles: Synthesis Methods, Properties and Applications. Int. J. Mater. Sci. Appl. 2015, 4, 325. [Google Scholar] [CrossRef]
- Doan, L.; Lu, Y.; Karatela, M.; Phan, V.; Jeffryes, C.; Benson, T.; Wujcik, E.K. Surface Modifications of Superparamagnetic Iron Oxide Nanoparticles with Polylactic Acid-Polyethylene Glycol Diblock Copolymer and Graphene Oxide for a Protein Delivery Vehicle. Eng. Sci. 2019, 7, 10–16. [Google Scholar] [CrossRef]
- Doan, L.; Nguyen, L.T.; Nguyen, N.T.N. Modifying superparamagnetic iron oxides nanoparticles for doxorubicin delivery carriers: A review. J. Nanoparticle Res. 2023, 25, 73. [Google Scholar] [CrossRef]
- Lu, Y.; Doan, L.; Bafana, A.; Yu, G.; Jeffryes, C.; Benson, T.; Wei, S.; Wujcik, E.K. Chapter 6—Multifunctional Nanocomposite Sensors for Environmental Monitoring. In Polymer-Based Multifunctional Nanocomposites and Their Applications; Song, K., Liu, C., Guo, J.Z., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 157–174. [Google Scholar] [CrossRef]
- Rajapaksha, P.; Orrell-Trigg, R.; Shah, D.; Cheeseman, S.; Vu, K.B.; Ngo, S.T.; Murdoch, B.J.; Choudhury, N.R.; Yin, H.; Cozzolino, D.; et al. Broad spectrum antibacterial zinc oxide-reduced graphene oxide nanocomposite for water depollution. Mater. Today Chem. 2023, 27, 101242. [Google Scholar] [CrossRef]
- Vu, K.B.; Phung, T.K.; Tran, T.T.T.; Mugemana, C.; Giang, H.N.; Nhi, T.L.P. Polystyrene nanoparticles prepared by nanoprecipitation: A recyclable template for fabricating hollow silica. J. Ind. Eng. Chem. 2021, 97, 307–315. [Google Scholar] [CrossRef]
- Medici, S.; Peana, M.; Nurchi, V.M.; Zoroddu, M.A. Medical Uses of Silver: History, Myths, and Scientific Evidence. J. Med. Chem. 2019, 62, 5923–5943. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, Y.-Y.; Huang, J.; Chen, C.-Y.; Wang, Z.-X.; Xie, H. Silver nanoparticles: Synthesis, medical applications and biosafety. Theranostics 2020, 10, 8996–9031. [Google Scholar] [CrossRef]
- Bouafia, A.; Laouini, S.E.; Ahmed, A.S.A.; Soldatov, A.V.; Algarni, H.; Feng Chong, K.; Ali, G.A.M. The Recent Progress on Silver Nanoparticles: Synthesis and Electronic Applications. Nanomaterials 2021, 11, 2318. [Google Scholar] [CrossRef]
- Alharbi, N.S.; Alsubhi, N.S.; Felimban, A.I. Green synthesis of silver nanoparticles using medicinal plants: Characterization and application. J. Radiat. Res. Appl. Sci. 2022, 15, 109–124. [Google Scholar] [CrossRef]
- Indiarto, R.; Indriana, L.P.A.; Andoyo, R.; Subroto, E.; Nurhadi, B. Bottom-up nanoparticle synthesis: A review of techniques, polyphenol-based core materials, and their properties. Eur. Food Res. Technol. 2022, 248, 1–24. [Google Scholar] [CrossRef]
- Güzel, R.; Erdal, G. Synthesis of Silver Nanoparticles. In Silver Nanoparticles—Fabrication, Characterization and Applications; Maaz, K., Ed.; InTech: Sydney, Australia, 2018. [Google Scholar] [CrossRef]
- Kalishwaralal, K.; Deepak, V.; Ramkumarpandian, S.; Nellaiah, H.; Sangiliyandi, G. Extracellular biosynthesis of silver nanoparticles by the culture supernatant of Bacillus licheniformis. Mater. Lett. 2008, 62, 4411–4413. [Google Scholar] [CrossRef]
- Lok, C.-N.; Ho, C.-M.; Chen, R.; He, Q.-Y.; Yu, W.-Y.; Sun, H.; Tam, P.K.-H.; Chiu, J.-F.; Che, C.-M. Silver nanoparticles: Partial oxidation and antibacterial activities. JBIC J. Biol. Inorg. Chem. 2007, 12, 527–534. [Google Scholar] [CrossRef]
- Mandal, D.; Kumar Dash, S.; Das, B.; Chattopadhyay, S.; Ghosh, T.; Das, D.; Roy, S. Bio-fabricated silver nanoparticles preferentially targets Gram positive depending on cell surface charge. Biomed. Pharmacother. 2016, 83, 548–558. [Google Scholar] [CrossRef]
- Zodrow, K.; Brunet, L.; Mahendra, S.; Li, D.; Zhang, A.; Li, Q.; Alvarez, P.J.J. Polysulfone ultrafiltration membranes impregnated with silver nanoparticles show improved biofouling resistance and virus removal. Water Res. 2009, 43, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Madhu; Sharma, R.; Bharti, R. A Review on the Synthesis and Photocatalytic Application of Silver Nano Particles. IOP Conf. Ser. Earth Environ. Sci. 2023, 1110, 012021. [Google Scholar] [CrossRef]
- Banerjee, K.; Ravishankar Rai, V. Silver nanoparticles synthesis mechanisms. In Green Synthesis of Silver Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2022; pp. 607–625. [Google Scholar] [CrossRef]
- Kruis, F.E.; Fissan, H.; Rellinghaus, B. Sintering and evaporation characteristics of gas-phase synthesis of size-selected PbS nanoparticles. Mater. Sci. Eng. B 2000, 69–70, 329–334. [Google Scholar] [CrossRef]
- Magnusson, M.H.; Deppert, K.; Malm, J.-O.; Bovin, J.-O.; Samuelson, L. Size-selected gold nanoparticles by aerosol technology. Nanostruct. Mater. 1999, 12, 45–48. [Google Scholar] [CrossRef]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic Basis of Antimicrobial Actions of Silver Nanoparticles. Front. Microbiol. 2016, 7, 01831. [Google Scholar] [CrossRef]
- Das, S.; Chakraborty, T. A review on green synthesis of silver nanoparticle and zinc oxide nanoparticle from different plants extract and their antibacterial activity against multi-drug resistant bacteria. J. Innov. Pharm. Biol. Sci. 2018, 5, 63–73. Available online: https://jipbs.com/index.php/journal/article/view/342 (accessed on 11 April 2023).
