Pulsed Electric Field Technology for Recovery of Proteins from Waste Plant Resources and Deformed Mushrooms: A Review
Abstract
:1. Introduction
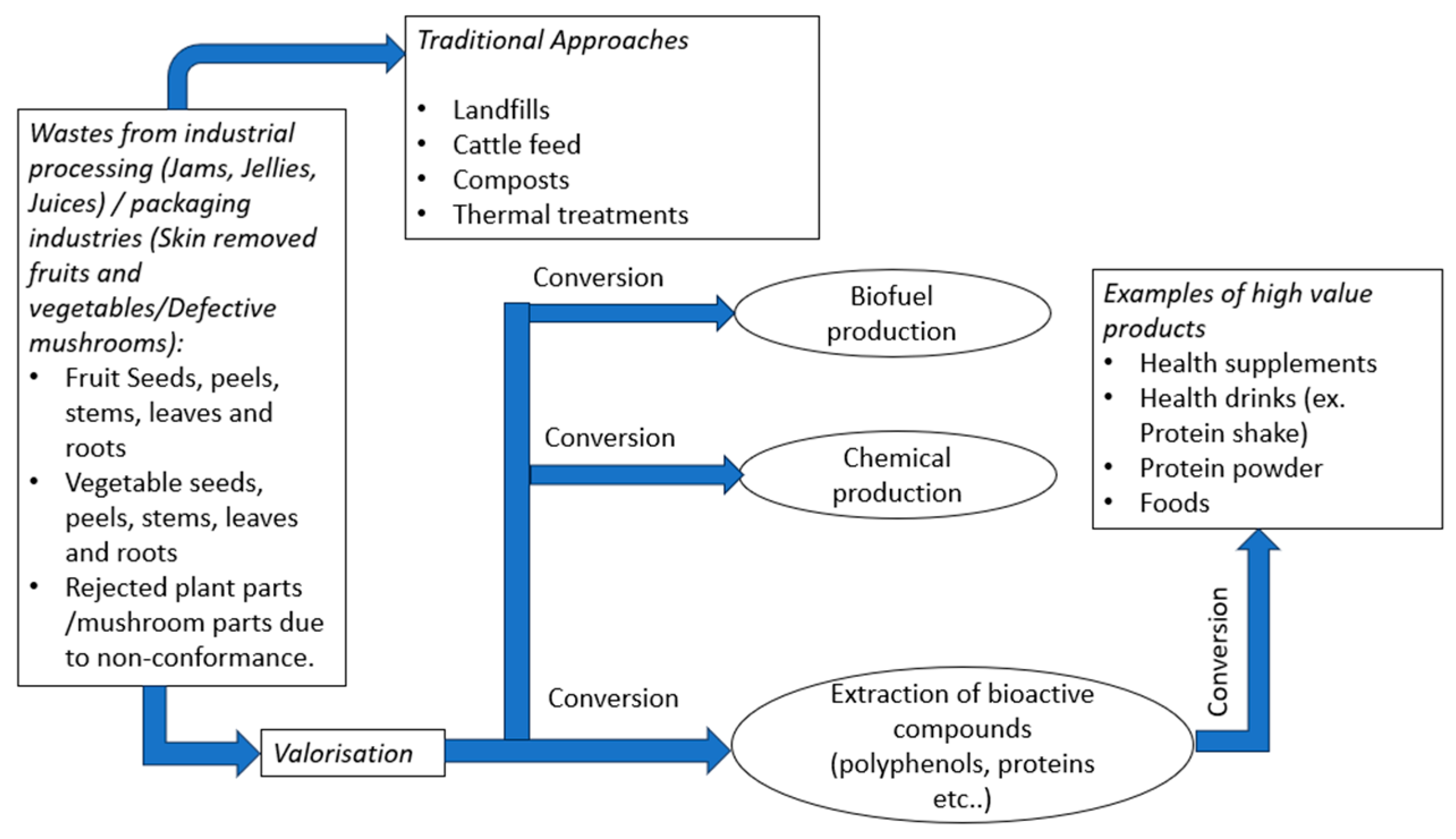
2. Waste Resources
2.1. Plant Wastes
2.2. Deformed Mushroom Wastes
3. Protein Extraction Methods
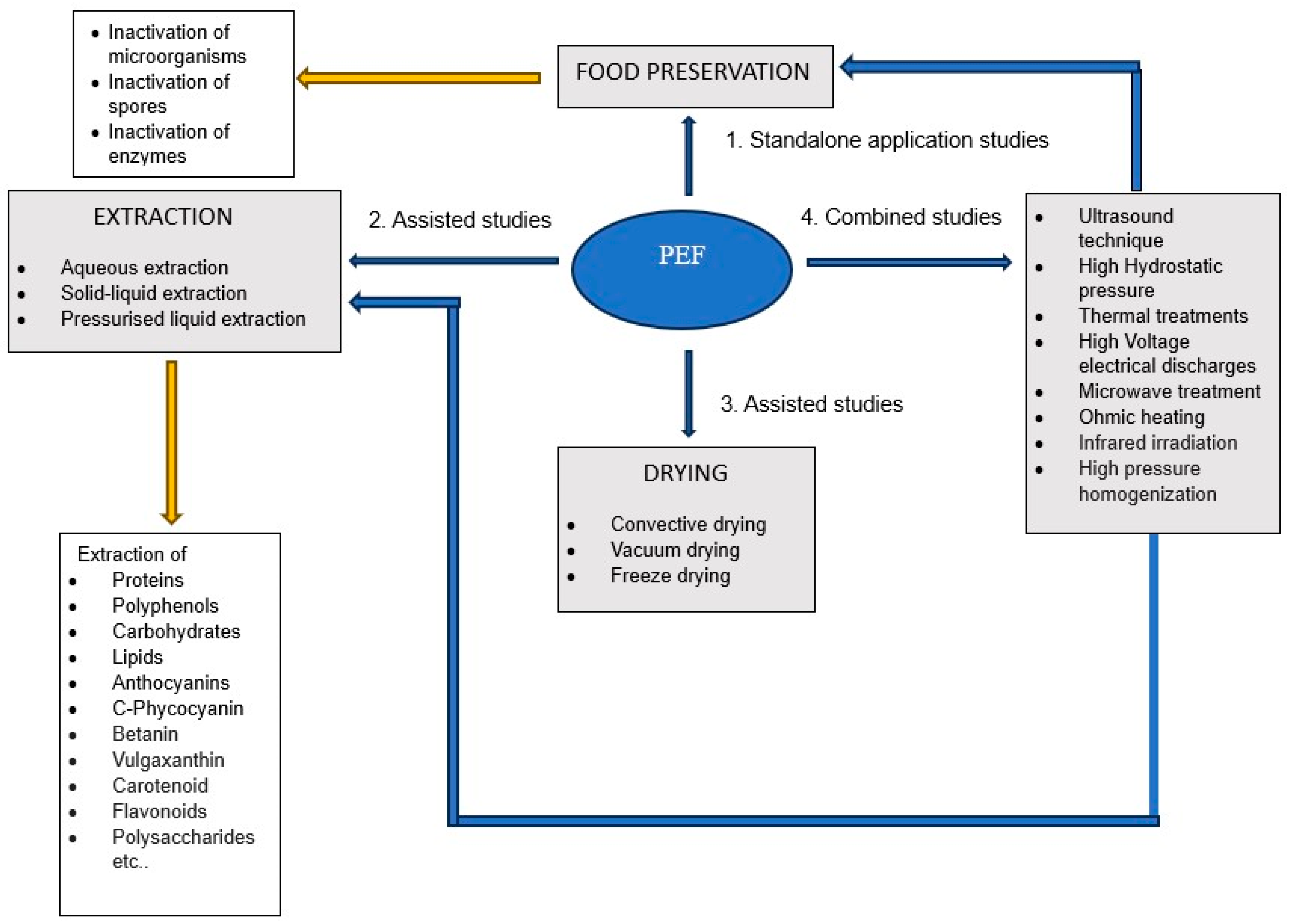
4. PEF Technique
4.1. General Features
4.2. PEF Setup
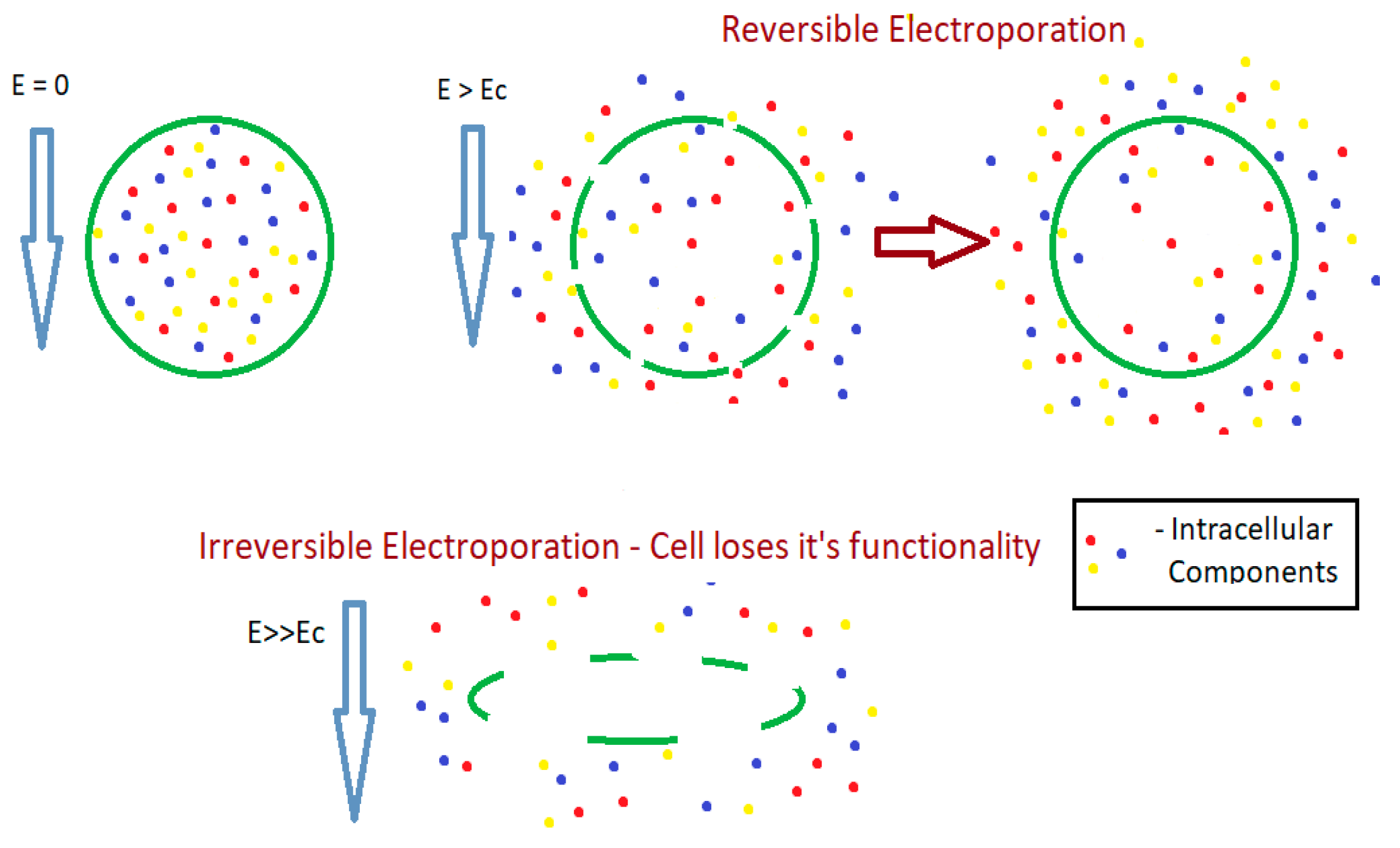
4.3. Mechanism of Extraction
5. PEF-Assisted Extraction of Proteins
6. Optimization of the PEF-Assisted Extraction Process
7. Industrial Processing
8. Existing Challenges for PEF Systems
9. Conclusions and Future Directions
- ○
- The protein extraction research on PEF process parameter optimization should be broadened to promote the applications in a large-scale manner.
- ○
- Deeper insights should be gained into PEF-assisted protein extraction in non-conforming botanical parts such as rejected stems, leaves, roots, flowers, fruits, and other green waste resources from industrial processing.
- ○
- Research on combining other green techniques with PEF to obtain the optimum protein yield should be extended.
- ○
- The research on electrolysis is underdeveloped in extraction studies and can be further improved to minimize the effects in the final byproducts.
- ○
- Advanced simulation studies should be conducted to find out the protein’s structural and functional changes due to electric stress, which would improve our understanding of the interaction between the protein molecule and electric field and consequently be beneficial for future experimental research.
Funding
Data Availability Statement
Conflicts of Interest
References
- Global Food Losses and Food Waste. Available online: https://www.fao.org (accessed on 22 November 2023).
- UNEP Food Waste Index Report 2021|UNEP—UN Environment Programme. Available online: https://www.unep.org (accessed on 22 November 2023).
