Preparation of Nickel-Based Bimetallic Catalyst and Its Activation of Persulfate for Degradation of Methyl Orange
Abstract
:1. Introduction
2. Materials and Methods
2.1. Medicine and Reagent
2.2. Instruments and Equipment
2.3. Method and Procedure
2.3.1. Catalyst Preparation
2.3.2. Optimization of Catalyst Preparation
2.3.3. Test Method for Influencing Factors of Degradation Reaction Efficiency
2.3.4. Research Methods of Catalyst Recovery and Reusability
3. Results and Discussion
3.1. Optimization of Preparation of New Activated Persulfate Catalyst
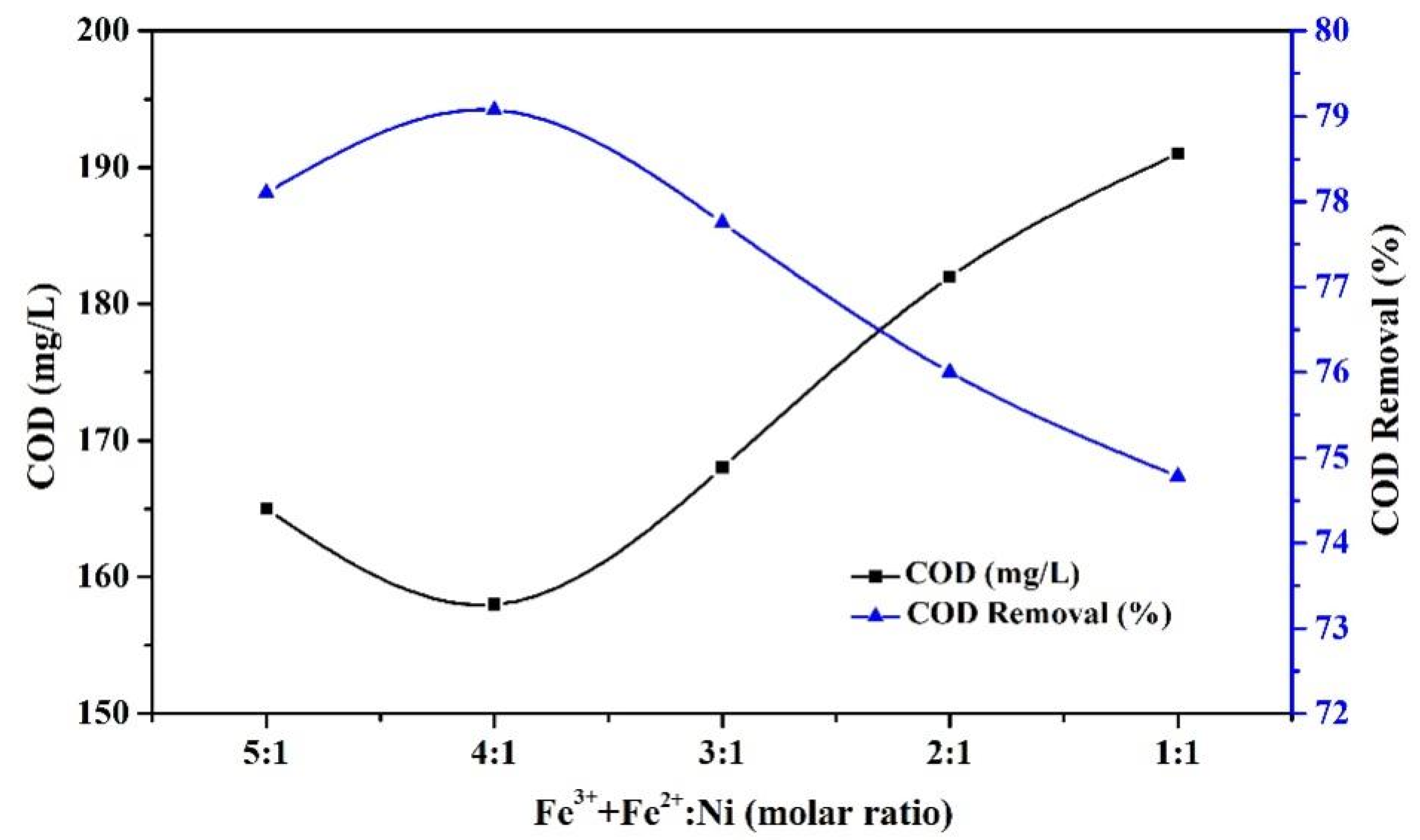
3.1.1. Effect of Ni Doping Amount on the Catalyst
3.1.2. Effect of PAC Addition to the Catalyst
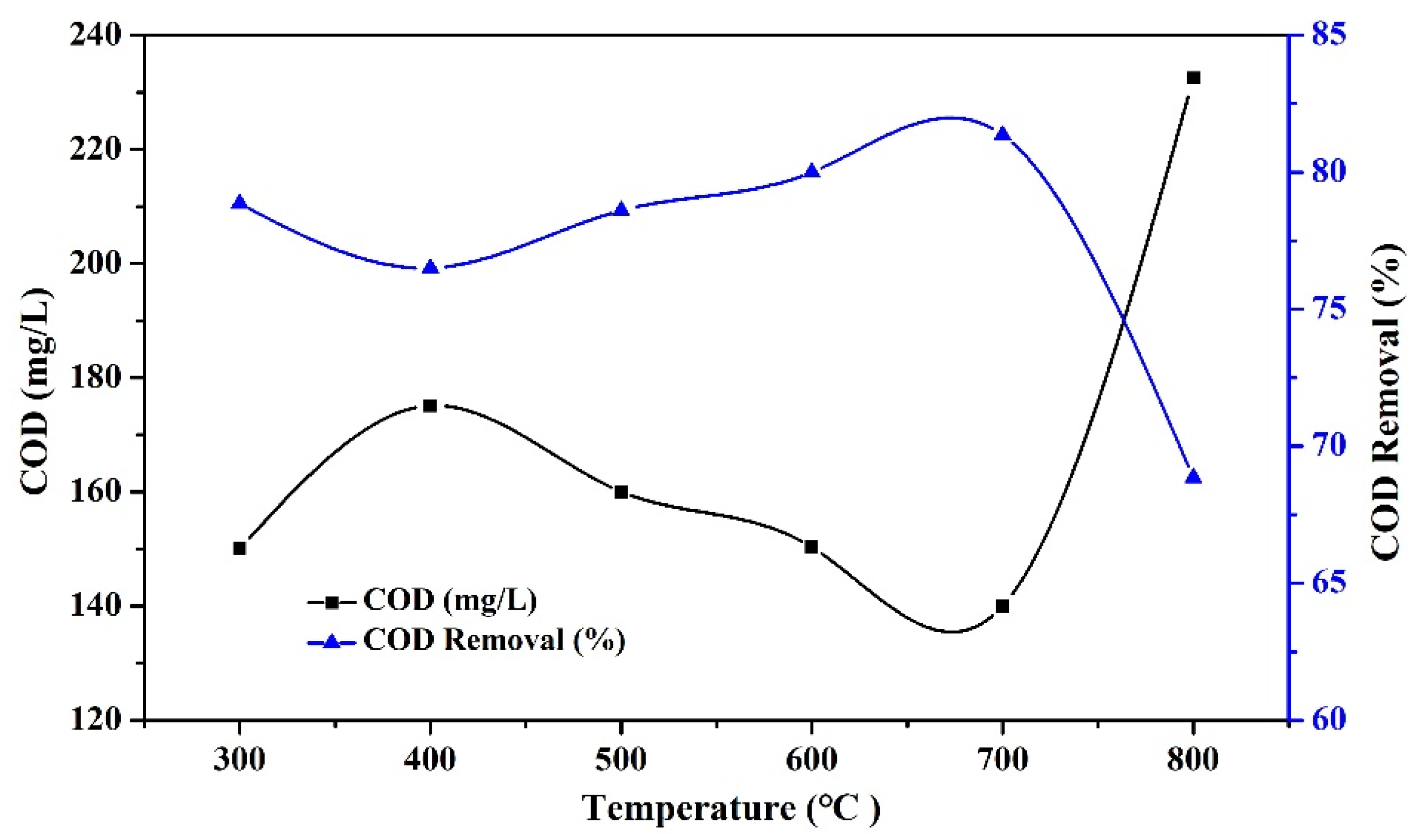
3.1.3. Effect of Calcination Temperature on the Catalyst
3.1.4. Effect of Calcination Time on the Catalyst
3.2. Characterization of Catalyst
3.2.1. SEM Analysis
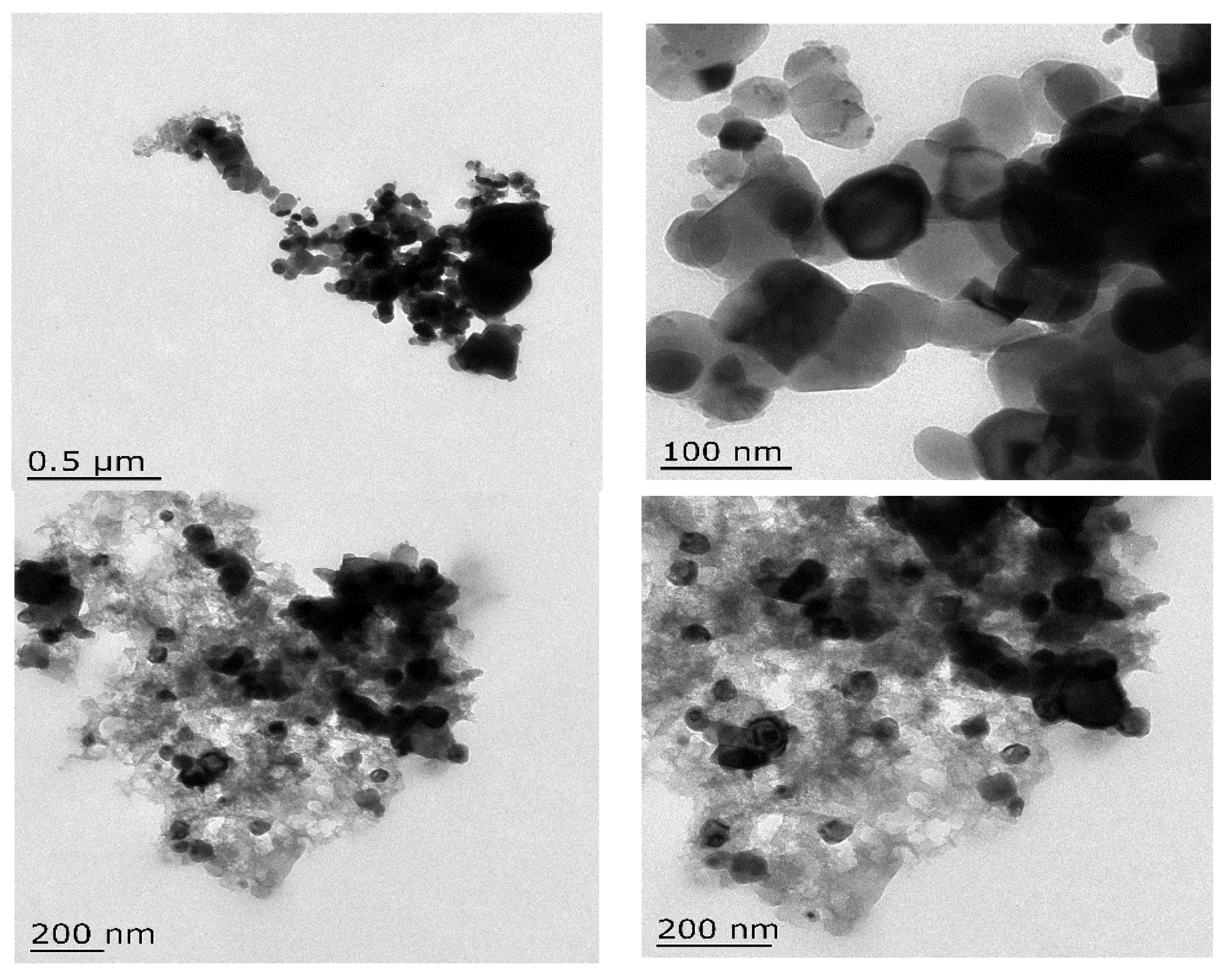
3.2.2. TEM Analysis
3.2.3. EDS Analysis
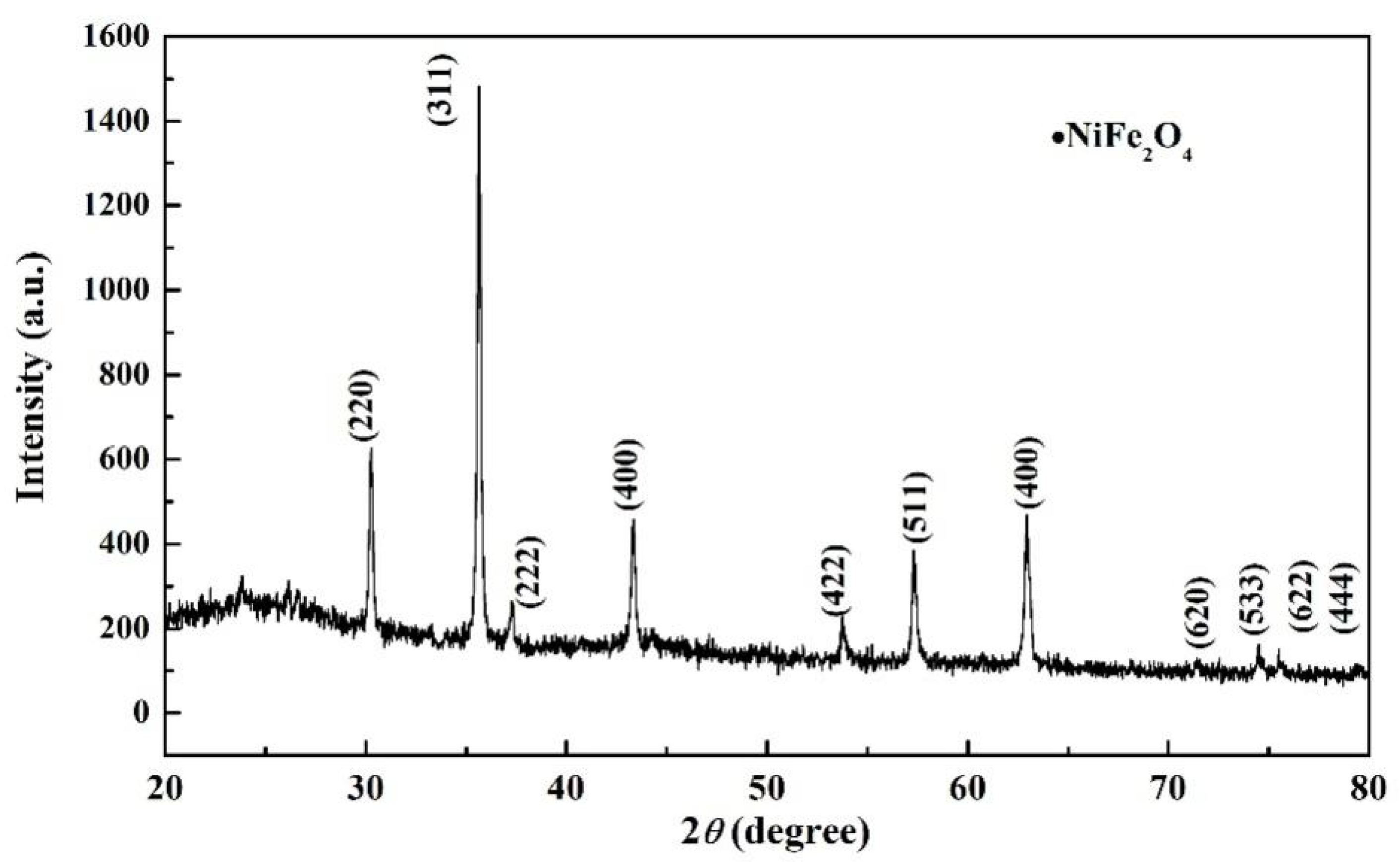
3.2.4. XRD Analysis
3.3. Factors Affecting Degradation Efficiency of Oxidation System
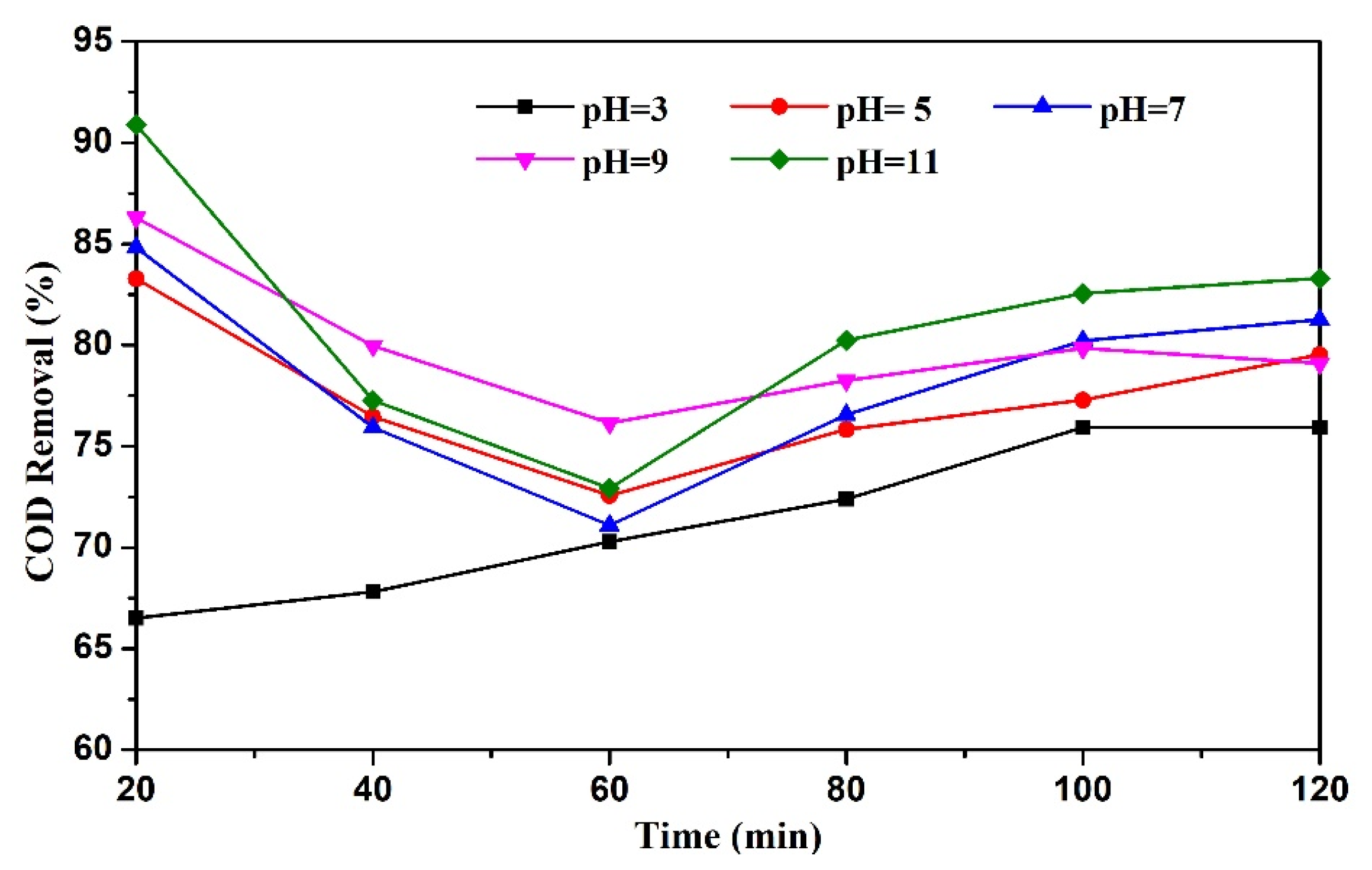
3.3.1. Effect of Initial pH on COD Removal Rate
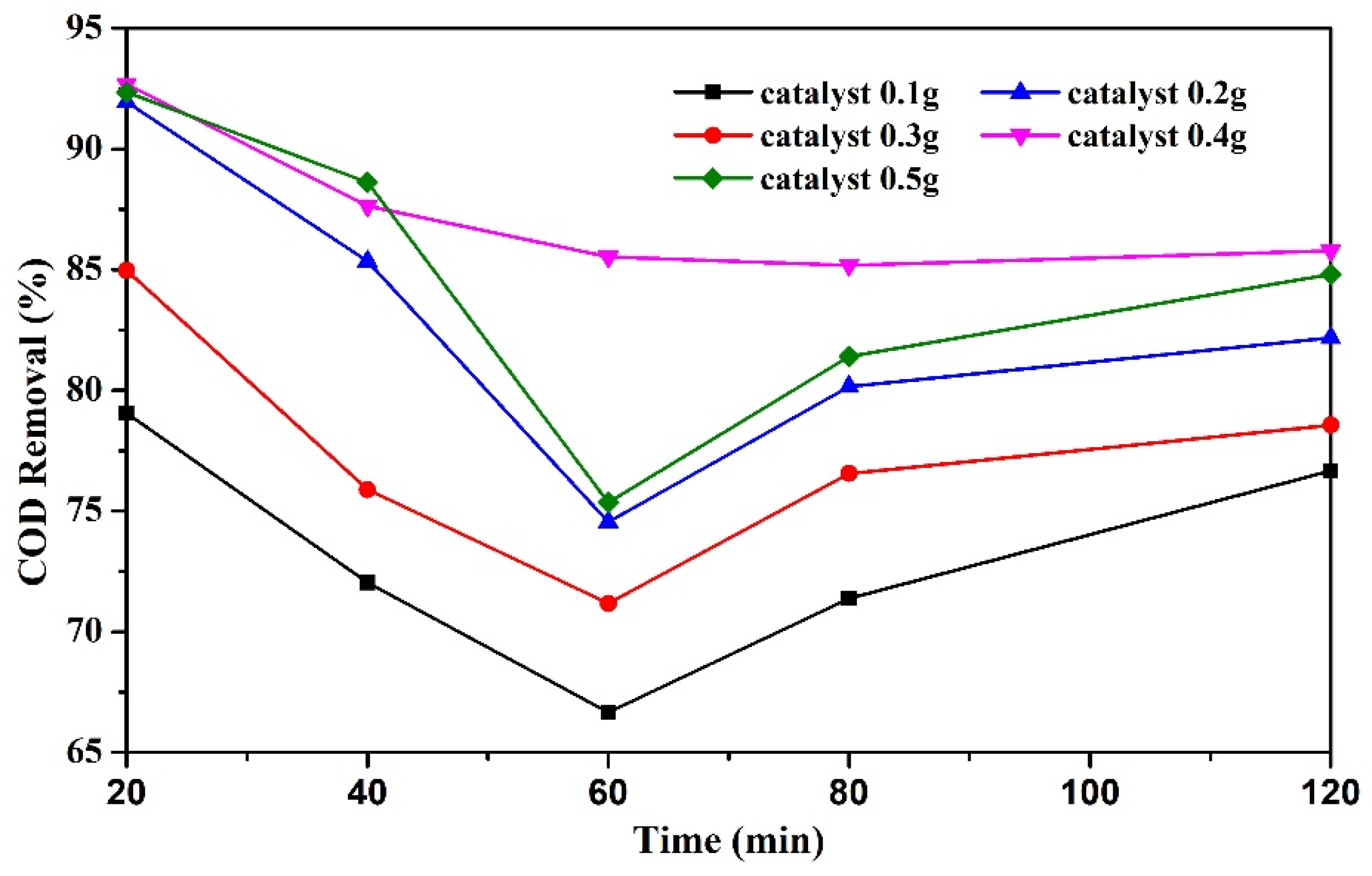
3.3.2. Effect of Catalyst Dosage on COD Removal Rate
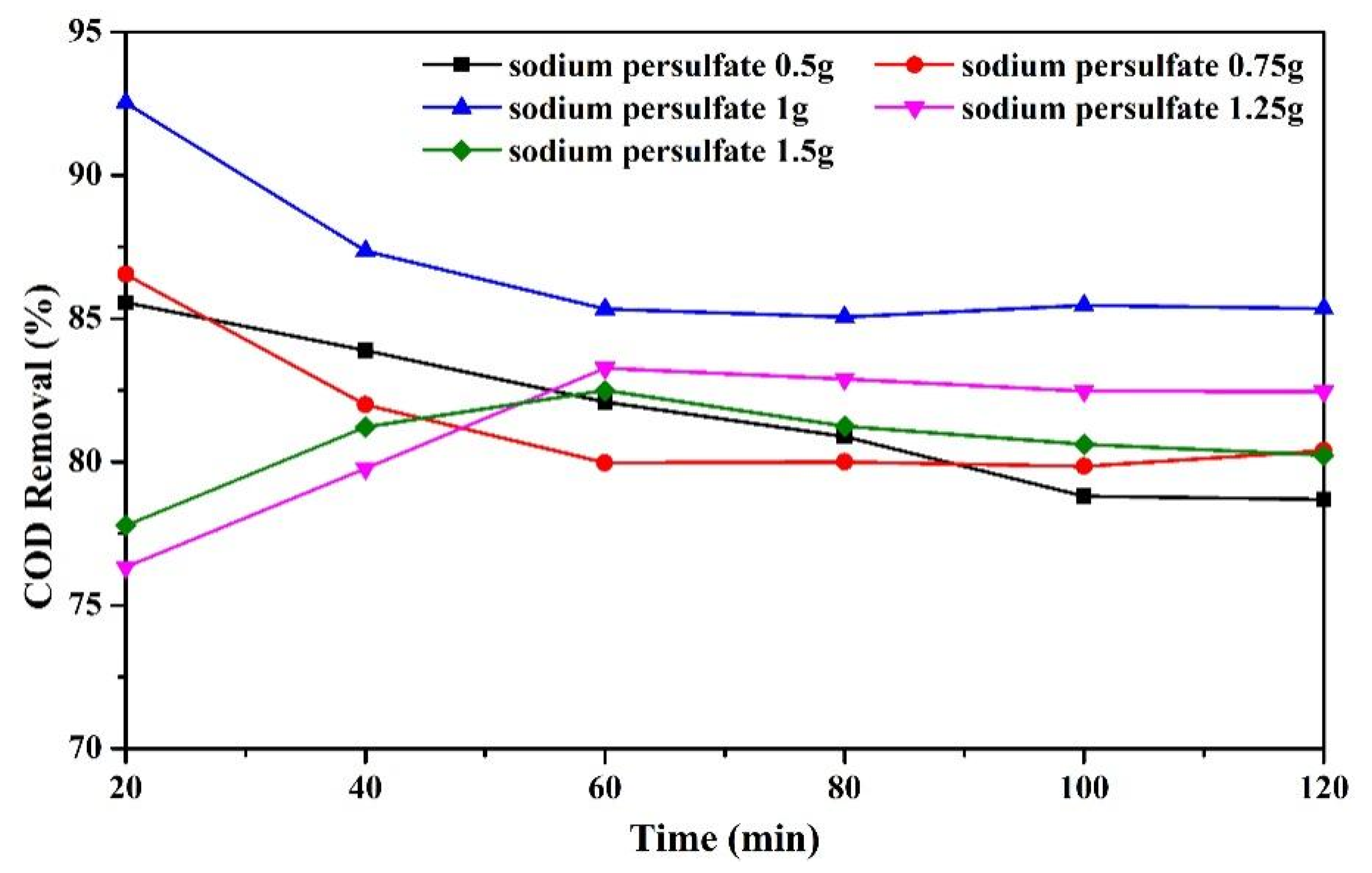
3.3.3. Effect of Sodium Persulfate Dosage on COD Removal Rate
3.4. Catalyst Recovery and Reusability
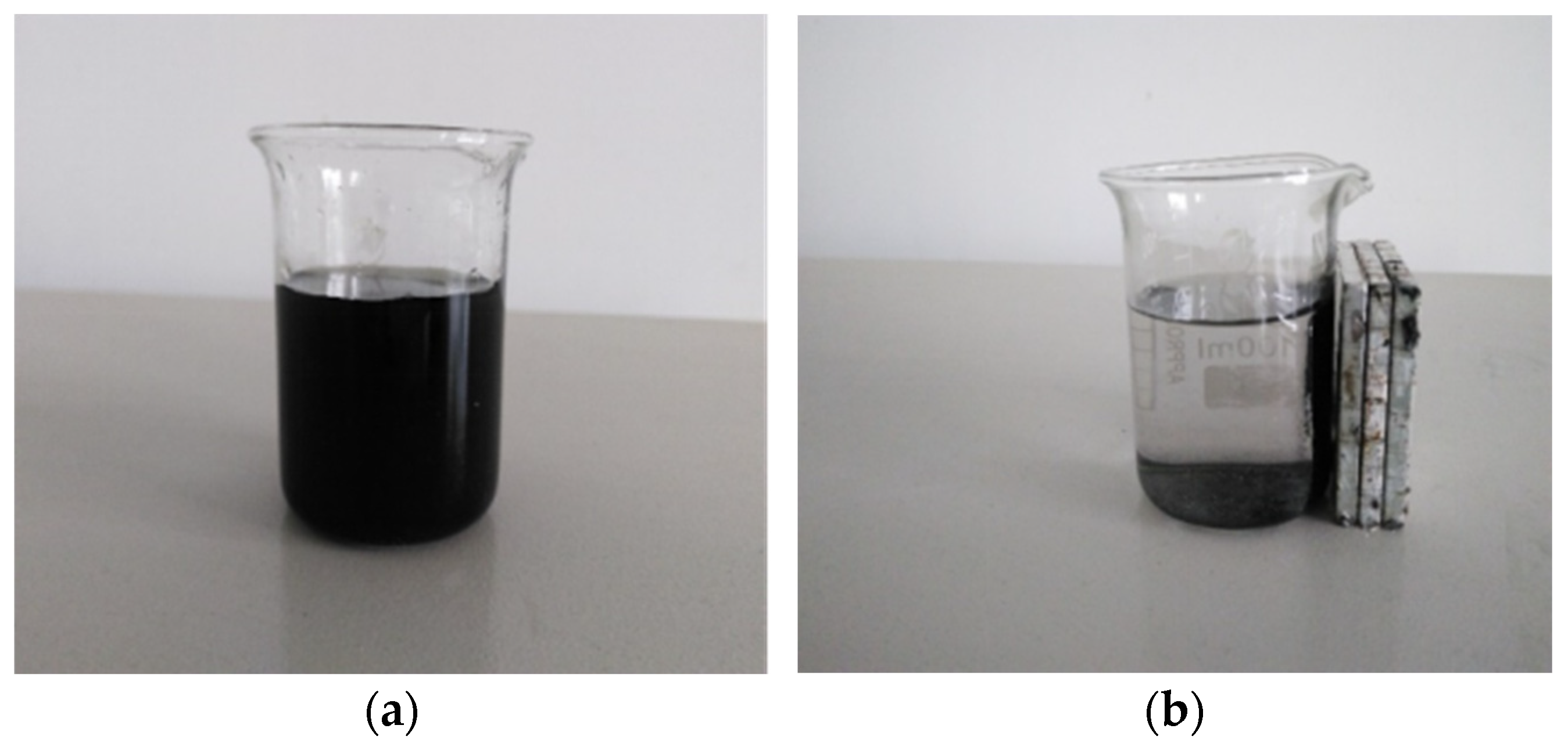
3.4.1. Recovery of Catalyst
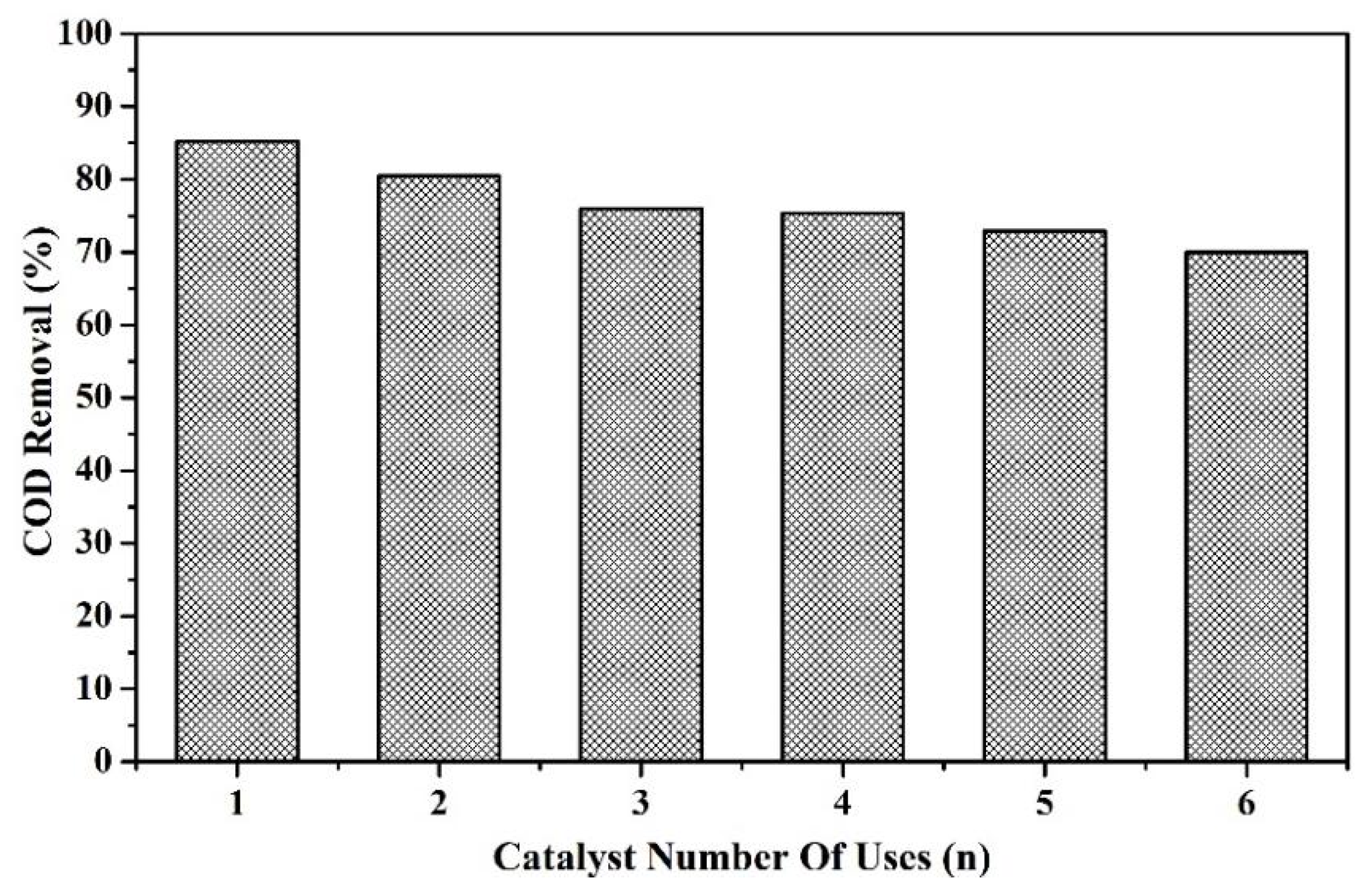
3.4.2. Reusability of the Catalyst