- Hussain, S.M.; Javorina, A.K.; Schrand, A.M.; Duhart, H.M.; Ali, S.F.; Schlager, J.J. The Interaction of Manganese Nanoparticles with PC-12 Cells Induces Dopamine Depletion. Toxicol. Sci. 2006, 92, 456–463. [Google Scholar] [CrossRef]
- Khatami, M.; Sharifi, I.; Nobre, M.A.L.; Zafarnia, N.; Aflatoonian, M.R. Waste-grass-mediated green synthesis of silver nanoparticles and evaluation of their anticancer, antifungal and antibacterial activity. Green Chem. Lett. Rev. 2018, 11, 125–134. [Google Scholar] [CrossRef]
- Paredes, D.; Ortiz, C.; Torres, R. Synthesis, characterization, and evaluation of antibacterial effect of Ag nanoparticles against Escherichia coli O157:H7 and methicillin-resistant Staphylococcus aureus (MRSA). Int. J. Nanomed. 2014, 9, 1717–1729. [Google Scholar] [CrossRef]
- Shameli, K.; Ahmad, M.B.; Yunis, W.Z.; Ibrahim, N.A.; Darroudi, M.; Gharayebi, Y.; Sedaghat, S. Synthesis of silver/montmorillonite nanocomposites using γ-irradiation. Int. J. Nanomed. 2010, 5, 1067–1077. [Google Scholar] [CrossRef]
- Xia, T.; Kovochich, M.; Brant, J.; Hotze, M.; Sempf, J.; Oberley, T.; Sioutas, C.; Yeh, J.I.; Wiesner, M.R.; Nel, A.E. Comparison of the Abilities of Ambient and Manufactured Nanoparticles To Induce Cellular Toxicity According to an Oxidative Stress Paradigm. Nano Lett. 2006, 6, 1794–1807. [Google Scholar] [CrossRef] [PubMed]
- Choi, O.; Hu, Z. Size Dependent and Reactive Oxygen Species Related Nanosilver Toxicity to Nitrifying Bacteria. Environ. Sci. Technol. 2008, 42, 4583–4588. [Google Scholar] [CrossRef]
- Choi, O.K.; Hu, Z.Q. Nitrification inhibition by silver nanoparticles. Water Sci. Technol. 2009, 59, 1699–1702. [Google Scholar] [CrossRef]
- nanoComposix Silver Nanoparticles: Physical Properties. Available online: https://nanocomposix.com/pages/silver-nanoparticles-physical-properties (accessed on 18 July 2022).
- Yaqoob, A.A.; Umar, K.; Ibrahim, M.N.M. Silver nanoparticles: Various methods of synthesis, size affecting factors and their potential applications—A review. Appl. Nanosci. 2020, 10, 1369–1378. [Google Scholar] [CrossRef]
- Li, Z.; Dong, H.; Wu, Z.; Shen, J.; Xu, D.; He, R.; Wan, L.; Zhang, S. Novel p-n type porous Ag2O/Bi5O7I heterojunction for Uv–Vis-NIR activated high efficient photocatalytic degradation of bisphenol A: Photoelectric properties and degradation mechanism. Appl. Surf. Sci. 2020, 529, 147162. [Google Scholar] [CrossRef]
- Kim, K.D.; Han, D.N.; Kim, H.T. Optimization of experimental conditions based on the Taguchi robust design for the formation of nano-sized silver particles by chemical reduction method. Chem. Eng. J. 2004, 104, 55–61. [Google Scholar] [CrossRef]
- Mallick, K.; Witcomb, M.J.; Scurrell, M.S. Polymer stabilized silver nanoparticles: A photochemical synthesis route. J. Mater. Sci. 2004, 39, 4459–4463. [Google Scholar] [CrossRef]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of silver nanoparticles: Chemical, physical and biological methods. Res. Pharm. Sci. 2014, 9, 385–406. [Google Scholar]
- Amir, D.; Nasaruddin, R.R.; Engliman, N.S.; Sulaiman, S.; Mastuli, M.S. Effect of Stabilizers in the Synthesis of Silver Nanoparticles and Methylene Blue Oxidation. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1192, 012031. [Google Scholar] [CrossRef]
- Gudikandula, K.; Charya Maringanti, S. Synthesis of silver nanoparticles by chemical and biological methods and their antimicrobial properties. J. Exp. Nanosci. 2016, 11, 714–721. [Google Scholar] [CrossRef]
- Suriati, G.; Mariatti, M.; Azizan, A. Synthesis of silver nanoparticles by chemical reduction method: Effect of reducing agent and surfactant concentration. Int. J. Automot. Mech. Eng. 2014, 10, 1920–1927. [Google Scholar] [CrossRef]
- Alviar-Agnew, M.; Agnew, H. Oxidation and Reduction—Some Definitions. In Introductory Chemistry; Libre Texts, 2022; p. 2. Available online: https://chem.libretexts.org/@go/page/47575?pdf (accessed on 23 April 2023).
