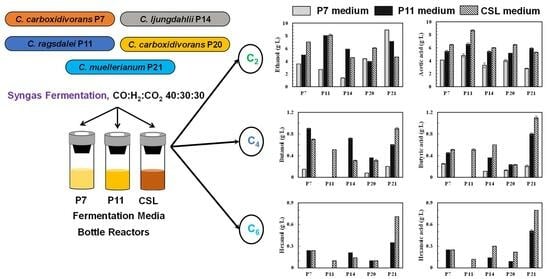
Characterizing Novel Acetogens for Production of C2–C6 Alcohols from Syngas
Abstract
:1. Introduction
2. Material and Methods
2.1. Microorganisms
2.2. Inoculum Preparation
2.3. Syngas Fermentation Medium Preparation
2.4. Analytical Procedures
2.4.1. Cell Mass
2.4.2. Solvent and Gas Analysis
2.4.3. Statistical Analysis and Product Yields
3. Results and Discussion
3.1. Syngas Fermentation in P7 Medium
3.2. Syngas Fermentation in P11 Medium
3.3. Syngas Fermentation in CSL Medium
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fernández-Naveira, Á.; Veiga, M.C.; Kennes, C. Selective anaerobic fermentation of syngas into either C2-C6 organic acids or ethanol and higher alcohols. Bioresour. Technol. 2019, 280, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.J.; Gong, G.; Ahn, J.H.; Ko, J.K.; Lee, S.M.; Um, Y. Effective hexanol production from carbon monoxide using extractive fermentation with Clostridium carboxidivorans P7. Bioresour. Technol. 2023, 367, 128201. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.; Huhnke, R.; Atiyeh, H. Syngas fermentation: A microbial conversion process of gaseous substrates to various products. Fermentation 2017, 3, 28. [Google Scholar] [CrossRef]
- EIA. Available online: https://www.eia.gov/todayinenergy/detail.php?id=53539 (accessed on 5 May 2023).
- Brandt, C.C.; Davis, M.R.; Davison, B.; Eaton, L.M.; Efroymson, R.A.; Hilliard, M.R.; Kline, K.; Langholtz, M.H.; Myers, A.; Sokhansanj, S.; et al. Billion-Ton Report: Advancing Domestic Resources for a Thriving Bioeconomy, Volume 1: Economic Availability of Feedstocks; Oak Ridge National Lab.: Oak Ridge, TN, USA, 2016. [Google Scholar]
- Atiyeh, H.K.; Lewis, R.S.; Phillips, J.R.; Huhnke, R.L. Method Improving Producer Gas Fermentation. U.S. Patents 10053711, 21 August 2018. [Google Scholar]
- Phillips, J.R.; Atiyeh, H.K.; Tanner, R.S.; Torres, J.R.; Saxena, J.; Wilkins, M.R.; Huhnke, R.L. Butanol and hexanol production in Clostridium carboxidivorans syngas fermentation: Medium development and culture techniques. Bioresour. Technol. 2015, 190, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Atiyeh, H.K.; Kumar, A.; Zhang, H.; Tanner, R.S. Biochar enhanced ethanol and butanol production by Clostridium carboxidivorans from syngas. Bioresour. Technol. 2018, 265, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Thunuguntla, R.; Zhang, H.; Atiyeh, H. Biochar amended microbial conversion of C1 gases to ethanol and butanol: Effects of biochar feedstock type and processing temperature. Bioresour. Technol. 2022, 360, 127573. [Google Scholar] [CrossRef]
- De Jong, S.; Antonissen, K.; Hoefnagels, R.; Lonza, L.; Wang, M.; Faaij, A.; Junginger, M. Life-cycle analysis of greenhouse gas emissions from renewable jet fuel production. Biotechnol. Biofuel. 2017, 10, 64. [Google Scholar] [CrossRef]
- Pimentel, D. Ethanol fuels: Energy balance, economics, and environmental impacts are negative. Nat. Resour. Res. 2003, 12, 127–134. [Google Scholar] [CrossRef]