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mi, J.; Tian, L.; Liu, L.; Qi, H.; Wang, Y.; Qin, Y.; Liu, Y. Research Progress on Persulfate Activation Method. Ind. Water Treat. 2020, 40, 12–17. [Google Scholar]
- Tian, K.; Hu, L.; Li, L.; Zheng, Q.; Xin, Y.; Zhang, G. Recent Advances in Persulfate-Based Advanced Oxidation Processes for Organic Wastewater Treatment. Chin. Chem. Lett. 2022, 33, 4461–4477. [Google Scholar] [CrossRef]
- Huang, W.; Xiao, S.; Zhong, H.; Yan, M.; Yang, X. Activation of Persulfates by Carbonaceous Materials: A Review. Chem. Eng. J. 2021, 418, 129297. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, Y.; Wang, S.; Gong, T.; Hua, M.; Qian, J.; Pan, B. Metal-Free Biomass with Abundant Carbonyl Groups as Efficient Catalyst for the Activation of Peroxymonosulfate and Degradation of Sulfamethoxazole. Chem. Eng. J. 2022, 430, 132767. [Google Scholar] [CrossRef]
- Ranjbari, A.; Kim, J.; Yu, J.; Kim, J.; Park, M.; Kim, N.; Demeestere, K.; Heynderickx, P. Effect of Oxygen Vacancy Modification of ZnO on Photocatalytic Degradation of Methyl Orange: A Kinetic Study. Catal. Today 2024, 427, 114413. [Google Scholar] [CrossRef]
- Rao, L.; Yang, Y.; Liu, X.; Huang, Y.; Chen, M.; Yao, Y.; Wang, W. Heterogeneous Activation of Persulfate by Supporting Ferric Oxalate onto Activated Carbon Fibers for Organic Contaminants Removal. Mater. Res. Bull. 2020, 130, 110919. [Google Scholar] [CrossRef]
- Bhat, A.; Gogate, P. Degradation of Nitrogen-Containing Hazardous Compounds Using Advanced Oxidation Processes: A Review on Aliphatic and Aromatic Amines, Dyes, and Pesticides. J. Hazard. Mater. 2021, 403, 123657. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Chen, L. Research Progress on Activation Generation of Sulfate Radical. Appl. Chem. Ind. 2021, 50, 3135–3139. [Google Scholar]