- Rodríguez-León, E.; Iñiguez-Palomares, R.; Navarro, R.E.; Herrera-Urbina, R.; Tánori, J.; Iñiguez-Palomares, C.; Maldonado, A. Synthesis of silver nanoparticles using reducing agents obtained from natural sources (Rumex hymenosepalus extracts). Nanoscale Res. Lett. 2013, 8, 318. [Google Scholar] [CrossRef] [PubMed]
- Naganthran, A.; Verasoundarapandian, G.; Khalid, F.E.; Masarudin, M.J.; Zulkharnain, A.; Nawawi, N.M.; Karim, M.; Che Abdullah, C.A.; Ahmad, S.A. Synthesis, Characterization and Biomedical Application of Silver Nanoparticles. Materials 2022, 15, 427. [Google Scholar] [CrossRef] [PubMed]
- Eka Putri, G.; Rahayu Gusti, F.; Novita Sary, A.; Zainul, R. Synthesis of silver nanoparticles used chemical reduction method by glucose as reducing agent. J. Phys. Conf. Ser. 2019, 1317, 012027. [Google Scholar] [CrossRef]
- Martins, C.; Araújo, A.; de Gouveia, L.; Prior, J. Minimizing the Silver Free Ion Content in Starch Coated Silver Nanoparticle Suspensions with Exchange Cationic Resins. Nanomaterials 2022, 12, 644. [Google Scholar] [CrossRef]
- Malik, M.A.; Wani, M.Y.; Hashim, M.A. Microemulsion method: A novel route to synthesize organic and inorganic nanomaterials. Arab. J. Chem. 2012, 5, 397–417. [Google Scholar] [CrossRef]
- Abou El-Nour, K.M.M.; Eftaiha, A.; Al-Warthan, A.; Ammar, R.A.A. Synthesis and applications of silver nanoparticles. Arab. J. Chem. 2010, 3, 135–140. [Google Scholar] [CrossRef]
- Miyazawa, T.; Itaya, M.; Burdeos, G.C.; Nakagawa, K.; Miyazawa, T. A Critical Review of the Use of Surfactant-Coated Nanoparticles in Nanomedicine and Food Nanotechnology. Int. J. Nanomed. 2021, 16, 3937–3999. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Qiao, X.; Chen, J. Synthesis of silver nanoparticles—Effects of concerned parameters in water/oil microemulsion. Mater. Sci. Eng. B 2007, 142, 1–15. [Google Scholar] [CrossRef]
- Zieliska-Jurek, A.; Reszczyska, J.; Grabowska, E.; Zalesk, A. Nanoparticles Preparation Using Microemulsion Systems. In Microemulsions—An Introduction to Properties and Applications; Najjar, R., Ed.; InTech: Sydney, Australia, 2012. [Google Scholar]
- Zielińska-Jurek, A.; Reszczyńska, J.; Grabowska, E.; Zaleska, A. Microemulsions—An Introduction to Properties and Applications; InTech: Sydney, Australia, 2012; p. 250. Available online: http://www.intechopen.com/books/microemulsions-an-introduction-to-properties-and-applications/nanoparticles-preparation-using-microemulsion-systems (accessed on 23 April 2023).