- Subramaniam, Y.; Masron, T.A.; Azman, N.H.N. The impact of biofuels on food security. Int. Econ. 2019, 160, 72–83. [Google Scholar] [CrossRef]
- Sun, X.; Atiyeh, H.K.; Huhnke, R.L.; Tanner, R.S. Syngas fermentation process development for production of biofuels and chemicals: A review. Bioresour. Technol. Rep. 2019, 7, 100279. [Google Scholar] [CrossRef]
- Wu, C.; Lo, J.; Urban, C.; Gao, X.; Yang, B.; Humphreys, J.; Shinde, S.; Wang, X.; Chou, K.J.; Maness, P.; et al. Acetyl-CoA synthesis through a bicyclic carbon-fixing pathway in gas-fermenting bacteria. Nat. Synth. 2022, 1, 615–625. [Google Scholar] [CrossRef]
- Han, Y.-F.; Xie, B.T.; Wu, G.X.; Guo, Y.Q.; Li, D.M.; Huang, Z.Y. Combination of trace metal to improve solventogenesis of Clostridium carboxidivorans P7 in syngas fermentation. Front Microbiol. 2020, 11, 577266. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Fang, B. Application of a two-stage temperature control strategy to enhance 1, 3-propanediol productivity by Clostridium butyricum. J. Chem. Technol. Biotechnol. 2013, 88, 853–857. [Google Scholar] [CrossRef]
- Bengelsdorf, F.R.; Beck, M.H.; Erz, C.; Hoffmeister, S.; Karl, M.M.; Riegler, P.; Wirth, S.; Poehlein, A.; Weuster-Botz, D.; Dürre, P. Bacterial anaerobic synthesis gas (syngas) and CO2+ H2 fermentation. Adv. Appl. Microbiol. 2018, 103, 143–221. [Google Scholar] [PubMed]
- Köpke, M.; Mihalcea, C.; Liew, F.; Tizard, J.H.; Ali, M.S.; Conolly, J.J.; Al-Sinawi, B.; Simpson, S.D. 2,3-Butanediol production by acetogenic bacteria, an alternative route to chemical synthesis, using industrial waste gas. Appl. Environ. Microbiol. 2011, 77, 5467–5475. [Google Scholar] [CrossRef] [PubMed]
- Saxena, J.; Tanner, R.S. Effect of trace metals on ethanol production from synthesis gas by the ethanologenic acetogen, Clostridiumragsdalei. J. Ind. Microbiol. Biotechnol. 2011, 38, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Maddipati, P.; Atiyeh, H.K.; Bellmer, D.D.; Huhnke, R.L. Ethanol production from syngas by Clostridium strain P11 using corn steep liquor as a nutrient replacement to yeast extract. Bioresour. Technol. 2011, 102, 6494–6501. [Google Scholar] [CrossRef]
- Panneerselvam, A. Effect of Glucose and Reducing Agents on Syngas Fermentation by Clostridia Species P11. Master’s Thesis, Department of Biosystems and Agricultural Engineering, Oklahoma State University, Stillwater, OK, USA, 2009; p. 82. [Google Scholar]
- Ramachandriya, K.D.; Kundiyana, D.K.; Wilkins, M.R.; Terrill, J.B.; Atiyeh, H.K.; Huhnke, R.L. Carbon dioxide conversion to fuels and chemicals using a hybrid green process. Appl. Energy. 2013, 112, 289–299. [Google Scholar] [CrossRef]
- Ukpong, M.N.; Atiyeh, H.K.; De Lorme, M.J.; Liu, K.; Zhu, X.; Tanner, R.S.; Wilkins, M.R.; Stevenson, B.S. Physiological response of Clostridium carboxidivorans during conversion of synthesis gas to solvents in a gas-fed bioreactor. Biotechnol. Bioeng. 2012, 109, 2720–2728. [Google Scholar] [CrossRef]
- Kundiyana, D.K.; Huhnke, R.L.; Wilkins, M.R. Effect of nutrient limitation and two-stage continuous fermentor design on productivities during “Clostridium ragsdalei” syngas fermentation. Bioresour. Technol. 2011, 102, 6058–6064. [Google Scholar] [CrossRef]