- Dominguez, C.; Romero, A.; Lorenzo, D.; Santos, A. Thermally Activated Persulfate for the Chemical Oxidation of Chlorinated Organic Compounds in Groundwater. J. Environ. Manag. 2020, 261, 110240. [Google Scholar] [CrossRef]
- Fang, Z.; Huang, R.; Chelme-Ayala, P.; Shi, Q.; Xu, C.; El-Din, M. Comparison of UV/Persulfate and UV/H2O2 for the Removal of Naphthenic Acids and Acute Toxicity towards Vibrio Fischeri from Petroleum Production Process Water. Sci. Total Environ. 2019, 694, 133686. [Google Scholar] [CrossRef]
- Wang, Q.; Shi, Y.; Lv, S.; Liang, Y.; Xiao, P. Application Progress of Carbon Material Supported Metal Catalyst in Activation of Persulfate. New Chem. Mater. 2021, 49, 64–67. [Google Scholar]
- Gao, Y.; Zou, D. Efficient Degradation of Levofloxacin by a Microwave-3D ZnCo2O4/Activated Persulfate Process: Effects, Degradation Intermediates, and Acute Toxicity. Chem. Eng. J. 2020, 393, 124795. [Google Scholar] [CrossRef]
- Gujar, S.; Divyapriya, G.; Gogate, P.; Nidheesh, P. Environmental Applications of Ultrasound Activated Persulfate/Peroxymonosulfate Oxidation Process in Combination with Other Activating Agents. Crit. Rev. Environ. Sci. Technol. 2023, 53, 780–802. [Google Scholar] [CrossRef]
- Shi, J.; Wang, L.; Gao, S.; Huang, J.; Yang, H.; Lu, H.; Cao, S. Degradation of Diclofenac by Loaded Solid Superbase-Activated Persulfate. Int. J. Mol. Sci. 2023, 24, 14313. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Wang, G.; Chen, S.; Yu, H.; Ye, F.; Quan, X. Heterogeneous Activation of Peroxymonosulfate by LaCo1-xCuxO3 Perovskites for Degradation of Organic Pollutants. J. Hazard. Mater. 2018, 353, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Huang, R.; Sun, R.; Yang, J.; Sillanpää, M. A Review on Persulfates Activation by Functional Biochar for Organic Contaminants Removal: Synthesis, Characterizations, Radical Determination, and Mechanism. J. Environ. Chem. Eng. 2021, 9, 106267. [Google Scholar] [CrossRef]
- Luo, H.; Zeng, Y.; He, D.; Pan, X. Application of Iron-Based Materials in Heterogeneous Advanced Oxidation Processes for Wastewater Treatment: A Review. Chem. Eng. J. 2021, 407, 127191. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, H.; Zhang, X.; Li, B.; Guo, R.; Cheng, Q.; Cheng, X. Synthesis of Magnetic CuO/MnFe2O4 Nanocompisite and Its High Activity for Degradation of Levofloxacin by Activation of Persulfate. Chem. Eng. J. 2019, 360, 848–860. [Google Scholar] [CrossRef]
- Xian, G.; Niu, L.; Zhang, G.; Zhou, N.; Long, Z.; Zhi, R. An Efficient CuO-γFe2O3 Composite Activates Persulfate for Organic Pollutants Removal: Performance, Advantages and Mechanism. Chemosphere 2020, 242, 125191. [Google Scholar] [CrossRef]
- Tian, D.; Zhou, H.; Zhang, H.; Zhou, P.; You, J.; Yao, G.; Pan, Z.; Liu, Y.; Lai, B. Heterogeneous Photocatalyst-Driven Persulfate Activation Process under Visible Light Irradiation: From Basic Catalyst Design Principles to Novel Enhancement Strategies. Chem. Eng. J. 2022, 428, 131166. [Google Scholar] [CrossRef]