- NPCS Team. India Emerging Business Opportunities: Cold Chain Sector (Why to Invest, Project Potential, Core Financials, Market Size & Industry Analysis); NIIR Project Consultancy Services: New Delhi, India, 2014; pp. 11–12. [Google Scholar]
- Yu, X.; Bals, O.; Grimi, N.; Vorobiev, E. A new way for the oil plant biomass valorization: Polyphenols and proteins extraction from rapeseed stems and leaves assisted by pulsed electric fields. Ind. Crops Prod. 2015, 74, 309–318. [Google Scholar] [CrossRef]
- Lonnie, M.; Hooker, E.; Brunstrom, J.M.; Corfe, B.M.; Green, M.A.; Watson, A.W.; Williams, E.A.; Stevenson, E.J.; Penson, S.; Johnstone, A.M. Protein for Life: Review of Optimal Protein Intake, Sustainable Dietary Sources and the Effect on Appetite in Ageing Adults. Nutrients 2018, 10, 360. [Google Scholar] [CrossRef] [PubMed]
- Pikosky, M.A.; Ragalie-Carr, J.; Miller, G.D. Recognizing the importance of protein quality in an era of food systems transformation. Front. Sustain. Food Syst. 2022, 6, 1–7. [Google Scholar] [CrossRef]
- Wolfe, R.R.; Cifelli, A.M.; Kostas, G.; Kim, I.-Y. Optimizing Protein Intake in Adults: Interpretation and Application of the Recommended Dietary Allowance Compared with the Acceptable Macronutrient Distribution Range. Adv. Nutr. 2017, 8, 266–275. [Google Scholar] [CrossRef] [PubMed]
- FAO; WHO; UNU. Technical Report Series 935. Protein and Amino Acid Requirements in Human Nutrition; WHO Press: Geneva, Switzerland, 2007; pp. 1–265. [Google Scholar]
- Institute of Medicine (IOM). Dietary Reference Intakes for Energy, Carbohydrates, Fiber, Fat, Fatty Acids, Cholesterol, Proteins, and Amino Acids; The National Academies Press: Washington, DC, USA, 2005. [Google Scholar]
- Naghshi, S.; Sadeghi, O.; Willett, W.C.; Esmaillzadeh, A. Dietary intake of total, animal, and plant proteins and risk of all cause, cardiovascular, and cancer mortality: Systematic review and dose-response meta-analysis of prospective cohort studies. BMJ 2020, 370, m2412. [Google Scholar] [CrossRef] [PubMed]
- Henchion, M.; Hayes, M.; Mullen, A.M.; Fenelon, M.; Tiwari, B. Future Protein Supply and Demand: Strategies and Factors Influencing a Sustainable Equilibrium. Foods 2017, 6, 53. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Yang, L.; Poe, N.; Huang, H. Integrated processing of plant-derived waste to produce value-added products based on the biorefinery concept. Trends Food Sci. Technol. 2018, 74, 119–131. [Google Scholar] [CrossRef]
- Basri, M.S.M.; Shah, N.N.A.K.; Sulaiman, A.; Tawakkal, I.S.M.A.; Nor, M.Z.M.; Ariffin, S.H.; Ghani, N.H.A.; Salleh, F.S.M. Progress in the Valorization of Fruit and Vegetable Wastes: Active Packaging, Biocomposites, By-Products, and Innovative Technologies Used for Bioactive Compound Extraction. Polymers 2021, 13, 3503. [Google Scholar] [CrossRef]
- Esparza, I.; Jiménez-Moreno, N.; Bimbela, F.; Ancín-Azpilicueta, C.; Gandía, L.M. Fruit and vegetable waste management: Conventional and emerging approaches. J. Environ. Manag. 2020, 265, 110510. [Google Scholar] [CrossRef]
- Rana, A.K. Green approaches in the valorization of plant wastes: Recent insights and future directions. Curr. Opin. Green Sustain. Chem. 2022, 38, 1–9. [Google Scholar] [CrossRef]
- Barbarino, E.; Lourenço, S.O. An evaluation of methods for extraction and quantification of protein from marine macro- and microalgae. J. Appl. Phycol. 2005, 17, 447–460. [Google Scholar] [CrossRef]
- Fleurence, J.; Le Coeur, C.; Mabeau, S.; Maurice, M.; Landrein, A. Comparison of different extractive procedures for proteins from the edible seaweeds Ulva rigida and Ulva rotundata. J. Appl. Phycol. 1995, 7, 577–582. [Google Scholar] [CrossRef]
- Urango, A.C.M.; Strieder, M.M.; Silva, E.K.; Meireles, M.A.A. Thermosonication Process Design for Recovering Bioactive Compounds from Fennel: A Comparative Study with Conventional Extraction Techniques. Appl. Sci. 2021, 11, 12104. [Google Scholar] [CrossRef]
- Harnedy, P.A.; FitzGerald, R.J. Extraction of protein from the macroalga Palmaria palmata. LWT—Food Sci. Technol. 2013, 51, 375–382. [Google Scholar] [CrossRef]
- Nowosad, K.; Sujka, M.; Pankiewicz, U.; Kowalski, R. The application of PEF technology in food processing and human nutrition. J. Food Sci. Technol. 2021, 58, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Lammerskitten, A.; Mykhailyk, V.; Wiktor, A.; Toepfl, S.; Nowacka, M.; Bialik, M.; Czyżewski, J.; Witrowa-Rajchert, D.; Parniakov, O. Impact of pulsed electric fields on physical properties of freeze-dried apple tissue. Innov. Food Sci. Emerg. Technol. 2019, 57, 1–7. [Google Scholar] [CrossRef]
- Min, S.; Evrendilek, G.A.; Zhang, H.Q. Pulsed Electric Fields: Processing System, Microbial and Enzyme Inhibition, and shelf life extension of Foods. IEEE Trans. Plasma Sci. 2007, 35, 59–73. [Google Scholar] [CrossRef]
- Dziadek, K.; Kopeć, A.; Dróżdż, T.; Kiełbasa, P.; Ostafin, M.; Bulski, K.; Oziembłowski, M. Effect of pulsed electric field treatment on shelf life and nutritional value of apple juice. J. Food Sci. Technol. 2019, 56, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Odriozola-Serrano, I.; Aguiló-Aguayo, I.; Soliva-Fortuny, R.; Martín-Belloso, O. Pulsed electric fields processing effects on quality and health-related constituents of plant-based foods. Trends Food Sci. Technol. 2013, 29, 98–107. [Google Scholar] [CrossRef]
- Kantala, C.; Supasin, S.; Intra, P.; Rattanadecho, P. Evaluation of Pulsed Electric Field and Conventional Thermal Processing for Microbial Inactivation in Thai Orange Juice. Foods 2022, 11, 1102. [Google Scholar] [CrossRef]
- Barsotti, L.; Dumay, E.; Mu, T.H.; Diaz, M.D.F.; Cheftel, J.C. Effects of high voltage electric pulses on protein-based food constituents and structures. Trends Food Sci. Technol. 2001, 12, 136–144. [Google Scholar] [CrossRef]
- Zulueta, A.; Esteve, M.J.; Frasquet, I.; Frígola, A. Fatty acid profile changes during orange juice-milk beverage processing by high-pulsed electric field. Eur. J. Lipid Sci. Technol. 2007, 109, 25–31. [Google Scholar] [CrossRef]
- Wouters, P.C.; Smelt, J.P. Inactivation of microorganisms with pulsed electric fields: Potential for food preservation. Food Biotechnol. 1997, 11, 193–229. [Google Scholar] [CrossRef]