- Orgill, J.J.; Atiyeh, H.K.; Devarapalli, M.; Phillips, J.R.; Lewis, R.S.; Huhnke, R.L. A comparison of mass transfer coefficients between trickle-bed, hollow fiber membrane and stirred tank reactors. Bioresour. Technol. 2013, 133, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Atiyeh, H.K.; Zhang, H.; Tanner, R.S.; Huhnke, R.L. Enhanced ethanol production from syngas by Clostridium ragsdalei in continuous stirred tank reactor using medium with poultry litter biochar. Appl. Energy 2019, 236, 1269–1279. [Google Scholar] [CrossRef]
- Karlson, B.; Bellavitis, C.; France, N. Commercializing LanzaTech, from waste to fuel: An effectuation case. J. Manag. Organ. 2021, 27, 175–196. [Google Scholar] [CrossRef]
- Köpke, M.; Mihalcea, C.; Bromley, J.C.; Simpson, S.D. Fermentative production of ethanol from carbon monoxide. Curr. Opin. Biotechnol. 2011, 22, 320–325. [Google Scholar] [CrossRef] [PubMed]
- LanzaTech. The LanzaTech Process. 2015. Available online: https://www.lanzajet.com/what-we-do (accessed on 3 March 2023).
- Kottenhahn, P.; Philipps, G.; Jennewein, S. Hexanol biosynthesis from syngas by Clostridium carboxidivorans P7–product toxicity, temperature dependence and in situ extraction. Heliyon 2021, 7, e07732. [Google Scholar] [CrossRef] [PubMed]
- Lauer, I.; Philipps, G.; Jennewein, S. Syngas Fermentation: Toxicity Analysis of Potential Products on Clostridium ljungdahlii; Clostridium XV: 18-19.09; TU Munich: Freising, Germany, 2018. [Google Scholar]
- Shen, S.; Gu, Y.; Chai, C.; Jiang, W.; Zhuang, Y.; Wang, Y. Enhanced alcohol titre and ratio in carbon monoxide-rich off-gas fermentation of Clostridium carboxidivorans through combination of trace metals optimization with variable-temperature cultivation. Bioresour. Technol. 2017, 239, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Huhnke, R.L.; Lewis, R.S.; Tanner, R.S. Isolation and Characterization of Novel Clostridial Species. U.S. Patent 7,704,723, 27 April 2010. [Google Scholar]
- Liou, J.S.; Balkwill, D.L.; Drake, G.R.; Tanner, R.S. Clostridium carboxidivorans sp. nov., a solvent-producing clostridium isolated from an agricultural settling lagoon, and reclassification of the acetogen Clostridium scatologenes strain SL1 as Clostridium drakei sp. nov. Int. J. Syst. Evol. Microbiol. 2005, 55, 2085–2091. [Google Scholar] [CrossRef]
- Doyle, D.A.; Smith, P.R.; Lawson, P.A.; Tanner, R.S. Clostridium muellerianum sp. nov., a carbon monoxide-oxidizing acetogen isolated from old hay. Int. J. Syst. Evol. Microbiol. 2022, 72, 005297. [Google Scholar] [CrossRef]
- Thunuguntla, R.; Atiyeh, H.K.; Huhnke, R.L.; Tanner, R.S. CO2-based production of C2-C6 acids and alcohols: The potential of novel Clostridia. Bioresour. Technol. Rep. 2023, 25, 101713. [Google Scholar] [CrossRef]
- Tanner, R.S. Cultivation of bacteria and fungi. In Manual of Environmental Microbiology, 3rd ed.; Hurst, C.J., Crawford, R.L., Mills, A.L., Garland, J.L., Stetzenbach, L.D., Lipson, D.A., Eds.; ASM Press: Washington, DC, USA, 2007; pp. 69–78. [Google Scholar]
