Experimental and Theoretical Analysis of the Thermostatic Drying Process in Wetted Porous Sand Beds with Different Pore Sizes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment
2.1.1. Experimental Methods
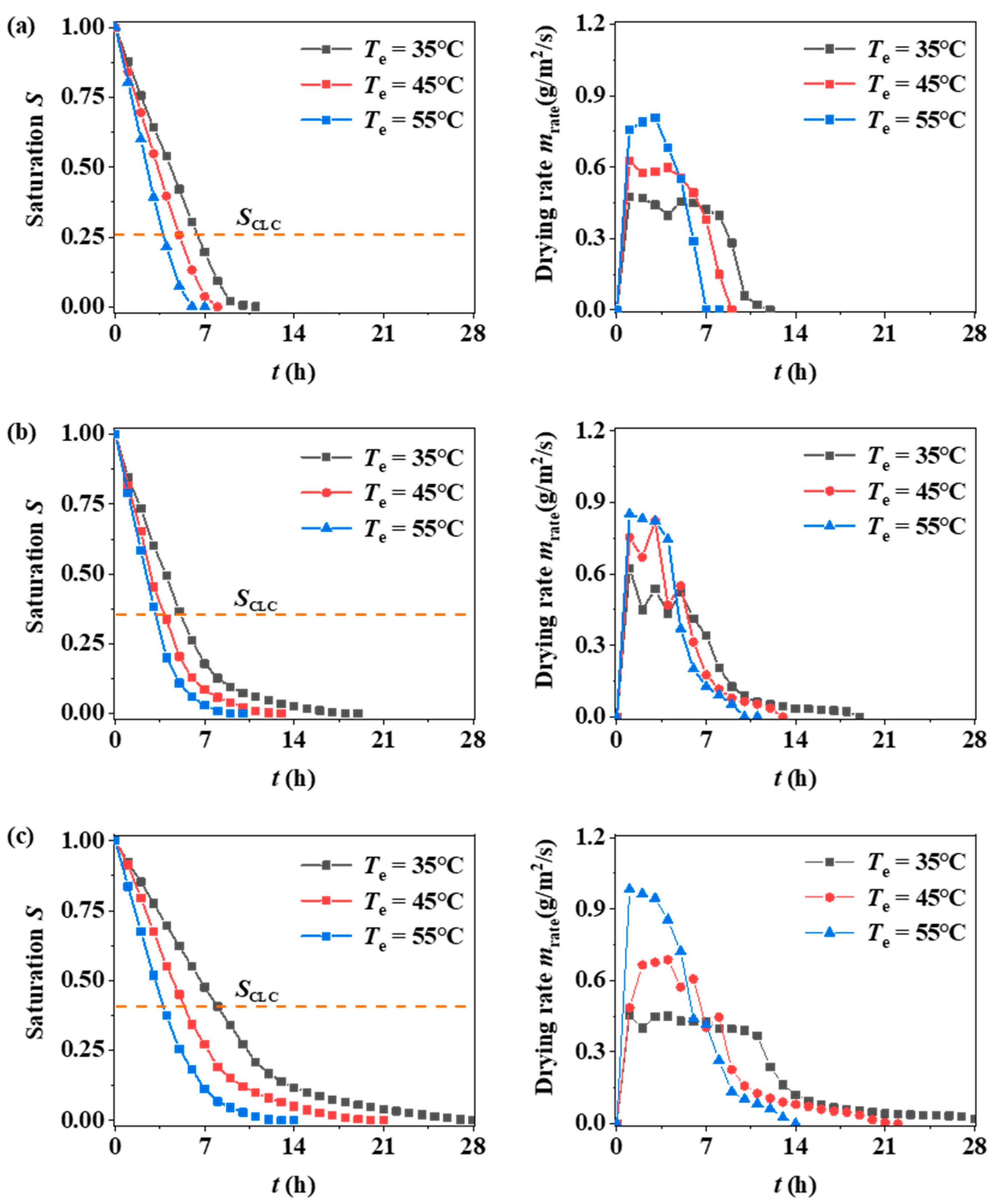
2.1.2. Experimental Data
2.2. Mathematical Model
2.2.1. Physical Model
2.2.2. Mass Conservation Equation
2.2.3. Momentum Equation
2.2.4. Energy Conservation Equation
2.2.5. Initial and Boundary Conditions
2.2.6. Mesh-Independent Verification
3. Results and Discussion
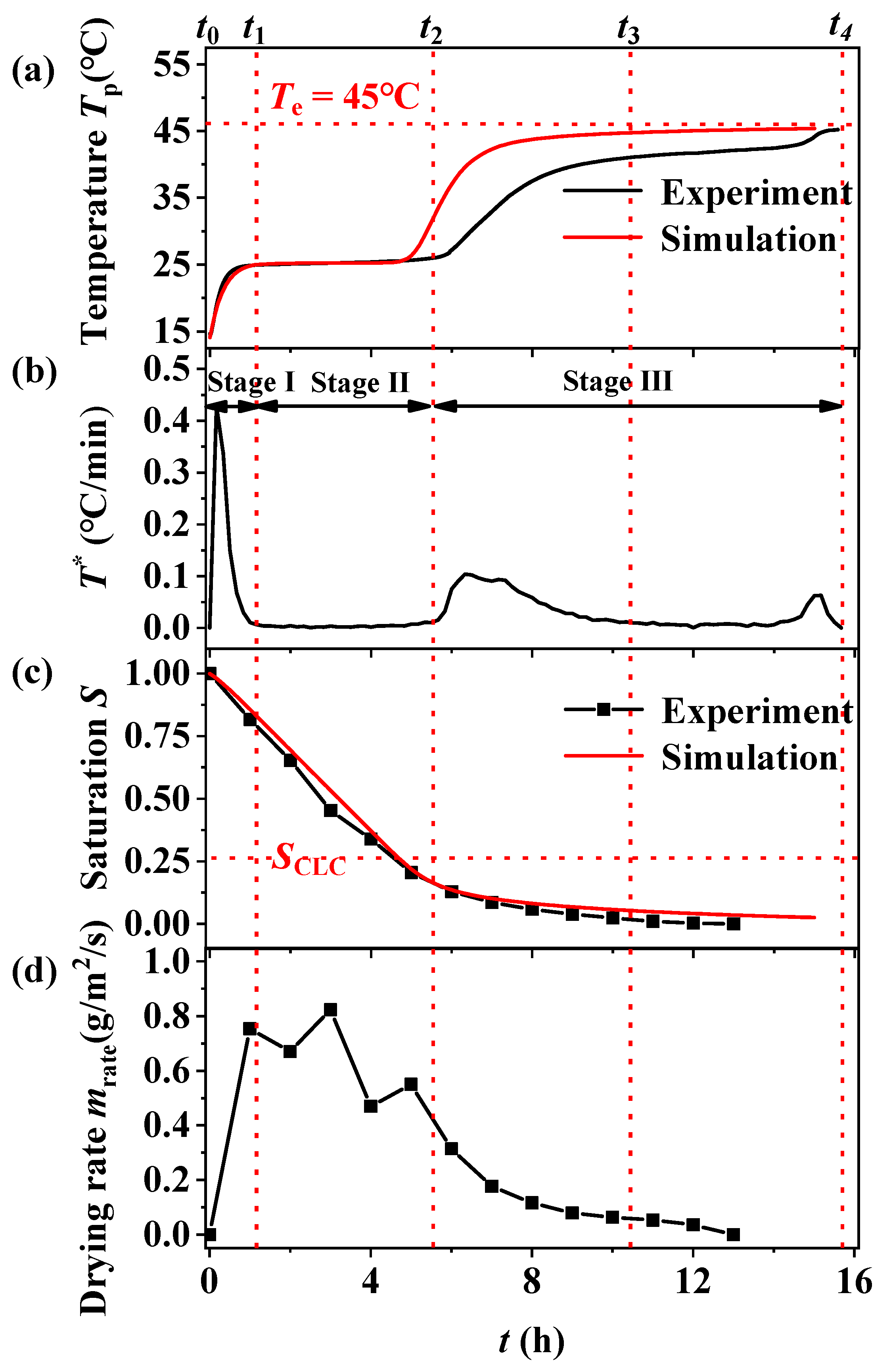
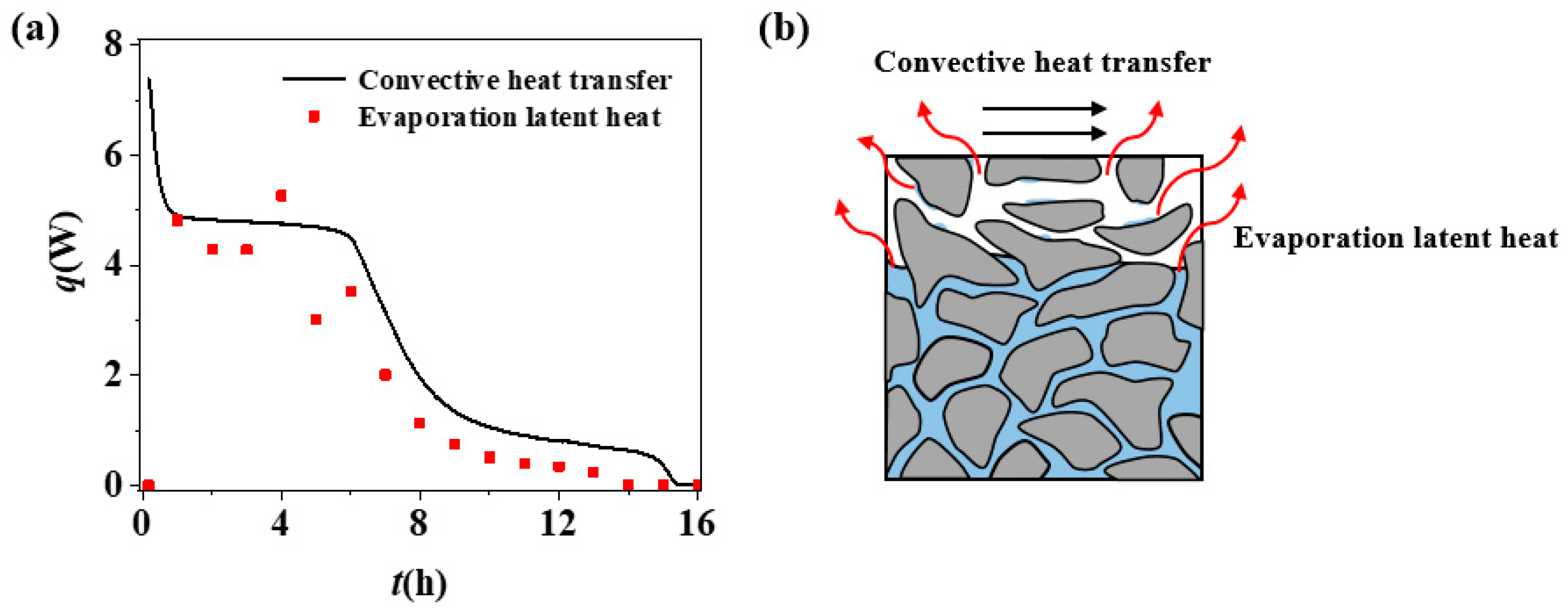
3.1. Drying Mechanism
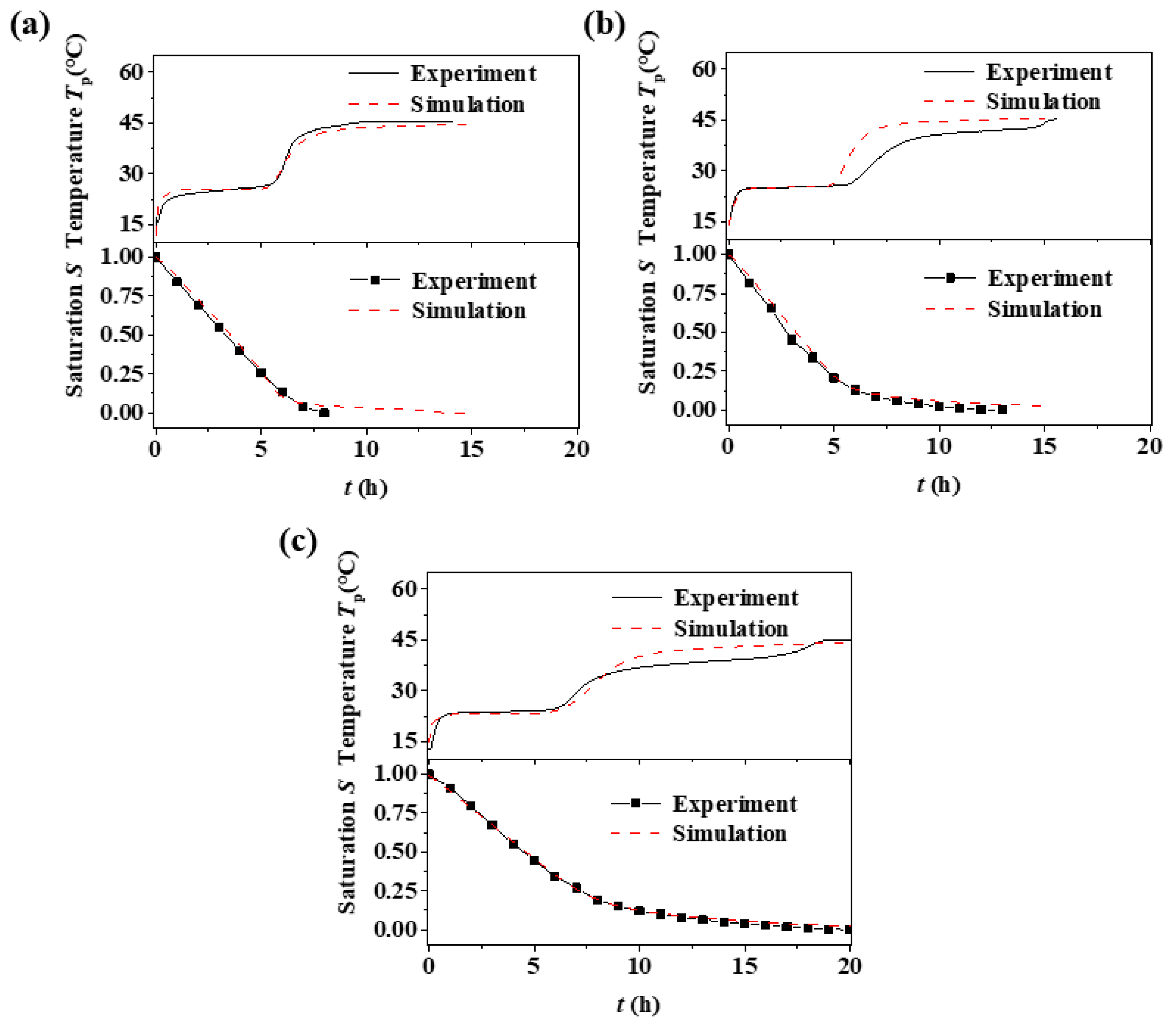
3.2. Sand Bed Particle Size Effect
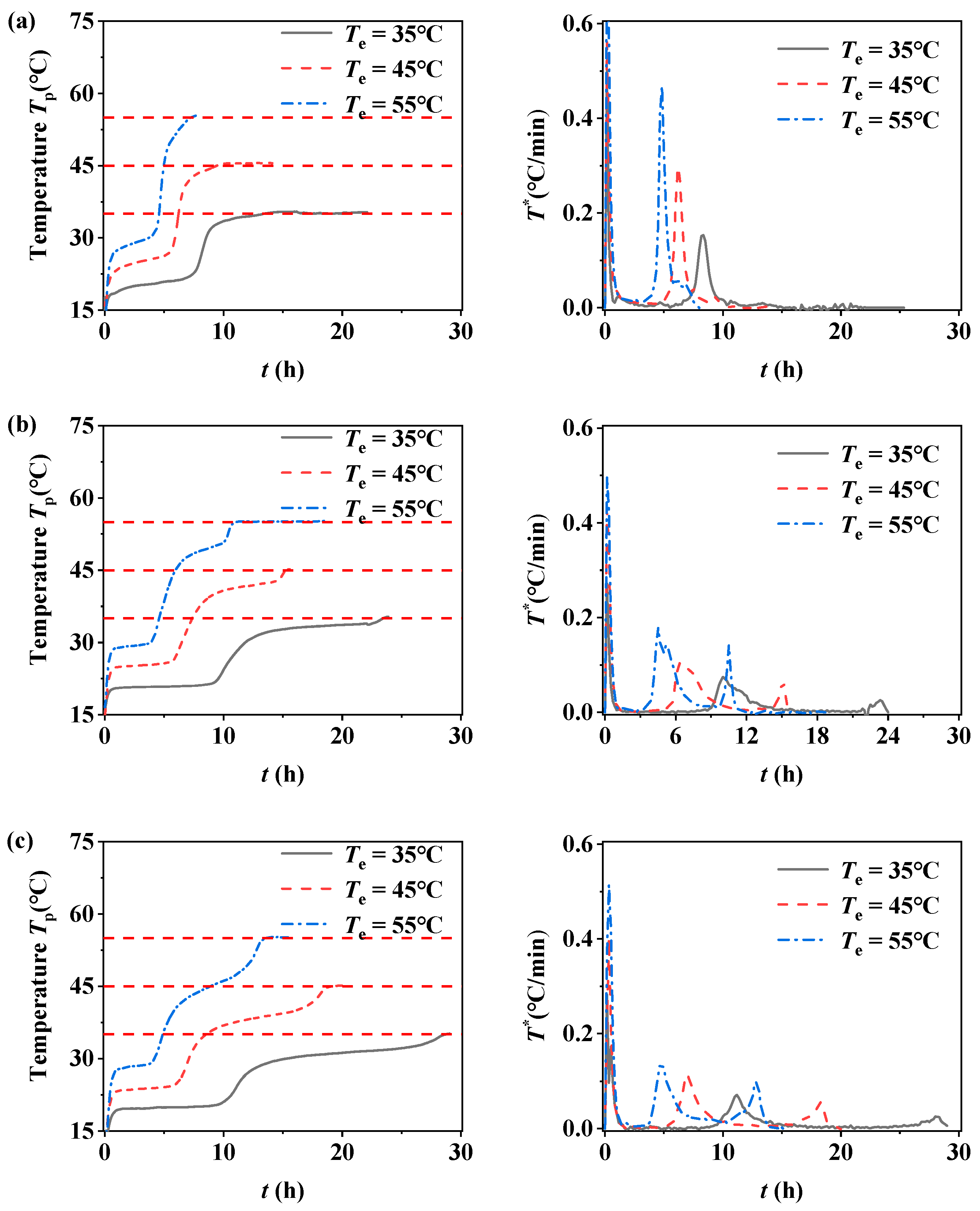
3.3. Drying Temperature Effect
4. Conclusions
- 1.
- There are three stages of porous media drying: Stage I, in the primary warming period, Stage II, in the constant temperature period, and Stage III, in the secondary warming period. With a reduction in the porous media particle size, a temperature plateau occurs in the secondary heating period (Stage III) and the maintenance time of the plateau period is extended with the reduction in the particle size of the filled bed.
- 2.
- Pore size is one of the factors affecting the drying process of porous media. With a reduction in pore size, the capillary effect inside the media is enhanced, gravity is constantly reduced, and the transport of moisture is mainly controlled by the capillary force gradient. This makes the critical liquid content inside the filled bed increase, the temperature rise rate within the second heating period (Stage III) slow down, and the drying rate increase.