- Ahn, Y.; Yun, E. Heterogeneous Metals and Metal-Free Carbon Materials for Oxidative Degradation through Persulfate Activation: A Review of Heterogeneous Catalytic Activation of Persulfate Related to Oxidation Mechanism. Korean J. Chem. Eng. 2019, 36, 1767–1779. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, G.; Chen, H.; Feng, L.; Wang, D. Research Progress on Heterogeneous Persulfate-Based Catalytic Materials. Mod. Chem. Ind. 2020, 40, 20–25. [Google Scholar]
- Matzek, L.; Carter, K. Activated Persulfate for Organic Chemical Degradation: A Review. Chemosphere 2016, 151, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Zhang, H.; Zhong, X.; Hou, L. Electrochemical Enhanced Heterogeneous Activation of Peroxydisulfate by Fe-Co/SBA-15 Catalyst for the Degradation of Orange II in Water. Water Res. 2014, 66, 473–485. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Yu, J.; Tao, Y.; Li, X.; Hou, J. Research Progress on the Degradation of Organic Pollutants in Water by Persulfate Activated with Different Catalysts. Environ. Pollut. Control 2021, 43, 1344–1349. [Google Scholar]
- Zhang, Y.; Zhang, Q.; Dong, Z.; Wu, L.; Hong, J. Structurally Modified CuFe2O4/Persulfate Process for Acetaminophen Scavenging: High Efficiency with Low Catalyst Addition. J. Chem. Technol. Biotechnol. 2019, 94, 785–794. [Google Scholar] [CrossRef]
- Wu, X.; Sun, D.; Ma, H.; Ma, C.; Zhang, X.; Hao, J. Activation of Peroxymonosulfate by Magnetic CuFe2O4@ZIF-67 Composite Catalyst for the Study on the Degradation of Methylene Blue. Colloids Surf. A Physicochem. Eng. Asp. 2022, 637, 128278. [Google Scholar] [CrossRef]
- Li, Y.; Sun, M.; Gao, B.; Hu, B.; Zhou, S.; Liu, B. 2D ZIF-L Arrays Supported on Zinc Foam to Activate Peroxymonosulfate for Degrading Sulfamethoxazole through Both Radical and Non-Radical Pathways. Sep. Purif. Technol. 2024, 330, 125656. [Google Scholar] [CrossRef]