- Liu, K.; Atiyeh, H.K.; Stevenson, B.S.; Tanner, R.S.; Wilkins, M.R.; Huhnke, R.L. Continuous syngas fermentation for the production of ethanol, n-propanol and n-butanol. Bioresour. Technol. 2014, 151, 69–77. [Google Scholar] [CrossRef]
- Hu, P.; Bowen, S.H.; Lewis, R.S. A thermodynamic analysis of electron production during syngas fermentation. Bioresour. Technol. 2011, 102, 8071–8076. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Atiyeh, H.K.; Stevenson, B.S.; Tanner, R.S.; Wilkins, M.R.; Huhnke, R.L. Mixed culture syngas fermentation and conversion of carboxylic acids into alcohols. Bioresour. Technol. 2014, 152, 337–346. [Google Scholar] [CrossRef]
- Devarapalli, M.; Atiyeh, H.K.; Phillips, J.R.; Lewis, R.S.; Huhnke, R.L. Ethanol production during semi-continuous syngas fermentation in a trickle bed reactor using Clostridium ragsdalei. Bioresour. Technol. 2016, 209, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Abubackar, H.N.; Veiga, M.C.; Kennes, C. Carbon monoxide fermentation to ethanol by Clostridium autoethanogenum in a bioreactor with no accumulation of acetic acid. Bioresour. Technol. 2015, 186, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Lauer, I.; Philipps, G.; Jennewein, S. Metabolic engineering of Clostridium ljungdahlii for the production of hexanol and butanol from CO2 and H2. Microb. Cell Factories 2022, 21, 85. [Google Scholar] [CrossRef]
- Kundiyana, D.K.; Huhnke, R.L.; Maddipati, P.; Atiyeh, H.K.; Wilkins, M.R. Feasibility of incorporating cotton seed extract in Clostridium ragsdalei fermentation medium during synthesis gas fermentation. Bioresour. Technol. 2010, 101, 9673–9680. [Google Scholar] [CrossRef]
- Shen, S.; Wang, G.; Zhang, M.; Tang, Y.; Gu, Y.; Jiang, W.; Wang, Y.; Zhuang, Y. Effect of temperature and surfactant on biomass growth and higher-alcohol production during syngas fermentation by Clostridium carboxidivorans P7. Bioresour. Bioprocess. 2020, 7, 56. [Google Scholar] [CrossRef]
- Ramió-Pujol, S.; Ganigué, R.; Bañeras, L.; Colprim, J. Incubation at 25 C prevents acid crash and enhances alcohol production in Clostridium carboxidivorans P7. Bioresour. Technol. 2015, 192, 296–303. [Google Scholar] [CrossRef]
- Oh, H.J.; Ko, J.K.; Gong, G.; Lee, S.M.; Um, Y. Production of hexanol as the main product through syngas fermentation by Clostridium carboxidivorans P7. Front. Bioeng. Biotechnol. 2022, 10, 850370. [Google Scholar] [CrossRef]
- Kim, J.; Kim, K.Y.; Ko, J.K.; Lee, S.M.; Gong, G.; Kim, K.H.; Um, Y. Characterization of a Novel Acetogen Clostridium sp. JS66 for Production of Acids and Alcohols: Focusing on Hexanoic Acid Production from Syngas. Biotechnol. Bioprocess. Eng. 2022, 27, 89–98. [Google Scholar] [CrossRef]
 ], P14 [▲], P20 [◊], and P21 [◯]. (A) pH; (B) cell mass; (C) acetic acid; (D) ethanol; (E) butyric acid; (F) butanol; (G) cumulative H2 uptake; (H) cumulative CO uptake.
], P14 [▲], P20 [◊], and P21 [◯]. (A) pH; (B) cell mass; (C) acetic acid; (D) ethanol; (E) butyric acid; (F) butanol; (G) cumulative H2 uptake; (H) cumulative CO uptake.