- 3.
- The drying environment temperature mainly affects the drying rate, so it has less effect on the critical liquid content controlled by the pore structure of the filled bed.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Huang, Q.; Jiang, Z.; Gu, X.; Zhang, W.; Guo, B. Numerical simulation of moisture transport in concrete based on a pore size distribution model. Cem. Concr. Res. 2015, 67, 31–43. [Google Scholar] [CrossRef]
- Pu, S.R.; Su, J.X.; Li, L.X.; Wang, H.S.; Chen, C.Y.; Hu, X.J. Bioinspired sweating with temperature sensitive hydrogel to passively dissipate heat from high-end wearable electronics. Energy Conv. Manag. 2019, 180, 747–756. [Google Scholar] [CrossRef]
- Lerouge, T.; Maillet, B.; Coutier-Murias, D.; Grande, D.; Le Droumaguet, B.; Pitois, O.; Coussot, P. Drying of a Compressible Biporous Material. Phys. Rev. Appl. 2020, 13, 044061. [Google Scholar] [CrossRef]
- Hnin, K.K.; Zhang, M.; Mujumdar, A.S.; Zhu, Y.L. Emerging food drying technologies with energy-saving characteristics: A review. Dry. Technol. 2019, 37, 1465–1480. [Google Scholar] [CrossRef]
- Radojčin, M.; Pavkov, I.; Bursać Kovačević, D.; Putnik, P.; Wiktor, A.; Stamenković, Z.; Kešelj, K.; Gere, A. Effect of Selected Drying Methods and Emerging Drying Intensification Technologies on the Quality of Dried Fruit: A Review. Processes 2021, 9, 132. [Google Scholar] [CrossRef]