- Barsotti, L.; Cheftel, J.C. Food processing by pulsed electric fields. II. Biological aspects. Food Rev. Int. 1999, 15, 181–213. [Google Scholar] [CrossRef]
- Jeyamkondan, S.; Jayas, D.S.; Holley, R.A. Pulsed electric field processing of foods: A review. J. Food Prot. 1999, 62, 1088–1096. [Google Scholar] [CrossRef]
- Zhang, Q.; Barbosa-Cánovas, G.V.; Swanson, B.G. Engineering aspects of pulsed electric field pasteurization. J. Food Eng. 1995, 25, 261–281. [Google Scholar] [CrossRef]
- Ho, S.; Mittal, G.S. High voltage pulsed electrical field for liquid food pasteurization. Food Rev. Int. 2000, 16, 395–434. [Google Scholar] [CrossRef]
- Dunn, J.E.; Pearlman, J.S.; La Costa, R. Methods and Apparatus for Extending the Shelf Life of Fluid Food Products. U.S. Patent 4,838,154, 22 September 1987. [Google Scholar]
- Dunn, J. Pulsed electric field processing: An overview. In Pulsed Electric Fields in Food Processing, Fundamental Aspects and Applications; Barbosa-Cánovas, G., Zhang, Q.H., Eds.; Technomic Press: Lancaster, PA, USA, 2001; p. 130. [Google Scholar]
- Sampedro, F.; Rodrigo, D.; Martínez, A.; Barbosa-Cánovas, G.V.; Rodrigo, M. Review: Application of Pulsed Electric Fields in Egg and Egg Derivatives. Food Sci. Technol. Int. 2006, 12, 397–405. [Google Scholar] [CrossRef]
- Góngora-Nieto, M.M.; Sepúlveda, D.R.; Pedrow, P.; Barbosa-Cánovas, G.V.; Swanson, B.G. Food Processing by Pulsed Electric Fields: Treatment Delivery, Inactivation Level, and Regulatory Aspects. LWT—Food Sci. Technol. 2002, 35, 375–388. [Google Scholar] [CrossRef]
- Puértolas, E.; Luengo, E.; Álvarez, I.; Raso, J. Improving Mass Transfer to Soften Tissues by Pulsed Electric Fields: Fundamentals and Applications. Annu. Rev. Food Sci. Technol. 2012, 3, 263–282. [Google Scholar] [CrossRef]
- Toepfl, S. Pulsed Electric Field food treatment—Scale up from lab to industrial scale. Procedia Food Sci. 2011, 1, 776–779. [Google Scholar] [CrossRef]
- Góngora-Nieto, M.M.; Pedrow, P.D.; Swanson, B.G.; Barbosa-Cánovas, G.V. Impact of air bubbles in a dielectric liquid when subjected to high field strengths. Innov. Food Sci. Emerg. Technol. 2003, 4, 57–67. [Google Scholar] [CrossRef]
- Mariotti, F. Animal and Plant Protein Sources and Cardiometabolic Health. Adv. Nutr. 2019, 10, S351–S366. [Google Scholar] [CrossRef] [PubMed]
- Rifna, E.J.; Misra, N.N.; Dwivedi, M. Recent advances in extraction technologies for recovery of bioactive compounds derived from fruit and vegetable waste peels: A review. Crit. Rev. Food Sci. Nutr. 2023, 63, 719–752. [Google Scholar] [CrossRef]
- Ganesh, K.S.; Sridhar, A.; Vishali, S. Utilization of fruit and vegetable waste to produce value-added products: Conventional utilization and emerging opportunities-A review. Chemosphere 2022, 287, 132221. [Google Scholar] [CrossRef]
- Mathews, A.D.; Tangirala, A.S.; Kumar, S.; Anandharaj, A.; Rawson, A. Extraction and modification of protein from sesame oil cake by the application of emerging technologies. Food Chem. Adv. 2023, 2, 100326. [Google Scholar] [CrossRef]
- Kumoro, A.C.; Alhanif, M.; Wardhani, D.H. A Critical Review on Tropical Fruits Seeds as Prospective Sources of Nutritional and Bioactive Compounds for Functional Foods Development: A Case of Indonesian Exotic Fruits. Int. J. Food Sci. 2020, 2020, 4051475. [Google Scholar] [CrossRef] [PubMed]
- Ruschioni, S.; Loreto, N.; Foligni, R.; Mannozzi, C.; Raffaelli, N.; Zamporlini, F.; Pasquini, M.; Roncolini, A.; Cardinali, F.; Osimani, A.; et al. Addition of Olive Pomace to Feeding Substrate Affects Growth Performance and Nutritional Value of Mealworm (Tenebrio Molitor L.) Larvae. Foods 2020, 9, 317. [Google Scholar] [CrossRef]
- Herchi, W.; Bahashwan, S.; Sakouhi, F.; Boukhchina, S. Influence of harvest year in the physicochemical properties and antioxidant activity of flaxseed hull oils from Tunisia. Food Sci. Technol. 2015, 35, 175–182. [Google Scholar] [CrossRef]
- Liadakis, G.; Kekes, T.; Frakolaki, G.; Giannou, V.; Tzia, C. Ingredients for Food Products in Mejdi Jeguirim. Tomato Processing By-Products; Zorpas, A., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 117–148. [Google Scholar]
- Wen, C.; Zhang, J.; Duan, Y.; Zhang, H.; Ma, H. A Mini-Review on Brewer’s Spent Grain Protein: Isolation, Physicochemical Properties, Application of Protein, and Functional Properties of Hydrolysates. J. Food Sci. 2019, 84, 3330–3340. [Google Scholar] [CrossRef]
- Mustafa, A.F.; Baurhoo, B. Evaluation of dried vegetables residues for poultry: II. Effects of feeding cabbage leaf residues on broiler performance, ileal digestibility and total tract nutrient digestibility. Poult. Sci. 2017, 96, 681–686. [Google Scholar] [CrossRef]
- Kaur, M.; Gautam, A.; Kaur, H. Nutritional, techno-functional, structural, and rheological properties of potato peel powder: A valuable biowaste being potential source of dietary fibre and antioxidants in cookie formulation. J. Food Process. Preserv. 2022, 46, 1–13. [Google Scholar] [CrossRef]
- Lobo, M.G.; Dorta, E. Utilization and management of horticultural waste in Elhadi. In Postharvest Technology of Perishable Horticultural Commodities; Yahia, M., Ed.; Woodhead Publishing: Cambridge, UK, 2019; pp. 639–666. [Google Scholar]
- Wikandari, R.; Nguyen, H.; Millati, R.; Niklasson, C.; Taherzadeh, M.J. Improvement of biogas production from orange peel waste by leaching of limonene. Biomed. Res. Int. 2015, 494182. [Google Scholar] [CrossRef] [PubMed]
- Cunha, T.J. Value of Feeds for Horses in Horse Feeding and Nutrition, 2nd ed.; Academic Press: San Diego, CA, USA, 2012; pp. 233–273. [Google Scholar]
- Hussain, A.; Kausar, T.; Sehar, S.; Sarwar, A.; Ashraf, A.H.; Jamil, M.A.; Noreen, S.; Rafique, A.; Iftikhar, K.; Quddoos, M.Y.; et al. A Comprehensive review of functional ingredients, especially bioactive compounds present in pumpkin peel, flesh and seeds, and their health benefits. Food Chem. Adv. 2022, 1, 100067. [Google Scholar] [CrossRef]
- Zaini, H.M.; Roslan, J.; Saallah, S.; Munsu, E.; Sulaiman, N.S.; Pindi, W. Banana peels as a bioactive ingredient and its potential application in the food industry. J. Funct. Foods 2022, 92, 105054. [Google Scholar] [CrossRef]
- Ain, H.B.U.; Tufail, T.; Bashir, S.; Ijaz, N.; Hussain, M.; Ikram, A.; Farooq, M.A.; Saewan, S.A. Nutritional importance and industrial uses of pomegranate peel: A critical review. Food Sci. Nutr. 2023, 11, 2589–2598. [Google Scholar] [CrossRef] [PubMed]