 ], P14 [▲], P20 [◊], and P21 [◯]. (A) pH; (B) cell mass; (C) acetic acid; (D) ethanol; (E) butyric acid; (F) butanol; (G) cumulative H2 uptake; (H) cumulative CO uptake.
], P14 [▲], P20 [◊], and P21 [◯]. (A) pH; (B) cell mass; (C) acetic acid; (D) ethanol; (E) butyric acid; (F) butanol; (G) cumulative H2 uptake; (H) cumulative CO uptake.
 ], P14 [▲], P20 [◊], and P21 [◯]. (A) pH; (B) cell mass; (C) acetic acid; (D) ethanol; (E) butyric acid; (F) butanol; (G) hexanoic acid; (H) hexanol; (I) cumulative H2 uptake; (J) cumulative CO uptake.
], P14 [▲], P20 [◊], and P21 [◯]. (A) pH; (B) cell mass; (C) acetic acid; (D) ethanol; (E) butyric acid; (F) butanol; (G) hexanoic acid; (H) hexanol; (I) cumulative H2 uptake; (J) cumulative CO uptake.
 ], P14 [▲], P20 [◊], and P21 [◯]. (A) pH; (B) cell mass; (C) acetic acid; (D) ethanol; (E) butyric acid; (F) butanol; (G) hexanoic acid; (H) hexanol; (I) cumulative H2 uptake; (J) cumulative CO uptake.
], P14 [▲], P20 [◊], and P21 [◯]. (A) pH; (B) cell mass; (C) acetic acid; (D) ethanol; (E) butyric acid; (F) butanol; (G) hexanoic acid; (H) hexanol; (I) cumulative H2 uptake; (J) cumulative CO uptake.

 ], P14 [▲], P20 [◊], and P21 [◯]. (A) pH; (B) cell mass; (C) acetic acid; (D) ethanol; (E) butyric acid; (F) butanol; (G) hexanoic acid; (H) hexanol; (I) cumulative H2 uptake; (J) cumulative CO uptake.
], P14 [▲], P20 [◊], and P21 [◯]. (A) pH; (B) cell mass; (C) acetic acid; (D) ethanol; (E) butyric acid; (F) butanol; (G) hexanoic acid; (H) hexanol; (I) cumulative H2 uptake; (J) cumulative CO uptake.
 ], P14 [▲], P20 [◊], and P21 [◯]. (A) pH; (B) cell mass; (C) acetic acid; (D) ethanol; (E) butyric acid; (F) butanol; (G) hexanoic acid; (H) hexanol; (I) cumulative H2 uptake; (J) cumulative CO uptake.
], P14 [▲], P20 [◊], and P21 [◯]. (A) pH; (B) cell mass; (C) acetic acid; (D) ethanol; (E) butyric acid; (F) butanol; (G) hexanoic acid; (H) hexanol; (I) cumulative H2 uptake; (J) cumulative CO uptake.

 ], P14 [▲], P20 [◊], and P21 [◯].
], P14 [▲], P20 [◊], and P21 [◯].
 ], P14 [▲], P20 [◊], and P21 [◯].
], P14 [▲], P20 [◊], and P21 [◯].
 ), P11 medium (
), P11 medium ( ), and CSL medium (
), and CSL medium ( ).
).
 ), P11 medium (
), P11 medium ( ), and CSL medium (
), and CSL medium ( ).
).