- Zhao, J.; Fu, Z.; Jia, X.; Cai, Y. Modeling conventional drying of wood: Inclusion of a moving evaporation interface. Dry. Technol. 2015, 34, 530–538. [Google Scholar] [CrossRef]
- Wu, R.; Cui, G.-M.; Chen, R. Pore network study of slow evaporation in hydrophobic porous media. Int. J. Heat Mass Transf. 2014, 68, 310–323. [Google Scholar] [CrossRef]
- Khaled, A.Y.; Kabutey, A.; Selvi, K.Ç.; Mizera, Č.; Hrabe, P.; Herák, D. Application of Computational Intelligence in Describing the Drying Kinetics of Persimmon Fruit (Diospyros kaki) During Vacuum and Hot Air Drying Process. Processes 2020, 8, 544. [Google Scholar] [CrossRef]
- Royen, M.J.; Noori, A.W.; Haydary, J. Experimental Study and Mathematical Modeling of Convective Thin-Layer Drying of Apple Slices. Processes 2020, 8, 1562. [Google Scholar] [CrossRef]
- Guzmán-Meza, M.; Laurindo, J.B.; Jarpa-Parra, M.; Segura-Ponce, L. Isothermal drying of plant-based food material: An approach using 2D polydimethylsiloxane (PDMS) micromodels. Chem. Eng. Sci. 2020, 215, 115385. [Google Scholar] [CrossRef]
- Coussot, P. Scaling approach of the convective drying of a porous medium. Eur. Phys. J. B 2000, 15, 557–566. [Google Scholar] [CrossRef]
- Kumar, N.; Arakeri, J.H. Evaporation From Layered Porous Medium in the Presence of Infrared Heating. Water Resour. Res. 2018, 54, 7670–7687. [Google Scholar] [CrossRef]