- Wu, P.; Wu, J.; Huang, X.; Wang, L.; Liu, M.; Wang, Z.; Wang, Z. Effect of Doping Ni on Microstructures and Properties of CoxNi1-xHCF Based Seawater Battery. Vacuum 2024, 220, 112822. [Google Scholar] [CrossRef]
- Heidarinejad, Z.; Dehghani, M.; Heidari, M.; Javedan, G.; Ali, I.; Sillanpää, M. Methods for Preparation and Activation of Activated Carbon: A Review. Environ. Chem. Lett. 2020, 18, 393–415. [Google Scholar] [CrossRef]
- Tan, X.; Liu, S.; Liu, Y.; Gu, Y.; Zeng, G.; Hua, X.; Wang, X.; Liu, S.; Jiang, L. Biochar as Potential Sustainable Precursors for Activated Carbon Production: Multiple Applications in Environmental Protection and Energy Storage. Bioresour. Technol. 2017, 227, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Sha, X.; Lei, Z.; Wang, R.; Zhang, L.; Shu, X. Influences of Different Preparation Conditions on Catalytic Activity of Ag2O-Co3O4/γ-Al2O3 for Hydrogenation of Coal Pyrolysis. J. Spectrosc. 2014, 2014, 272819. [Google Scholar]
- Babyszko, A.; Wanag, A.; Kusiak-Nejman, E.; Morawski, A. Effect of Calcination Temperature of SiO2/TiO2 Photocatalysts on UV-VIS and VIS Removal Efficiency of Color Contaminants. Catalysts 2023, 13, 186. [Google Scholar] [CrossRef]
- Ge, C.; Wang, L.; Liu, G.; Wu, R. Effects of Calcination Temperature on the Electromagnetic Properties of Carbon Nanotubes/Indium Tin Oxide Composites. J. Alloys Compd. 2019, 775, 647–656. [Google Scholar] [CrossRef]