- Das, M.; Patowary, K.; Vidya, R.; Malipeddi, H. Microemulsion synthesis of silver nanoparticles using biosurfactant extracted from Pseudomonas aeruginosa MKVIT3 strain and comparison of their antimicrobial and cytotoxic activities. IET Nanobiotechnol. 2016, 10, 411–418. [Google Scholar] [CrossRef]
- Chen, S.; Ju, Y.; Guo, Y.; Xiong, C.; Dong, L. In-site synthesis of monodisperse, oleylamine-capped Ag nanoparticles through microemulsion approach. J. Nanoparti. Res. 2017, 19, 88. [Google Scholar] [CrossRef]
- Jara, N.; Milán, N.S.; Rahman, A.; Mouheb, L.; Boffito, D.C.; Jeffryes, C.; Dahoumane, S.A. Photochemical Synthesis of Gold and Silver Nanoparticles—A Review. Molecules 2021, 26, 4585. [Google Scholar] [CrossRef]
- dos Santos, M.A.; Paterno, L.G.; Moreira, S.G.C.; Sales, M.J.A. Original photochemical synthesis of Ag nanoparticles mediated by potato starch. SN Appl. Sci. 2019, 1, 554. [Google Scholar] [CrossRef]
- Raveendran, P.; Fu, J.; Wallen, S.L. Completely “Green” Synthesis and Stabilization of Metal Nanoparticles. J. Am. Chem. Soc. 2003, 125, 13940–13941. [Google Scholar] [CrossRef]
- Pencheva, D.; Bryaskova, R.; Kantardjiev, T. Polyvinyl alcohol/silver nanoparticles (PVA/AgNps) as a model for testing the biological activity of hybrid materials with included silver nanoparticles. Mater. Sci. Eng. C 2012, 32, 2048–2051. [Google Scholar] [CrossRef]
- Pinzaru, I.; Coricovac, D.; Dehelean, C.; Moacă, E.-A.; Mioc, M.; Baderca, F.; Sizemore, I.; Brittle, S.; Marti, D.; Calina, C.D.; et al. Stable PEG-coated silver nanoparticles—A comprehensive toxicological profile. Food Chem. Toxicol. 2018, 111, 546–556. [Google Scholar] [CrossRef]
- Abdel-Halim, E.S.; El-Rafie, M.H.; Al-Deyab, S.S. Polyacrylamide/guar gum graft copolymer for preparation of silver nanoparticles. Carbohydr. Polym. 2011, 85, 692–697. [Google Scholar] [CrossRef]
- Mavani, K.; Shah, M. Synthesis of Silver Nanoparticles by using Sodium Borohydride as a Reducing Agent. Int. J. Eng. Res. Technol. 2013, 2, 1–4. Available online: https://www.ijert.org/research/synthesis-of-silver-nanoparticles-by-using-sodium-borohydride-as-a-reducing-agent-IJERTV2IS3605.pdf (accessed on 8 May 2023).
- Zienkiewicz-Strzałka, M.; Deryło-Marczewska, A.; Skorik, Y.A.; Petrova, V.A.; Choma, A.; Komaniecka, I. Silver Nanoparticles on Chitosan/Silica Nanofibers: Characterization and Antibacterial Activity. Int. J. Mol. Sci. 2019, 21, 166. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.-S.; Wang, L.-S.; Wang, C.-Y.; Yang, C.-H.; Hsieh, C.-L.; Chen, S.-Y.; Shen, C.-Y.; Wang, J.-J. Synthesis and anti-fungal effect of silver nanoparticles–chitosan composite particles. Int. J. Nanomed. 2015, 10, 2685. [Google Scholar] [CrossRef]
- Huq, M.A.; Ashrafudoulla, M.; Parvez, M.A.K.; Balusamy, S.R.; Rahman, M.M.; Kim, J.H.; Akter, S. Chitosan-Coated Polymeric Silver and Gold Nanoparticles: Biosynthesis, Characterization and Potential Antibacterial Applications: A Review. Polymers 2022, 14, 5302. [Google Scholar] [CrossRef]
- Sofy, A.R.; Hmed, A.A.; Abd El Haliem, N.F.; Zein, M.A.-E.; Elshaarawy, R.F.M. Polyphosphonium-oligochitosans decorated with nanosilver as new prospective inhibitors for common human enteric viruses. Carbohydr. Polym. 2019, 226, 115261. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Krishnan, S.; Hii, Y.S.; Pan, S.; Chan, Y.S.; Acquah, C.; Danquah, M.K.; Rodrigues, J. Synthesis approach-dependent antiviral properties of silver nanoparticles and nanocomposites. J. Nanostruct. Chem. 2022, 12, 809–831. [Google Scholar] [CrossRef]
- Kuntyi, O.I.; Kytsya, A.R.; Bondarenko, A.B.; Mazur, A.S.; Mertsalo, I.P.; Bazylyak, L.I. Microplasma synthesis of silver nanoparticles in PVP solutions using sacrificial silver anodes. Colloid Polym. Sci. 2021, 299, 855–863. [Google Scholar] [CrossRef]
- Nadagouda, M.N.; Speth, T.F.; Varma, R.S. Microwave-Assisted Green Synthesis of Silver Nanostructures. Acc. Chem. Res. 2011, 44, 469–478. [Google Scholar] [CrossRef]
- Onwudiwe, D.C. Microwave-assisted synthesis of PbS nanostructures. Heliyon 2019, 5, e01413. [Google Scholar] [CrossRef]
- Athawale, A.A.; Desai, P.A. Silver doped lanthanum chromites by microwave combustion method. Ceram. Int. 2011, 37, 3037–3043. [Google Scholar] [CrossRef]
- Rahimi, H.-R.; Doostmohammadi, M. Nanoparticle Synthesis, Applications, and Toxicity. In Applications of Nanobiotechnology; Stoytcheva, M., Zlatev, R., Eds.; IntechOpen: Sydney, Australia, 2020. [Google Scholar] [CrossRef]
- Charan Teja, V.R.; Harini Chowdary, V.; Prasanna Raju, Y.; Surendra, N.; Vishnu Vardhan, R.; Kiran Kumar Reddy, B. A glimpse on solid lipid nanoparticles as drug delivery systems. J. Glob. Trends Pharm. Sci. 2014, 5, 1649–1657. Available online: https://www.jgtps.com/admin/uploads/EbgVcK.pdf (accessed on 8 May 2023).