- Gómez-García, R.; Campos, D.A.; Oliveira, A.; Aguilar, C.N.; Madureira, A.R.; Pintado, M. A chemical valorisation of melon peels towards functional food ingredients: Bioactives profile and antioxidant properties. Food Chem. 2021, 335, 127579. [Google Scholar] [CrossRef] [PubMed]
- Maloney, K.P.; Truong, V.D.; Allen, J.C. Susceptibility of sweet potato (Ipomoea batatas) peel proteins to digestive enzymes. Food Sci. Nutr. 2014, 2, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Dotto, J.M.; Chacha, J.S. The potential of pumpkin seeds as a functional food ingredient: A review. Sci. Afr. 2020, 10, e00575. [Google Scholar] [CrossRef]
- Apprich, S.; Tirpanalan, O.; Hell, J.; Reisinger, M.; Böhmdorfer, S.; Siebenhandl-Ehn, S.; Novalin, S.; Kneifel, W. Wheat bran-based biorefinery 2: Valorization of products. LWT—Food Sci. Technol. 2014, 56, 222–231. [Google Scholar] [CrossRef]
- Kalpanadevi, C.; Muthukumar, S.P.; Govindaraju, K.; Subramanian, R. Rice bran protein: An alternative plant-based protein to ameliorate protein malnourishment. J. Cereal Sci. 2021, 97, 103154. [Google Scholar] [CrossRef]
- Kumar, M.; Barbhai, M.D.; Hasan, M.; Punia, S.; Dhumal, S.; Radha; Rais, N.; Chandran, D.; Pandiselvam, R.; Kothakota, A.; et al. Onion (Allium cepa L.) peels: A review on bioactive compounds and biomedical activities. Biomed. Pharmacother. 2022, 146, 112498. [Google Scholar] [CrossRef]
- Kamal, H.; Le, C.F.; Salter, A.M.; Ali, A. Extraction of protein from food waste: An overview of current status and opportunities. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2455–2475. [Google Scholar] [CrossRef]
- Kumar, M.; Tomar, M.; Punia, S.; Dhakane-Lad, J.; Dhumal, S.; Changan, S.; Senapathy, M.; Berwal, M.K.; Sampathrajan, V.; Sayed, A.A.; et al. Plant-based proteins and their multifaceted industrial applications. LWT 2022, 154, 112620. [Google Scholar] [CrossRef]
- Ayimbila, F.; Keawsompong, S. Nutritional Quality and Biological Application of Mushroom Protein as a Novel Protein Alternative. Curr. Nutr. Rep. 2023, 12, 290–307. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, M.; Wu, S.; Liao, X.; Wang, J.; Wu, Q.; Zhuang, M.; Ding, Y. A review on mushroom-derived bioactive peptides: Preparation and biological activities. Food Res. Int. 2020, 134, 109230. [Google Scholar] [CrossRef]
- Meenu, M.; Xu, B. Application of vibrational spectroscopy for classification, authentication and quality analysis of mushroom: A concise review. Food Chem. 2019, 289, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.M.; Zhang, J.; Wu, L.H.; Zhao, Y.L.; Li, T.; Li, J.Q.; Wang, Y.Z.; Liu, H.G. A mini-review of chemical composition and nutritional value of edible wild-grown mushroom from China. Food Chem. 2014, 151, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Elhusseiny, S.M.; El-Mahdy, T.S.; Awad, M.F.; Elleboudy, N.S.; Farag, M.M.S.; Aboshanab, K.M.; Yassien, M.A. Antiviral, Cytotoxic, and Antioxidant Activities of Three Edible Agaricomycetes Mushrooms: Pleurotus columbinus, Pleurotus sajor-caju, and Agaricus bisporus. J. Fungi—Open Access Mycol. J. 2021, 7, 645. [Google Scholar] [CrossRef]
- González, A.; Nobre, C.; Simões, L.S.; Cruz, M.; Loredo, A.; Rodríguez-Jasso, R.M.; Contreras, J.; Texeira, J.; Belmares, R. Evaluation of functional and nutritional potential of a protein concentrate from Pleurotus ostreatus mushroom. Food Chem. 2021, 346, 128884. [Google Scholar] [CrossRef]
- Miles, P.G.; Chang, S.-T. Mushrooms: Cultivation, Nutritional Value, Medicinal Effect, and Environmental Impact, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar] [CrossRef]
- Rangsinth, P.; Sillapachaiyaporn, C.; Nilkhet, S.; Tencomnao, T.; Ung, A.T.; Chuchawankul, S. Mushroom-derived bioactive compounds potentially serve as the inhibitors of SARS-CoV-2 main protease: An in silico approach. J. Tradit. Complement. Med. 2021, 11, 158–172. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Belwal, T.; Devkota, H.P.; Li, L.; Luo, Z. Trends of utilizing mushroom polysaccharides (MPs) as potent nutraceutical components in food and medicine: A comprehensive review. Trends Food Sci. Technol. 2019, 92, 94–110. [Google Scholar] [CrossRef]
- Lu, X.; Brennan, M.A.; Serventi, L.; Liu, J.; Guan, W.; Brennan, C.S. Addition of mushroom powder to pasta enhances the antioxidant content and modulates the predictive glycaemic response of pasta. Food Chem. 2018, 264, 199–209. [Google Scholar] [CrossRef]
- Venturella, G.; Ferraro, V.; Cirlincione, F.; Gargano, M.L. Medicinal Mushrooms: Bioactive Compounds, Use, and Clinical Trials. Int. J. Mol. Sci. 2021, 22, 634. [Google Scholar] [CrossRef]
- Das, A.K.; Nanda, P.K.; Dandapat, P.; Bandyopadhyay, S.; Gullón, P.; Sivaraman, G.K.; McClements, D.J.; Gullón, B.; Lorenzo, J.M. Edible Mushrooms as Functional Ingredients for Development of Healthier and More Sustainable Muscle Foods: A Flexitarian Approach. Molecules 2021, 26, 2463. [Google Scholar] [CrossRef]
- Jacinto-Azevedo, B.; Valderrama, N.; Henríquez, K.; Aranda, M.; Aqueveque, P. Nutritional value and biological properties of Chilean wild and commercial edible mushrooms. Food Chem. 2021, 356, 129651. [Google Scholar] [CrossRef] [PubMed]
- Ghose, A.; Mitra, S. Spent waste from edible mushrooms offers innovative strategies for the remediation of persistent organic micropollutants: A review. Environ. Pollut. 2022, 305, 119285. [Google Scholar] [CrossRef] [PubMed]
- Atallah, E.; Zeaiter, J.; Ahmad, M.N.; Leahy, J.J.; Kwapinski, W. Hydrothermal carbonization of spent mushroom compost waste compared against torrefaction and pyrolysis. Fuel Process. Technol. 2021, 216, 106795. [Google Scholar] [CrossRef]
- Leong, Y.K.; Ma, T.-W.; Chang, J.-S.; Yang, F.-C. Recent advances and future directions on the valorization of spent mushroom substrate (SMS): A review. Bioresour. Technol. 2021, 344, 126157. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Murtaza, G.; Ditta, A. Nutritional, Medicinal, and Cosmetic Value of Bioactive Compounds in Button Mushroom (Agaricus bisporus): A Review. Appl. Sci. 2021, 11, 5943. [Google Scholar] [CrossRef]