- Kumar, N.; Arakeri, J.H. Investigation on the effect of temperature on evaporative characteristic length of a porous medium. Dry. Technol. 2019, 38, 1194–1206. [Google Scholar] [CrossRef]
- Ceaglske, N.H.; Hougen, O.A. Drying Granular Solids. Ind. Eng. Chem. 2002, 29, 805–813. [Google Scholar] [CrossRef]
- Thiery, J.; Rodts, S.; Weitz, D.A.; Coussot, P. Drying regimes in homogeneous porous media from macro- to nanoscale. Phys. Rev. Fluids 2017, 2, 074201. [Google Scholar] [CrossRef]
- Lehmann, P.; Assouline, S.; Or, D. Characteristic lengths affecting evaporative drying of porous media. Phys. Rev. E Stat. Nonlin Soft Matter Phys. 2008, 77, 056309. [Google Scholar] [CrossRef]
- Kumar, N.; Arakeri, J.H. Evaporation From Confined Porous Media Due to Controlled IR Heating From Above. Transp. Porous Media 2018, 125, 311–340. [Google Scholar] [CrossRef]
- Tang, Y.; Min, J. Water film coverage model and its application to the convective air-drying simulation of a wet porous medium. Int. J. Heat Mass Transf. 2019, 131, 999–1008. [Google Scholar] [CrossRef]
- Prommas, R. Theoretical and experimental study of heat and mass transfer mechanism during convective drying of multi-layered porous packed bed. Int. Commun. Heat Mass Transf. 2011, 38, 900–905. [Google Scholar] [CrossRef]
- Jean-François Daian, J.S. Determination d’un réseau aleatoire de pores pour modéliser la sorption et la migration d’humidité dans un mortier de ciment. Int. J. Heat Mass Transf. 1991, 34, 2081–2096. [Google Scholar] [CrossRef]