- Ambrozic, G.; Orel, Z.C.; Zigon, M. Microwave-Assisted Non-Aqueous Synthesis of ZnO Nanoparticles. Mater. Technol. 2011, 45, 173–177. Available online: https://www.researchgate.net/publication/283837760_Microwave-assisted_non-aqueous_synthesis_of_ZnO_nanoparticles (accessed on 8 May 2023).
- Vishwanath, R.; Negi, B. Conventional and green methods of synthesis of silver nanoparticles and their antimicrobial properties. Curr. Res. Green Sustain. Chem. 2021, 4, 100205. [Google Scholar] [CrossRef]
- Rafique, M.; Sadaf, I.; Rafique, M.S.; Tahir, M.B. A review on green synthesis of silver nanoparticles and their applications. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1272–1291. [Google Scholar] [CrossRef] [PubMed]
- Chugh, D.; Viswamalya, V.S.; Das, B. Green synthesis of silver nanoparticles with algae and the importance of capping agents in the process. J. Genet. Eng. Biotechnol. 2021, 19, 126. [Google Scholar] [CrossRef]
- Srikar, S.K.; Giri, D.D.; Pal, D.B.; Mishra, P.K.; Upadhyay, S.N. Green Synthesis of Silver Nanoparticles: A Review. Green Sustain. Chem. 2016, 6, 34–56. [Google Scholar] [CrossRef]
- Kumar, P.; Mahato, D.K.; Kamle, M.; Mohanta, T.K.; Kang, S.G. Aflatoxins: A global concern for food safety, human health and their management. Front. Microbiol. 2017, 7, 2170. [Google Scholar] [CrossRef]
- Epifani, M.; Giannini, C.; Tapfer, L.; Vasanelli, L. Sol-Gel Synthesis and Characterization of Ag and Au Nanoparticles in SiO2, TiO2, and ZrO2 Thin Films. J. Am. Ceram. Soc. 2004, 83, 2385–2393. [Google Scholar] [CrossRef]
- Mustapha, T.; Misni, N.; Ithnin, N.R.; Daskum, A.M.; Unyah, N.Z. A Review on Plants and Microorganisms Mediated Synthesis of Silver Nanoparticles, Role of Plants Metabolites and Applications. Int. J. Environ. Res. Public Health 2022, 19, 674. [Google Scholar] [CrossRef]
- Castillo-Henríquez, L.; Alfaro-Aguilar, K.; Ugalde-Álvarez, J.; Vega-Fernández, L.; Montes de Oca-Vásquez, G.; Vega-Baudrit, J.R. Green Synthesis of Gold and Silver Nanoparticles from Plant Extracts and Their Possible Applications as Antimicrobial Agents in the Agricultural Area. Nanomaterials 2020, 10, 1763. [Google Scholar] [CrossRef]
- Zuhrotun, A.; Oktaviani, D.J.; Hasanah, A.N. Biosynthesis of Gold and Silver Nanoparticles Using Phytochemical Compounds. Molecules 2023, 28, 3240. [Google Scholar] [CrossRef] [PubMed]
- Kuppusamy, P.; Yusoff, M.M.; Maniam, G.P.; Govindan, N. Biosynthesis of metallic nanoparticles using plant derivatives and their new avenues in pharmacological applications—An updated report. Saudi Pharm. J. 2016, 24, 473–484. [Google Scholar] [CrossRef]
- Sreelekha, E.; George, B.; Shyam, A.; Sajina, N.; Mathew, B. A Comparative Study on the Synthesis, Characterization, and Antioxidant Activity of Green and Chemically Synthesized Silver Nanoparticles. BioNanoScience 2021, 11, 489–496. [Google Scholar] [CrossRef]
- Naveed, M.; Batool, H.; Rehman, S.U.; Javed, A.; Makhdoom, S.I.; Aziz, T.; Mohamed, A.A.; Sameeh, M.Y.; Alruways, M.W.; Dablool, A.S.; et al. Characterization and Evaluation of the Antioxidant, Antidiabetic, Anti-Inflammatory, and Cytotoxic Activities of Silver Nanoparticles Synthesized Using Brachychiton populneus Leaf Extract. Processes 2022, 10, 1521. [Google Scholar] [CrossRef]
- Kapoor, R.T.; Salvadori, M.R.; Rafatullah, M.; Siddiqui, M.R.; Khan, M.A.; Alshareef, S.A. Exploration of Microbial Factories for Synthesis of Nanoparticles—A Sustainable Approach for Bioremediation of Environmental Contaminants. Front. Microbiol. 2021, 12, 658294. [Google Scholar] [CrossRef] [PubMed]
- Klaus, T.; Joerger, R.; Olsson, E.; Granqvist, C.-G. Silver-based crystalline nanoparticles, microbially fabricated. Proc. Natl. Acad. Sci. USA 1999, 96, 13611–13614. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, L.; Chopade, B. Plasmid mediated silver resistance in Acinetobacter baumannii. Biometals 1994, 7, 49–56. [Google Scholar] [CrossRef]
- Naseer, Q.A.; Xue, X.; Wang, X.; Dang, S.; Din, S.U.; Kalsoom; Jamil, J. Synthesis of silver nanoparticles using Lactobacillus bulgaricus and assessment of their antibacterial potential. Braz. J. Biol. 2022, 82, e232434. [Google Scholar] [CrossRef]