- Wang, M.; Li, C.; Liu, B.; Qin, W.; Xie, Y. Influence of Calcination Temperature on Photocatalyst Performances of Floral Bi2O3/TiO2 Composite. Catalysts 2022, 12, 1635. [Google Scholar] [CrossRef]
- Cao, B.; Liu, Q.; Zhang, D. Synthesis and Characterization of Porous Carbon/Fe Nanocomposite. J. Inorg. Mater. 2010, 25, 457–462. [Google Scholar] [CrossRef]
- Alhalili, Z.; Smiri, M. The Influence of the Calcination Time on Synthesis of Nanomaterials with Small Size, High Crystalline Nature and Photocatalytic Activity in the TiO2 Nanoparticles Calcined at 500 °C. Crystals 2022, 12, 1629. [Google Scholar] [CrossRef]
- Hu, L.; Yan, Z.; Zhang, J.; Peng, X.; Mo, X.; Wang, A.; Chen, L. Surfactant Aggregates within Deep Eutectic Solvent-Assisted Synthesis of Hierarchical ZIF-8 with Tunable Porosity and Enhanced Catalytic Activity. J. Mater. Sci. 2019, 54, 11009–11023. [Google Scholar] [CrossRef]
- Wang, J.; Liu, H. The Effect of the Support Structure on Catalytic Activity: A Case Study on Hollow and Solid MoO3/SiO2. RSC Adv. 2016, 6, 2374–2378. [Google Scholar] [CrossRef]
- Fischbacher, A.; von Sonntag, C.; Schmidt, T. Hydroxyl Radical Yields in the Fenton Process under Various pH, Ligand Concentrations and Hydrogen Peroxide/Fe(II) Ratios. Chemosphere 2017, 182, 738–744. [Google Scholar] [CrossRef]
- Zhao, Q.; Mao, Q.; Zhou, Y.; Wei, J.; Liu, X.; Yang, J.; Luo, L.; Zhang, J.; Chen, H.; Chen, H.; et al. Metal-Free Carbon Materials-Catalyzed Sulfate Radical-Based Advanced Oxidation Processes: A Review on Heterogeneous Catalysts and Applications. Chemosphere 2017, 189, 224–238. [Google Scholar] [CrossRef]