| Components | P7 (g/L) | P11 and CSL (g/L) |
|---|---|---|
| Minerals solution | ||
| NH4Cl | 100 | 100 |
| KH2PO4 | 10 | 10 |
| KCl | 10 | 10 |
| CaCl2∙2H2O | 4 | 4 |
| MgSO4∙7H2O | 20 | 20 |
| Vitamin solution | ||
| Pyridoxine | - | 0.010 |
| Riboflavin | - | 0.005 |
| Thiamine | - | 0.005 |
| Thioctic acid | - | 0.005 |
| Nicotinic acid | - | 0.005 |
| Vitamin B12 | - | 0.005 |
| 2-Mercaptoethanesulfonic acid sodium salt (MESNA) | - | 0.010 |
| Calcium pantothenate | 0.005 | 0.005 |
| p-(4)-Aminobenzoic Acid | 0.005 | 0.005 |
| Biotin | 0.002 | 0.002 |
| Trace Metal Solution | ||
| Nitrilotriacetic acid | 2.00 | 2.00 |
| Fe(NH4)2(SO4)2∙6H2O | 0.80 | 0.80 |
| ZnSO4∙7H2O | 0.20 | 1.00 |
| MnSO4∙H2O | 1.00 | 1.00 |
| NiCl2∙6H2O | 0.02 | 0.20 |
| Na2SeO4 | 0.02 | 0.10 |
| Na2WO4∙2H2O | 0.02 | 0.20 |
| CoCl2∙6H2O | 0.20 | 0.20 |
| Na2MoO4∙2H2O | 0.20 | 0.02 |
| Media | P7 | P11 | CSL |
|---|---|---|---|
| Concentration | mL/L | ||
| Mineral solution a | 20 | 25 | 25 |
| Trace metal solution a | 10 | 10 | 10 |
| Vitamin solution a | 10 | 10 | 10 |
| Resazurin | 1 | 1 | 1 |
| Cysteine-sulfide | 5 | 10 | 10 |
| Others | g/L | ||
| Yeast extract | 0.5 | 0.5 | 0.0 |
| MES monohydrate b | 10 | 10 | 10 |
| CSL b | 0 | 0 | 20 |
| Fermentation Parameters/Strains | P7 | P11 | P14 | P20 | P21 |
|---|---|---|---|---|---|
| P7 Medium | |||||
| Cell mass yield (g/mol) i | 0.9 ± 0.1 A,b | 0.8 ± 0.0 B,c | 0.7 ± 0.0 E,c | 0.7 ± 0.1 C,c | 0.7 ± 0.1 D,c |
| Ethanol yield (%) ii | 54.8 ± 2.3 C,c | 43.6 ± 0.8 D,c | 25.6 ± 0.8 E,c | 61.2 ± 6.4 B,c | 91.6 ± 7.2 A,b |
| Butanol yield (%) ii | 16.7 ± 2.6 A,c | 0.0 ± 0.0 D,b | 0.0 ± 0.0 D,c | 14.5 ± 3.1 B,c | 10.5 ± 1.2 C,c |
| Hexanol yield (%) ii | 0.0 ± 0.0 A,c | 0.0 ± 0.0 A,b | 0.0 ± 0.0 A,c | 0.0 ± 0.0 A,c | 0.0 ± 0.0 A,c |
| EtOH/HAc (mol/mol) iii | 1.4 ± 0.1 C,c | 1.0 ± 0.0 D,c | 0.6 ± 0.0 E,c | 2.1 ± 0.3 B,a | 9.4 ± 1.5 A,a |
| BuOH/HBua (mol/mol) iii | 0.7 ± 0.1 B,c | 0.0 ± 0.0 D,b | 0.0 ± 0.0 D,c | 0.7 ± 0.1 C,c | 1.0 ± 0.1 A,b |
| HeOH/Hhex (mol/mol) iii | 0.0 ± 0.0 A,b | 0.0 ± 0.0 A,b | 0.0 ± 0.0 A,c | 0.0 ± 0.0 A,c | 0.0 ± 0.0 A,c |