- John, M.S.; Nagoth, J.A.; Ramasamy, K.P.; Mancini, A.; Giuli, G.; Miceli, C.; Pucciarelli, S. Synthesis of Bioactive Silver Nanoparticles Using New Bacterial Strains from an Antarctic Consortium. Mar. Drugs 2022, 20, 558. [Google Scholar] [CrossRef]
- Nanda, A.; Saravanan, M. Biosynthesis of silver nanoparticles from Staphylococcus aureus and its antimicrobial activity against MRSA and MRSE. Nanomed. Nanotechnol. Biol. Med. 2009, 5, 452–456. [Google Scholar] [CrossRef]
- Yuan, Q.; Bomma, M.; Xiao, Z. Enhanced Silver Nanoparticle Synthesis by Escherichia Coli Transformed with Candida Albicans Metallothionein Gene. Materials 2019, 12, 4180. [Google Scholar] [CrossRef] [PubMed]
- Menaa, F.; Wijesinghe, U.; Thiripuranathar, G.; Althobaiti, N.A.; Albalawi, A.E.; Khan, B.A.; Menaa, B. Marine Algae-Derived Bioactive Compounds: A New Wave of Nanodrugs? Mar. Drugs 2021, 19, 484. [Google Scholar] [CrossRef] [PubMed]
- Uzair, B.; Liaqat, A.; Iqbal, H.; Menaa, B.; Razzaq, A.; Thiripuranathar, G.; Fatima Rana, N.; Menaa, F. Green and Cost-Effective Synthesis of Metallic Nanoparticles by Algae: Safe Methods for Translational Medicine. Bioengineering 2020, 7, 129. [Google Scholar] [CrossRef] [PubMed]
- Kathiraven, T.; Sundaramanickam, A.; Shanmugam, N.; Balasubramanian, T. Green synthesis of silver nanoparticles using marine algae Caulerpa racemosa and their antibacterial activity against some human pathogens. Appl. Nanosci. 2015, 5, 499–504. [Google Scholar] [CrossRef]
- Somasundaram, C.K.; Atchudan, R.; Edison, T.N.J.I.; Perumal, S.; Vinodh, R.; Sundramoorthy, A.K.; Babu, R.S.; Alagan, M.; Lee, Y.R. Sustainable Synthesis of Silver Nanoparticles Using Marine Algae for Catalytic Degradation of Methylene Blue. Catalysts 2021, 11, 1377. [Google Scholar] [CrossRef]
- Sinha, S.N.; Paul, D.; Halder, N.; Sengupta, D.; Patra, S.K. Green synthesis of silver nanoparticles using fresh water green alga Pithophora oedogonia (Mont.) Wittrock and evaluation of their antibacterial activity. Appl. Nanosci. 2015, 5, 703–709. [Google Scholar] [CrossRef]
- Volk, T.J. Fungi. In Encyclopedia of Biodiversity; Elsevier: Amsterdam, The Netherlands, 2013; pp. 624–640. [Google Scholar] [CrossRef]
- Guilger-Casagrande, M.; Lima, R.D. Synthesis of Silver Nanoparticles Mediated by Fungi: A Review. Front. Bioeng. Biotechnol. 2019, 7, 287. [Google Scholar] [CrossRef]
- Mahmudin, L.; Suharyadi, E.; Utomo, A.B.S.; Abraha, K. Influence of Stabilizing Agent and Synthesis Temperature on the Optical Properties of Silver Nanoparticles as Active Materials in Surface Plasmon Resonance (SPR) Biosensor; AIP Publishing: Minneapolis, MN, USA, 2016; p. 020041. [Google Scholar]
- Ondari Nyakundi, E.; Padmanabhan, M.N. Green chemistry focus on optimization of silver nanoparticles using response surface methodology (RSM) and mosquitocidal activity: Anopheles stephensi (Diptera: Culicidae). Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2015, 149, 978–984. [Google Scholar] [CrossRef]
- Thangaraj, V.; Mahmud, S.; Li, W.; Yang, F.; Liu, H. Greenly synthesised silver-alginate nanocomposites for degrading dyes and bacteria. IET Nanobiotechnol. 2018, 12, 47–51. [Google Scholar] [CrossRef]
- Gunnarsson, R.; Pilch, I.; Boyd, R.D.; Brenning, N.; Helmersson, U. The influence of pressure and gas flow on size and morphology of titanium oxide nanoparticles synthesized by hollow cathode sputtering. J. Appl. Phys. 2016, 120, 044308. [Google Scholar] [CrossRef]
- Zikmund, T.; Bulíř, J.; Novotný, M.; Fekete, L.; Chertopalov, S.; Irimiciuc, S.A.; Klementová, M.; Balogová, J.; Lančok, J. Silver Nanoparticles for Fluorescent Nanocomposites by High-Pressure Magnetron Sputtering. Materials 2023, 16, 1591. [Google Scholar] [CrossRef]
- Htwe, Y.Z.N.; Chow, W.S.; Suda, Y.; Mariatti, M. Effect of Silver Nitrate Concentration on the Production of Silver Nanoparticles by Green Method. Mater. Today Proc. 2019, 17, 568–573. [Google Scholar] [CrossRef]