- Nowicki, S.C.; Davis, H.T.; Scriven, L.E. Microscopic determination of transport paraheters in drying porous media. Dry. Technol. 1992, 10, 925–946. [Google Scholar] [CrossRef]
- Whitaker, S. Simultaneous Heat, Mass, and Momentum Transfer in Porous Media: A Theory of Drying. In Advances in Heat Transfer; Academic Press: Cambridge, MA, USA, 1977; Volume 13, pp. 119–203. [Google Scholar]
- Prat, M. Recent advances in pore-scale models for drying of porous media. Chem. Eng. J. 2002, 86, 153–164. [Google Scholar] [CrossRef]
- Whitaker, S. The Method of Volume Averaging; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1998; Volume 13. [Google Scholar]
- Lu, X.; Tsotsas, E.; Kharaghani, A. Scale transition: Pore network study of how pore structure affects the macroscopic parameters of the continuum model for drying. Dry. Technol. 2023, 41, 948–967. [Google Scholar] [CrossRef]
- Vu, H.T.; Tsotsas, E. A Framework and Numerical Solution of the Drying Process in Porous Media by Using a Continuous Model. Int. J. Chem. Eng. 2019, 2019, 9043670. [Google Scholar] [CrossRef]
- Li, Z.; Vanderborght, J.; Smits, K.M. Evaluation of model concepts to describe water transport in shallow subsurface soil and across the soil–air interface. Transp. Porous Media 2019, 128, 945–976. [Google Scholar] [CrossRef]
- Nuske, P.; Joekar-Niasar, V.; Helmig, R. Non-equilibrium in multiphase multicomponent flow in porous media: An evaporation example. Int. J. Heat Mass Transf. 2014, 74, 128–142. [Google Scholar] [CrossRef]