| Total alcohols (g/L) | 3.7 ± 0.0 C,c | 2.7 ± 0.1 D,c | 1.4 ± 0.0 E,c | 4.5 ± 0.4 B,b | 9.1 ± 0.1 A,a |
| Total acids (g/L) | 3.5 ± 0.3 A,c | 3.3 ± 0.0 C,c | 3.4 ± 0.1 B,b | 2.9 ± 0.2 D,c | 1.5 ± 0.2 E,c |
| Sp. alcohol yield (galcol/gx) | 10.4 ± 0.4 C,c | 7.0 ± 0.1 D,c | 4.9 ± 0.1 E,c | 12.5 ± 1.3 B,a | 18.6 ± 0.1 A,a |
| Sp. acid yield (gacid/gx) | 9.9 ± 0.5 B,c | 8.9 ± 0.1 C,b | 12.1 ± 0.3 A,b | 8.0 ± 0.4 D,c | 3.0 ± 0.4 E,c |
| CO consumption (%) | 37.6 ± 1.3 C,c | 35.1 ± 0.9 D,c | 31.3 ± 0.7 E,c | 39.0 ± 0.7 B,c | 44.2 ± 1.0 A,b |
| H2 consumption (%) | 19.4 ± 1.3 E,c | 30.0 ± 1.0 C,c | 20.1 ± 0.7 D,c | 34.6 ± 1.2 B,a | 42.4 ± 0.8 A,a |
| P11 Medium | |||||
| Cell mass yield (g/mol) i | 0.9 ± 0.0 A,b | 0.9 ± 0.0 B,b | 0.8 ± 0.0 C,b | 0.9 ± 0.0 AB,b | 0.9 ± 0.1 A,b |
| Ethanol yield (%) ii | 92.2 ± 1.8 C,b | 97.4 ± 0.8 A,a | 90.5 ± 1.8 D,a | 63.4 ± 1.7 E,b | 93.7 ± 1.6 B,a |
| Butanol yield (%) ii | 23.1 ± 0.6 A,b | 0.0 ± 0.0 E,b | 16.5 ± 0.2 C,a | 15.1 ± 1.4 D,b | 17.2 ± 0.5 B,b |
| Hexanol yield (%) ii | 8.7 ± 1.0 B,b | 0.0 ± 0.0 E,b | 5.8 ± 0.1 C,b | 2.8 ± 0.2 D,b | 11.6 ± 0.4 A,b |
| EtOH/HAc (mol/mol) iii | 1.6 ± 0.0 D,a | 3.0 ± 0.1 B,a | 3.5 ± 0.1 A,a | 1.3 ± 0.0 E,b | 2.5 ± 0.0 C,b |
| BuOH/HBua (mol/mol) iii | 2.3 ± 0.0 B,a | 0.0 ± 0.0 E,b | 2.4 ± 0.1 A,a | 1.9 ± 0.2 C,a | 0.9 ± 0.0 D,c |
| HeOH/Hhex (mol/mol) iii | 1.1 ± 0.1 B,a | 0.0 ± 0.0 D,b | 1.7 ± 0.1 A,a | 1.1 ± 0.1 B,a | 0.8 ± 0.0 C,b |
| Total alcohols (g/L) | 6.1 ± 0.0 C,b | 8.1 ± 0.0 A,b | 6.8 ± 0.1 B,a | 4.4 ± 0.1 D,c | 8.1 ± 0.1 A,b |
| Total acids (g/L) | 4.7 ± 0.0 B,b | 3.5 ± 0.2 D,b | 2.7 ± 0.1 E,c | 4.5 ± 0.1 C,b | 5.0 ± 0.1 A,b |
| Sp. alcohol yield (galcol/gx) | 17.0 ± 0.6 C,a | 21.4 ± 0.1 B,a | 24.3 ± 0.5 A,a | 12.3 ± 0.3 E,a | 16.5 ± 0.1 D,b |
| Sp. acid yield (gacid/gx) | 13.1 ± 0.4 A,a | 9.4 ± 0.4 D,b | 9.6 ± 0.3 D,c | 12.6 ± 0.3 B,a | 10.3 ± 0.0 C,b |
| CO consumption (%) | 42.5 ± 0.9 B,a | 47.6 ± 0.9 A,b | 41.8 ± 0.6 C,a | 40.6 ± 0.9 D,b | 47.4 ± 0.8 A,a |
| H2 consumption (%) | 23.1 ± 1.4 E,b | 47.2 ± 1.0 A,a | 28.4 ± 0.8 C,a | 25.6 ± 1.2 D,b | 40.8 ± 1.2 B,b |
| CSL Medium | |||||