- Bamsaoud, S.F.; Basuliman, M.M.; Bin-Hameed, E.A.; Balakhm, S.M.; Alkalali, A.S. The effect of volume and concentration of AgNO3 aqueous solutions on silver nanoparticles synthesized using Ziziphus Spina–Christi leaf extract and their antibacterial activity. J. Phys. Conf. Ser. 2021, 1900, 012005. [Google Scholar] [CrossRef]
- Sobczak-Kupiec, A.; Malina, D.; Wzorek, Z.; Zimowska, M. Influence of silver nitrate concentration on the properties of silver nanoparticles. Micro Nano Lett. 2011, 6, 656. [Google Scholar] [CrossRef]
- Stevenson, A.P.; Blanco Bea, D.; Civit, S.; Antoranz Contera, S.; Iglesias Cerveto, A.; Trigueros, S. Three strategies to stabilise nearly monodispersed silver nanoparticles in aqueous solution. Nanoscale Res. Lett. 2012, 7, 151. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-W.; Yang, T.-H.; Yang, W.-S.; Cheng, T.-W.; Chiu, W.-Y.; Don, T.-M. Improved stability and film formability of oil-based silver nanoparticle suspensions by addition of polystyrene. Mater. Chem. Phys. 2022, 282, 125930. [Google Scholar] [CrossRef]
- Rahman, A.; Kumar, S.; Bafana, A.; Dahoumane, S.; Jeffryes, C. Biosynthetic Conversion of Ag+ to highly Stable Ag0 Nanoparticles by Wild Type and Cell Wall Deficient Strains of Chlamydomonas reinhardtii. Molecules 2018, 24, 98. [Google Scholar] [CrossRef]
- Kang, H.; Buchman, J.T.; Rodriguez, R.S.; Ring, H.L.; He, J.; Bantz, K.C.; Haynes, C.L. Stabilization of Silver and Gold Nanoparticles: Preservation and Improvement of Plasmonic Functionalities. Chem. Rev. 2019, 119, 664–699. [Google Scholar] [CrossRef]
- Betancourt, A.P.; Goswami, D.Y.; Bhethanabotla, V.R.; Kuhn, J.N. Scalable and stable silica-coated silver nanoparticles, produced by electron beam evaporation and rapid thermal annealing, for plasmon-enhanced photocatalysis. Catal. Commun. 2021, 149, 106213. [Google Scholar] [CrossRef]
- Bordbar, M.; Mortazavimanesh, N. Biosynthesis of waste pistachio shell supported silver nanoparticles for the catalytic reduction processes. IET Nanobiotechnol. 2018, 12, 939–945. [Google Scholar] [CrossRef]
- Wojtysiak, S.; Solla-Gullón, J.; Dłużewski, P.; Kudelski, A. Synthesis of core–shell silver–platinum nanoparticles, improving shell integrity. Colloids Surf. Physicochem. Eng. Asp. 2014, 441, 178–183. [Google Scholar] [CrossRef]
- Salazar-Bryam, A.M.; Yoshimura, I.; Santos, L.P.; Moura, C.C.; Santos, C.C.; Silva, V.L.; Lovaglio, R.B.; Costa Marques, R.F.; Jafelicci Junior, M.; Contiero, J. Silver nanoparticles stabilized by ramnolipids: Effect of pH. Colloids Surf. B Biointerfaces 2021, 205, 111883. [Google Scholar] [CrossRef]
- Akhtar, N.; Mohammed, H.A.; Yusuf, M.; Al-Subaiyel, A.; Sulaiman, G.M.; Khan, R.A. SPIONs Conjugate Supported Anticancer Drug Doxorubicin’s Delivery: Current Status, Challenges, and Prospects. Nanomaterials 2022, 12, 3686. [Google Scholar] [CrossRef]
- Wojnicki, M.; Tokarski, T.; Hessel, V.; Fitzner, K.; Luty-Błocho, M. Continuous, monodisperse silver nanoparticles synthesis using microdroplets as a reactor. J. Flow Chem. 2019, 9, 1–7. [Google Scholar] [CrossRef]
- Salem, S.S.; Fouda, A. Green Synthesis of Metallic Nanoparticles and Their Prospective Biotechnological Applications: An Overview. Biol. Trace Elem. Res. 2021, 199, 344–370. [Google Scholar] [CrossRef]
- Pandit, C.; Roy, A.; Ghotekar, S.; Khusro, A.; Islam, M.N.; Emran, T.B.; Lam, S.E.; Khandaker, M.U.; Bradley, D.A. Biological agents for synthesis of nanoparticles and their applications. J. King Saud Univ.-Sci. 2022, 34, 101869. [Google Scholar] [CrossRef]
- Yang, C.; Jung, S.; Yi, H. A biofabrication approach for controlled synthesis of silver nanoparticles with high catalytic and antibacterial activities. Biochem. Eng. J. 2014, 89, 10–20. [Google Scholar] [CrossRef]
- Tehri, N.; Vashishth, A.; Gahlaut, A.; Hooda, V. Biosynthesis, antimicrobial spectra and applications of silver nanoparticles: Current progress and future prospects. Inorg. Nano-Met. Chem. 2020, 52, 1–19. [Google Scholar] [CrossRef]
- Jorge De Souza, T.A.; Rosa Souza, L.R.; Franchi, L.P. Silver nanoparticles: An integrated view of green synthesis methods, transformation in the environment, and toxicity. Ecotoxicol. Environ. Saf. 2019, 171, 691–700. [Google Scholar] [CrossRef]