- Stull, D.R. Vapor pressure of pure substances. Organic and inorganic compounds. Ind. Eng. Chem. 1947, 39, 517–540. [Google Scholar] [CrossRef]
- Hegde, V.H.; Doherty, M.F.; Squires, T.M. A two-phase model that unifies and extends the classical models of membrane transport. Science 2022, 377, 186–191. [Google Scholar] [CrossRef]
- Rogers, J.A.; Kaviany, M. Funicular and evaporative-front regimes in convective drying of granular beds. Int. J. Heat Mass Transf. 1992, 35, 469–480. [Google Scholar] [CrossRef]
- Ullman, W.J.; Aller, R.C. Diffusion-coefficients in nearshore marine-sediments. Limnol. Oceanogr. 1982, 27, 552–556. [Google Scholar] [CrossRef]
- Devries, D.A. The theory of heat and moisture transfer in porous-media revisited. Int. J. Heat Mass Transf. 1987, 30, 1343–1350. [Google Scholar] [CrossRef]
- Bruggeman, V.D. Berechnung verschiedener physikalischer Konstanten von heterogenen Substanzen. I. Dielektrizitätskonstanten und Leitfähigkeiten der Mischkörper aus isotropen Substanzen. Ann. Phys. 1935, 416, 636–664. [Google Scholar] [CrossRef]
- Shen, L.; Chen, Z.X. Critical review of the impact of tortuosity on diffusion. Chem. Eng. Sci. 2007, 62, 3748–3755. [Google Scholar] [CrossRef]
- Moseley, W.A.; Dhir, V.K. Capillary pressure-saturation relations in porous media including the effect of wettability. J. Hydrol. 1996, 178, 33–53. [Google Scholar] [CrossRef]