- Carrasco-González, J.A.; Serna-Saldívar, S.O.; Gutiérrez-Uribe, J.A. Nutritional composition and nutraceutical properties of the Pleurotus fruiting bodies: Potential use as food ingredient. J. Food Compos. Anal. 2017, 58, 69–81. [Google Scholar] [CrossRef]
- Abidin, M.H.Z.; Abdullah, N.; Abidin, N.Z. Therapeutic properties of Pleurotus species (oyster mushrooms) for atherosclerosis: A review. Int. J. Food Prop. 2017, 20, 1251–1261. [Google Scholar] [CrossRef]
- Valverde, M.E.; Hernández-Pérez, T.; Paredes-López, O.; Hernández-Pérez, T.; Paredes-López, O. Edible Mushrooms: Improving Human Health and Promoting Quality Life. Int. J. Microbiol. 2015, 2015, 376387. [Google Scholar] [CrossRef]
- Li, Y.; Chen, H.; Zhang, X. Cultivation, nutritional value, bioactive compounds of morels, and their health benefits: A systematic review. Front. Nutr. 2023, 10, 1159029. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, C.; Fan, G.; Li, T.; Sun, Y. Improvement of antioxidant activity of Morchella esculenta protein hydrolysate by optimized glycosylation reaction. CyTA—J. Food 2018, 16, 238–246. [Google Scholar] [CrossRef]
- Das, S.; Prakash, B. Edible mushrooms: Nutritional composition and medicinal benefits for improvement in quality life. In Research and Technological Advances in Food Science; Prakash, B., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 269–300. [Google Scholar]
- Spim, S.R.V.; Castanho, N.R.C.M.; Pistila, A.M.H.; Jozala, A.F.; Oliveira Júnior, J.M.; Grotto, D. Lentinula edodes mushroom as an ingredient to enhance the nutritional and functional properties of cereal bars. J. Food Sci. Technol. 2021, 58, 1349–1357. [Google Scholar] [CrossRef]
- Bisen, P.S.; Baghel, R.K.; Sanodiya, B.S.; Thakur, G.S.; Prasad, G.B. Lentinus edodes: A macrofungus with pharmacological activities. Curr. Med. Chem. 2010, 17, 2419–2430. [Google Scholar] [CrossRef]
- Sun, X.; Yang, C.; Ma, Y.; Zhang, J.; Wang, L. Research progress of Auricularia heimuer on cultivation physiology and molecular biology. Front. Microbiol. 2022, 13, 148249. [Google Scholar] [CrossRef]
- Hrudayanath, T.; Sameer, K.S. Diversity, nutritional composition and medicinal potential of Indian mushrooms: A review. Afr. J. Biotechnol. 2014, 13, 523–545. [Google Scholar] [CrossRef]
- Tang, C.; Hoo, P.C.; Tan, L.T.; Pusparajah, P.; Khan, T.M.; Lee, L.H.; Goh, B.H.; Chan, K.G. Golden Needle Mushroom: A Culinary Medicine with Evidenced-Based Biological Activities and Health Promoting Properties. Front. Pharmacol. 2016, 7, 474. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, J.M.; Tang, Y.J.; Ma, K.; Li, B.; Zeng, X.; Liu, X.-B.; Li, Y.; Yang, Z.-L.; Xu, W.-N.; et al. Genome-wide analysis and prediction of genes involved in the biosynthesis of polysaccharides and bioactive secondary metabolites in high-temperature-tolerant wild Flammulina filiformis. BMC Genom. 2020, 21, 719. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, J.; Xu, F.; Han, R.; Quan, M.; Wang, L. Effect of Tremella fuciformis on dough structure and rheology, noodle flavor, and quality characteristics. LWT 2022, 172, 114180. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Sun, W.; Cheng, Q.; Khoshkharam, M. Exploring the quality of foods from ancient China based on traditional Chinese medicine. In Functional Foods and Nutraceuticals in Metabolic and Non-Communicable Diseases; Singh, R.B., Watanabe, S., Isaza, A.A., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 87–105. [Google Scholar] [CrossRef]
- Rajarathnam, S.; Shashirekha, M.N. Mushrooms and Truffles|Use of Wild Mushrooms. In Encyclopaedia of Food Sciences and Nutrition, 2nd ed.; Academic Press: Cambridge, MA, USA, 2003; pp. 4048–4054. [Google Scholar] [CrossRef]
- Mikola, E.; Geösel, A.; Stefanovits-Bányai, É.; Fodor, M. Quantitative determination of macro components and classification of some cultivated mushrooms using near-infrared spectroscopy. J. Food Process Preserv. 2020, 44, e14540. [Google Scholar] [CrossRef]
- Chauhan, G.; Prasad, S.; Himanshi, R.; Satyawati, S. Nutritional profiling and value addition of products from Hypsizygus tessellatus. Food Biol. 2017, 6, 1–6. [Google Scholar] [CrossRef]
- Li, W.; Chen, W.; Ma, H.; Wang, J.; Li, Z.; Wang, Q.; Zhang, Z.; Wu, D.; Zhang, J.; Yang, Y. Study on the relationship between structure and taste activity of the umami peptide of Stropharia rugosoannulata prepared by ultrasound. Ultrason. Sonochem. 2022, 90, 106206. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Kakumyan, P.; Bandara, A.; Peter, M. The nutrition, cultivation and biotechnology of Stropharia rugosoannulata. Fungal Biotec. 2021, 1, 13–25. [Google Scholar] [CrossRef]
- Petrović, J.; Glamočlija, J.; Stojković, D.; Ćirić, A.; Barros, L.; Ferreira, I.C.F.R.; Soković, M. Nutritional value, chemical composition, antioxidant activity and enrichment of cream cheese with chestnut mushroom Agrocybe aegerita (Brig.) Sing. J. Food Sci. Technol. 2015, 52, 6711–6718. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Gu, B.; Huang, J.; Jiang, S.; Chen, Y.; Yin, Y.; Pan, Y.; Yu, G.; Li, Y.; Wong, B.H.C.; et al. Transcriptome and Proteome Exploration to Provide a Resource for the Study of Agrocybe aegerita. PLoS ONE 2013, 8, e56686. [Google Scholar] [CrossRef]
- Bandura, I.; Kulyk, A.; Makohon, S.; Khareba, O.; Khareba, V. Influence of the substrate composition on the yield and nutritional value of the fruiting bodies of the edible mushrooms Pleurotus citrinopileatus and Cyclocybe aegerita. Plant Var. Stud. Prot. 2021, 17, 130–138. [Google Scholar] [CrossRef]
- Friedman, M. Chemistry, Nutrition, and Health-Promoting Properties of Hericium erinaceus (Lion’s Mane) Mushroom Fruiting Bodies and Mycelia and Their Bioactive Compounds. J. Agric. Food Chem. 2015, 63, 7108–7123. [Google Scholar] [CrossRef] [PubMed]
- Chaiyasut, C.; Sivamaruthi, B.S. Anti-hyperglycemic property of Hericium erinaceus—A mini review. Asian Pac. J. Trop. Biomed. 2017, 7, 1036–1040. [Google Scholar] [CrossRef]
- Sitinjak, R. The Nutritional Content of the Mushroom Phallus indusiatus Vent., which Grows in the Cocoa Plantation, Gaperta-Ujung, Medan. Der Pharma Chem. 2017, 9, 44–47. [Google Scholar]
- Roselló-Soto, E.; Barba, F.J.; Parniakov, O.; Galanakis, C.M.; Lebovka, N.; Grimi, N.; Vorobiev, E. High Voltage Electrical Discharges, Pulsed Electric Field, and Ultrasound Assisted Extraction of Protein and Phenolic Compounds from Olive Kernel. Food Bioprocess. Technol. 2015, 8, 885–894. [Google Scholar] [CrossRef]