- Fabrega, J.; Luoma, S.N.; Tyler, C.R.; Galloway, T.S.; Lead, J.R. Silver nanoparticles: Behaviour and effects in the aquatic environment. Environ. Int. 2011, 37, 517–531. [Google Scholar] [CrossRef]
- Bianchini, A.; Wood, C.M. Does sulfide or water hardness protect against chronic silver toxicity in Daphnia magna? A critical assessment of the acute-to-chronic toxicity ratio for silver. Ecotoxicol. Environ. Saf. 2008, 71, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Luoma, S.N.; Ho, Y.B.; Bryan, G.W. Fate, bioavailability and toxicity of silver in estuarine environments. Mar. Pollut. Bull. 1995, 31, 44–54. [Google Scholar] [CrossRef]
- Luoma, S.N. Bioavailability of trace metals to aquatic organisms—A review. Sci. Total Environ. 1983, 28, 1–22. [Google Scholar] [CrossRef]
- Adams, N.W.H.; Kramer, J.R. Reactivity of Ag+ ion with thiol ligands in the presence of iron sulfide. Environ. Toxicol. Chem. 1998, 17, 625–629. [Google Scholar] [CrossRef]
- Ratte, H.T. Bioaccumulation and toxicity of silver compounds: A review. Environ. Toxicol. Chem. 1999, 18, 89–108. [Google Scholar] [CrossRef]
- Liao, C.; Li, Y.; Tjong, S. Bactericidal and Cytotoxic Properties of Silver Nanoparticles. Int. J. Mol. Sci. 2019, 20, 449. [Google Scholar] [CrossRef]
- Levard, C.; Hotze, E.M.; Lowry, G.V.; Brown, G.E. Environmental Transformations of Silver Nanoparticles: Impact on Stability and Toxicity. Environ. Sci. Technol. 2012, 46, 6900–6914. [Google Scholar] [CrossRef]
- Tortella, G.R.; Rubilar, O.; Durán, N.; Diez, M.C.; Martínez, M.; Parada, J.; Seabra, A.B. Silver nanoparticles: Toxicity in model organisms as an overview of its hazard for human health and the environment. J. Hazard. Mater. 2020, 390, 121974. [Google Scholar] [CrossRef] [PubMed]
- Souza, L.R.R.; Da Silva, V.S.; Franchi, L.P.; De Souza, T.A.J. Toxic and Beneficial Potential of Silver Nanoparticles: The Two Sides of the Same Coin. In Cellular and Molecular Toxicology of Nanoparticles; Saquib, Q., Faisal, M., Al-Khedhairy, A.A., Alatar, A.A., Eds.; Springer International Publishing: Cham, Switzerland, 2018; Volume 1048, pp. 251–262. [Google Scholar] [CrossRef]
- Liang, D.; Fan, W.; Wu, Y.; Wang, Y. Effect of organic matter on the trophic transfer of silver nanoparticles in an aquatic food chain. J. Hazard. Mater. 2022, 438, 129521. [Google Scholar] [CrossRef]



| No. | Method | Advantages | Disadvantages | References |
|---|---|---|---|---|
| 1 | Chemical reduction | Operate easily Low cost | Toxic and hazardous chemicals | [68] |
| 2 | Microemulsion techniques | Low input of mechanical force Theoretical consistency | Exceptionally susceptible to change Extensive formulation effort Low concentrations of AgNPs | [69] |
| 3 | Photochemical method | In situ highly fast dissolving AgNPs in the luminescence region Utilize at ambient temperature No dangerous or potent reducing agents Not rely on costly equipment or highly trained personnel | Long time duration expensive equipment experimental environment | [52] |
| 4 | Electrochemical reduction | Metal ions come from sarcrificial anodes to reduce the quantity of precursors. Simple reaction control, moderate reaction conditions, and less pollution | Unsuitable for large-scale AgNP production | [64] |
| 5 | Microwave-assisted method | Efficacy of energy conversion at a high level Time-saving Cleanliness, convenience Produce on a large scale AgNPs with maximum dispersal | Expensive equipment Unfeasible for reaction monitoring Unsuitable for scale-up | [70] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, N.P.U.; Dang, N.T.; Doan, L.; Nguyen, T.T.H. Synthesis of Silver Nanoparticles: From Conventional to ‘Modern’ Methods—A Review. Processes 2023, 11, 2617. https://doi.org/10.3390/pr11092617
Nguyen NPU, Dang NT, Doan L, Nguyen TTH. Synthesis of Silver Nanoparticles: From Conventional to ‘Modern’ Methods—A Review. Processes. 2023; 11(9):2617. https://doi.org/10.3390/pr11092617
Chicago/Turabian StyleNguyen, Ngoc Phuong Uyen, Ngoc Tung Dang, Linh Doan, and Thi Thu Hoai Nguyen. 2023. "Synthesis of Silver Nanoparticles: From Conventional to ‘Modern’ Methods—A Review" Processes 11, no. 9: 2617. https://doi.org/10.3390/pr11092617