- Huang, C.; Siang, H.; Best, C. Heat and moisture transfer in concrete slabs. Int. J. Heat Mass Transf. 1979, 22, 257–266. [Google Scholar] [CrossRef]
- Ilic, M.; Turner, I.W. Convective drying of a consolidated slab of wet porous material. Int. J. Heat Mass Transf. 1989, 32, 2351–2362. [Google Scholar] [CrossRef]
- Autengruber, M.; Lukacevic, M.; Füssl, J. Finite-element-based moisture transport model for wood including free water above the fiber saturation point. Int. J. Heat Mass Transf. 2020, 161, 120228. [Google Scholar] [CrossRef]
- Sherwood, T.K. The drying of solids—II. Ind. Eng. Chem. 1929, 21, 976–980. [Google Scholar] [CrossRef]






| Stage | Particle Size dp(μm) | Starting Saturation |
|---|---|---|
| Stage III | 4–6 | 0.342 |
| 48–75 | 0.338 | |
| 430–700 | 0.257 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, W.; Cao, X.; Deng, Z. Experimental and Theoretical Analysis of the Thermostatic Drying Process in Wetted Porous Sand Beds with Different Pore Sizes. Processes 2024, 12, 337. https://doi.org/10.3390/pr12020337
Su W, Cao X, Deng Z. Experimental and Theoretical Analysis of the Thermostatic Drying Process in Wetted Porous Sand Beds with Different Pore Sizes. Processes. 2024; 12(2):337. https://doi.org/10.3390/pr12020337
Chicago/Turabian StyleSu, Weijie, Xiang Cao, and Zilong Deng. 2024. "Experimental and Theoretical Analysis of the Thermostatic Drying Process in Wetted Porous Sand Beds with Different Pore Sizes" Processes 12, no. 2: 337. https://doi.org/10.3390/pr12020337