- Antunes, F.; Marçal, S.; Taofiq, O.; Morais, A.M.M.B.; Freitas, A.C.; Ferreira, I.C.F.R.; Pintado, M. Valorization of Mushroom By-Products as a Source of Value-Added Compounds and Potential Applications. Molecules 2020, 25, 2672. [Google Scholar] [CrossRef] [PubMed]
- Jayachandran, M.; Xiao, J.; Xu, B.A. Critical Review on Health Promoting Benefits of Edible Mushrooms through Gut Microbiota. Int. J. Mol. Sci. 2017, 18, 1934. [Google Scholar] [CrossRef] [PubMed]
- Welti-Chanes, J.; Vergara-Balderas, F.; Bermúdez-Aguirre, D. Transport phenomena in food engineering: Basic concepts and advances. J. Food Eng. 2005, 67, 113–128. [Google Scholar] [CrossRef]
- Barba, F.J.; Parniakov, O.; Pereira, S.A.; Wiktor, A.; Grimi, N.; Boussetta, N.; Saraiva, J.A.; Raso, J.; Martin-Belloso, O.; Witrowa-Rajchert, D.; et al. Current applications and new opportunities for the use of pulsed electric fields in food science and industry. Food Res. Int. 2015, 77, 773–798. [Google Scholar] [CrossRef]
- Barba, F.J.; Zhu, Z.; Koubaa, M.; Sant’Ana, A.S.; Orlien, V. Green alternative methods for the extraction of antioxidant bioactive compounds from winery wastes and by-products: A review. Trends Food Sci. Technol. 2016, 49, 96–109. [Google Scholar] [CrossRef]
- Zhang, Q.W.; Lin, L.G.; Ye, W.C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef]
- Chua, L.S. A review on plant-based rutin extraction methods and its pharmacological activities. J. Ethnopharmacol. 2013, 150, 805–817. [Google Scholar] [CrossRef]
- Chandran, A.S.; Suri, S.; Choudhary, P. Sustainable plant protein: An up-to-date overview of sources, extraction techniques and utilization. Sustain. Food Technol. 2023, 1, 466–483. [Google Scholar] [CrossRef]
- Nadar, S.S.; Rao, P.; Rathod, V.K. Enzyme assisted extraction of biomolecules as an approach to novel extraction technology: A review. Food Res. Int. 2018, 108, 309–330. [Google Scholar] [CrossRef]
- Chakka, A.K.; Sriraksha, M.S.; Ravishankar, C.N. Sustainability of emerging green non-thermal technologies in the food industry with food safety perspective: A review. LWT 2021, 151, 112140. [Google Scholar] [CrossRef]
- Soquetta, M.B.; Terra, L.d.M.; Bastos, C.P. Green technologies for the extraction of bioactive compounds in fruits and vegetables. CyTA—J. Food 2018, 16, 400–412. [Google Scholar] [CrossRef]
- Xue, D.; Farid, M.F. Pulsed Electric Field Extraction of Valuable Compounds from White Button Mushroom (Agaricus Bisporus). Innov. Food Sci. Emerg. Technol. 2015, 29, 178–186. [Google Scholar] [CrossRef]
- Jadhav, H.B.; Annapure, U.S.; Deshmukh, R.R. Non-thermal Technologies for Food Processing. Front. Nutr. 2021, 8, 657090. [Google Scholar] [CrossRef] [PubMed]
- Galanakis, C.M.; Schieber, A. Editorial. Food Res. Int. 2014, 65, 299–300. [Google Scholar] [CrossRef]
- Reddy, V.Y.; Koruth, J.; Jais, P.; Petru, J.; Timko, F.; Skalsky, I.; Hebeler, R.; Labrousse, L.; Barandon, L.; Kralovec, S.; et al. Ablation of Atrial Fibrillation with Pulsed Electric Fields: An Ultra-Rapid, Tissue-Selective Modality for Cardiac Ablation. JACC Clin. Electrophysiol. 2018, 4, 987–995. [Google Scholar] [CrossRef]
- Nuccitelli, R. Application of Pulsed Electric Fields to Cancer Therapy. Bioelectricity 2019, 1, 30–34. [Google Scholar] [CrossRef]
- Buchmann, L.; Brändle, I.; Haberkorn, I.; Hiestand, M.; Mathys, A. Pulsed electric field based cyclic protein extraction of microalgae towards closed-loop biorefinery concepts. Bioresour. Technol. 2019, 291, 121870. [Google Scholar] [CrossRef]
- Rios-Corripio, G.; Morales-de la Peña, M.; Welti-Chanes, J.; Ángel Guerrero-Beltrán, J. Pulsed electric field processing of a pomegranate (Punica granatum L.) fermented beverage. Innov. Food Sci. Emerg. Technol. 2022, 79, 103045. [Google Scholar] [CrossRef]
- Naliyadhara, N.; Kumar, A.; Girisa, S.; Daimary, U.D.; Hegde, M.; Kunnumakkara, A.B. Pulsed electric field (PEF): Avant-garde extraction escalation technology in food industry. Trends Food Sci. Technol. 2022, 122, 238–255. [Google Scholar] [CrossRef]
- Punthi, F.; Yudhistira, B.; Gavahian, M.; Chang, C.-K.; Cheng, K.-C.; Hou, C.-Y.; Hsieh, C.-W. Pulsed electric field-assisted drying: A review of its underlying mechanisms, applications, and role in fresh produce plant-based food preservation. Compr. Rev. Food Sci. Food Saf. 2022, 21, 5109–5130. [Google Scholar] [CrossRef]
- Navarro, A.; Ruiz-Méndez, M.-V.; Sanz, C.; Martínez, M.; Rego, D.; Pérez, A.G. Application of Pulsed Electric Fields to Pilot and Industrial Scale Virgin Olive Oil Extraction: Impact on Organoleptic and Functional Quality. Foods 2022, 11, 2022. [Google Scholar] [CrossRef] [PubMed]
- Parniakov, O.; Barba, F.J.; Grimi, N.; Lebovka, N.; Vorobiev, E. Extraction assisted by pulsed electric energy as a potential tool for green and sustainable recovery of nutritionally valuable compounds from mango peels. Food Chem. 2016, 192, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Käferböck, A.; Smetana, S.; de Vos, R.; Schwarz, C.; Toepfl, S.; Parniakov, O. Sustainable extraction of valuable components from Spirulina assisted by pulsed electric fields technology. Algal Res. 2020, 48, 101914. [Google Scholar] [CrossRef]
- Kumari, B.; Tiwari, B.K.; Hossain, M.B.; Brunton, N.P.; Rai, D.K. Recent Advances on Application of Ultrasound and Pulsed Electric Field Technologies in the Extraction of Bioactives from Agro-Industrial By-products. Food Bioprocess. Technol. 2018, 11, 223–241. [Google Scholar] [CrossRef]
- Saldaña, G.; Puértolas, E.; Álvarez, I.; Meneses, N.; Knorr, D.; Raso, J. Evaluation of a static treatment chamber to investigate kinetics of microbial inactivation by pulsed electric fields at different temperatures at quasi-isothermal conditions. J. Food Eng. 2010, 100, 349–356. [Google Scholar] [CrossRef]
- Golberg, A.; Sack, M.; Teissie, J.; Pataro, G.; Pliquett, U.; Saulis, G.; Stefan, T.; Miklavcic, D.; Vorobiev, E.; Frey, W. Energy-efficient biomass processing with pulsed electric fields for bioeconomy and sustainable development. Biotechnol. Biofuels 2016, 9, 1–22. [Google Scholar] [CrossRef]