| Cell mass yield (g/mol) i | 1.3 ± 0.0 C,a | 1.5 ± 0.1 A,a | 1.2 ± 0.0 D,a | 1.4 ± 0.1 B,a | 1.1 ± 0.1 E,a |
| Ethanol yield (%) ii | 98.1 ± 0.9 A,a | 96.8 ± 0.7 B,b | 85.8 ± 0.8 E,b | 89.7 ± 1.4 C,a | 86.5 ± 1.0 D,c |
| Butanol yield (%) ii | 25.7 ± 1.1 B,a | 17.1 ± 0.9 D,a | 15.8 ± 0.3 E,b | 18.4 ± 0.3 C,a | 30.7 ± 1.4 A,a |
| Hexanol yield (%) ii | 12.3 ± 1.7 B,a | 3.7 ± 0.2 E,a | 8.2 ± 0.4 C,a | 3.9 ± 0.2 D,a | 25.6 ± 0.9 A,a |
| EtOH/HAc (mol/mol) iii | 1.5 ± 0.0 A,b | 1.3 ± 0.0 C,b | 1.0 ± 0.0 E,b | 1.3 ± 0.0 B,b | 1.20± 0.0 D,c |
| BuOH/HBua (mol/mol) iii | 1.5 ± 0.0 B,b | 1.2 ± 0.0 C,a | 0.6 ± 0.0 E,b | 1.6 ± 0.1 A,b | 1.1 ± 0.1 D,a |
| HeOH/Hhex (mol/mol) iii | 1.2 ± 0.1 A,a | 0.8 ± 0.0 C,a | 0.5 ± 0.0 D,b | 0.5 ± 0.0 D,b | 1.1 ± 0.0 B,a |
| Total alcohols (g/L) | 7.9 ± 0.0 B,a | 8.7 ± 0.1 A,a | 5.0 ± 0.0 E,b | 6.5 ± 0.0 C,a | 6.2 ± 0.1 D,c |
| Total acids (g/L) | 7.0 ± 0.1 B,a | 9.0 ± 0.2 A,a | 6.8 ± 0.0 D,a | 6.7 ± 0.1 E,a | 6.8 ± 0.1 C,a |
| Sp. alcohol yield (galcol/gx) | 14.0 ± 0.3 B,b | 14.4 ± 0.2 B,b | 13.2 ± 0.1 C,b | 11.0 ± 0.1 D,b | 16.3 ± 0.8 A,b |
| Sp. acid yield (gacid/gx) | 12.3 ± 0.4 C,b | 14.9 ± 0.4 B,a | 17.7 ± 0.2 A,a | 11.3 ± 0.2 D,b | 17.8 ± 0.9 A,a |
| CO consumption (%) | 41.2 ± 1.0 B,b | 48.0 ± 0.9 A,a | 36.3 ± 0.6 C,b | 42.8 ± 0.8 B,a | 35.2 ± 0.8 D,c |
| H2 consumption (%) | 32.9 ± 1.0 C,a | 45.6 ± 0.9 A,b | 22.0 ± 1.0 E,b | 34.8 ± 0.8 B,a | 25.3 ± 1.1 D,c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thunuguntla, R.; Atiyeh, H.K.; Huhnke, R.L.; Tanner, R.S. Characterizing Novel Acetogens for Production of C2–C6 Alcohols from Syngas. Processes 2024, 12, 142. https://doi.org/10.3390/pr12010142
Thunuguntla R, Atiyeh HK, Huhnke RL, Tanner RS. Characterizing Novel Acetogens for Production of C2–C6 Alcohols from Syngas. Processes. 2024; 12(1):142. https://doi.org/10.3390/pr12010142
Chicago/Turabian StyleThunuguntla, Rahul, Hasan K. Atiyeh, Raymond L. Huhnke, and Ralph S. Tanner. 2024. "Characterizing Novel Acetogens for Production of C2–C6 Alcohols from Syngas" Processes 12, no. 1: 142. https://doi.org/10.3390/pr12010142