- Oh, H.; Ko, Y. Effect of Polymerization Conditions on the Polymer Properties of CO2-Cyclohexene Oxide Copolymer Prepared by Double Metal Cyanide Catalyst. J. Ind. Eng. Chem. 2013, 19, 1939–1943. [Google Scholar] [CrossRef]
- Eisavi, R.; Ghadernejad, S. NiFe2O4@SiO2-Cu as a Novel and Efficient Magnetically Recoverable Nanocatalyst for Regioselective Synthesis of β-Thiol-1,2,3-Triazoles under Benign Conditions. RSC Adv. 2023, 13, 27984–27996. [Google Scholar] [CrossRef]
- Elansary, M.; Belaiche, M.; Oulhakem, O.; Alaoui, K.B.; Lemine, O.; Mouhib, Y.; Iffer, E.; Salameh, B.; Alsmadi, A. In-Depth Study of the Photocatalytic Performance of Novel Magnetic Catalysts for Efficient Photocatalytic Degradation of the Dye Orange G. Mater. Res. Bull. 2024, 170, 112598. [Google Scholar] [CrossRef]




| Weight/% | ||||
| Element | C | O | Fe | Ni |
| Proportion | 72.84 | 16.76 | 8.37 | 2.03 |
| Atom/% | ||||
| Element | C | O | Fe | Ni |
| Proportion | 83.11 | 14.36 | 2.05 | 0.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Li, J.; Xu, Z.; Xu, X.; Wu, C. Preparation of Nickel-Based Bimetallic Catalyst and Its Activation of Persulfate for Degradation of Methyl Orange. Processes 2024, 12, 322. https://doi.org/10.3390/pr12020322
Zhang B, Li J, Xu Z, Xu X, Wu C. Preparation of Nickel-Based Bimetallic Catalyst and Its Activation of Persulfate for Degradation of Methyl Orange. Processes. 2024; 12(2):322. https://doi.org/10.3390/pr12020322
Chicago/Turabian StyleZhang, Bo, Jiale Li, Zhizhi Xu, Xiaohong Xu, and Chundu Wu. 2024. "Preparation of Nickel-Based Bimetallic Catalyst and Its Activation of Persulfate for Degradation of Methyl Orange" Processes 12, no. 2: 322. https://doi.org/10.3390/pr12020322