- Weaver, J.C. Electroporation of cells and tissues. IEEE Trans. Plasma Sci. 2000, 28, 24–33. [Google Scholar] [CrossRef]
- Wang, M.-S.; Wang, L.-H.; Bekhit, A.E.-D.A.; Yang, J.; Hou, Z.-P.; Wang, Y.-Z.; Dai, Q.-Z.; Zeng, X.-A. A review of sublethal effects of pulsed electric field on cells in food processing. J. Food Eng. 2018, 223, 32–41. [Google Scholar] [CrossRef]
- Napotnik, T.B.; Polajžer, T.; Miklavčič, D. Cell death due to electroporation—A review. Bioelectrochemistry 2021, 141, 107871. [Google Scholar] [CrossRef]
- Boussetta, N.; Lesaint, O.; Vorobiev, E. A study of mechanisms involved during the extraction of polyphenols from grape seeds by pulsed electrical discharges. Innov. Food Sci. Emerg. Technol. 2013, 19, 124–132. [Google Scholar] [CrossRef]
- Redondo, D.; Venturini, M.E.; Luengo, E.; Raso, J.; Arias, E. Pulsed electric fields as a green technology for the extraction of bioactive compounds from thinned peach by-products. Innov. Food Sci. Emerg. Technol. 2018, 45, 335–343. [Google Scholar] [CrossRef]
- Sánchez-Vega, R.; Elez-Martínez, P.; Martín-Belloso, O. Influence of high-intensity pulsed electric field processing parameters on antioxidant compounds of broccoli juice. Innov. Food Sci. Emerg. Technol. 2015, 29, 70–77. [Google Scholar] [CrossRef]
- Carullo, D.; Abera, B.D.; Casazza, A.A.; Donsì, F.; Perego, P.; Ferrari, G.; Pataro, G. Effect of pulsed electric fields and high pressure homogenization on the aqueous extraction of intracellular compounds from the microalgae Chlorella vulgaris. Algal Res. 2018, 31, 60–69. [Google Scholar] [CrossRef]
- Postma, P.R.; Pataro, G.; Capitoli, M.; Barbosa, M.J.; Wijffels, R.H.; Eppink, M.H.M.; Olivieri, G.; Ferrari, G. Selective extraction of intracellular components from the microalga Chlorella vulgaris by combined pulsed electric field–temperature treatment. Bioresour. Technol. 2016, 203, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Carullo, D.; Donsì, F.; Ferrari, G.; Pataro, G. Extraction improvement of water-soluble compounds from Arthrospira platensis through the combination of high-shear homogenization and pulsed electric fields. Algal Res. 2021, 57, 102341. [Google Scholar] [CrossRef]
- Calleja-Gómez, M.; Castagnini, J.M.; Carbó, E.; Ferrer, E.; Berrada, H.; Barba, F.J. Evaluation of Pulsed Electric Field-Assisted Extraction on the Microstructure and Recovery of Nutrients and Bioactive Compounds from Mushroom (Agaricus bisporus). Separations 2022, 9, 302. [Google Scholar] [CrossRef]
- Moreira, S.A.; Alexandre, E.M.; Pintado, M.; Saraiva, J.A. Effect of emergent non-thermal extraction technologies on bioactive individual compounds profile from different plant materials. Food Res. Int. 2019, 115, 177–190. [Google Scholar] [CrossRef]
- Thongkong, S.; Klangpetch, W.; Unban, K.; Tangjaidee, P.; Phimolsiripol, Y.; Rachtanapun, P.; Jantanasakulwong, K.; Schönlechner, R.; Thipchai, P.; Phongthai, S. Impacts of Electroextraction Using the Pulsed Electric Field on Properties of Rice Bran Protein. Foods 2023, 12, 835. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, R. Pulsed Electric Field Induced Aggregation of Food Proteins: Ovalbumin and Bovine Serum Albumin. Food Bioprocess. Technol. 2012, 5, 1706–1714. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Zeng, X.A.; Deng, Z.; Yu, S.J.; Yamasaki, S. Effect of pulsed electric field on the secondary structure and thermal properties of soy protein isolate. Eur. Food Res. Technol. 2011, 233, 841–850. [Google Scholar] [CrossRef]
- Wu, L.; Zhao, W.; Yang, R.; Chen, X. Effects of pulsed electric fields processing on stability of egg white proteins. J. Food Eng. 2014, 139, 13–18. [Google Scholar] [CrossRef]
- Xiang, B.Y.; Simpson, M.V.; Ngadi, M.O.; Simpson, B.K. Effect of pulsed electric field on the rheological and colour properties of soy milk. Int. J. Food Sci. Nutr. 2011, 62, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Xiang, B.Y.; Ngadi, M.O.; Ochoa-Martinez, L.A.; Simpson, M.V. Pulsed Electric Field-Induced Structural Modification of Whey Protein Isolate. Food Bioprocess Technol. 2011, 4, 1341–1348. [Google Scholar] [CrossRef]
- Cullen, P.J.; Tiwari, B.K.; Valdramidis, V. Novel Thermal and Non-Thermal Technologies for Fluid Foods; Academic Press: Cambridge, MA, USA, 2011; pp. 65–66. [Google Scholar] [CrossRef]
- El-Hag, A.H.; Rodriguez Gonzalez, O.; Jayaram, S.H.; Griffiths, M.W. A Performance Study of a Multilevel Electrode Treatment Chamber for Food Processing. IEEE Trans. Ind. Appl. 2013, 49, 1091–1097. [Google Scholar] [CrossRef]
- Huang, K.; Wang, J. Designs of Pulsed Electric Fields Treatment Chambers for Liquid Foods Pasteurization Process: A Review. J. Food Eng. 2009, 95, 227–239. [Google Scholar] [CrossRef]
- Qin, S.; Timoshkin, I.V.; Maclean, M.; Wilson, M.P.; Given, M.J.; Wang, T.; Anderson, J.G.; Macgregor, S.J. Pulsed electric field treatment of saccharomyces cerevisiae using different waveforms. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 1841–1848. [Google Scholar] [CrossRef]
- Qin, S.; Timoshkin, I.V.; Maclean, M.; Wilson, M.P.; Given, M.J.; Wang, T.; Anderson, J.G.; Macgregor, S.J. TiO2-Coated Electrodes for Pulsed Electric Field Treatment of Microorganisms. IEEE Trans. Plasma Sci. 2016, 44, 2121–2128. [Google Scholar] [CrossRef]
- Achayuthakan, P.; Wongsagonsup, R.; Sriprablom, J.; Suphantharika, M.; Intra, P. Effect of Pulsed Electric Field Treatment on the Protein, Digestibility, and Physicochemical Properties of Starch Granules in Wheat Flour. Polymers 2023, 15, 4087. [Google Scholar] [CrossRef]
- Raso, J.; Frey, W.; Ferrari, G.; Pataro, G.; Knorr, D.; Teissie, J.; Miklavčič, D. Recommendations guidelines on the key information to be reported in studies of application of PEF technology in food and biotechnological processes. Innov. Food Sci. Emerg. Technol. 2016, 37, 312–321. [Google Scholar] [CrossRef]
- Andreou, V.; Dimopoulos, G.; Dermesonlouoglou, E.; Taoukis, P. Application of pulsed electric fields to improve product yield and waste valorization in industrial tomato processing. J. Food Eng. 2020, 270, 109778. [Google Scholar] [CrossRef]
- Parniakov, O.; Roselló-Soto, E.; Barba, F.J.; Grimi, N.; Lebovka, N.; Vorobiev, E. New approaches for the effective valorization of papaya seeds: Extraction of proteins, phenolic compounds, carbohydrates, and isothiocyanates assisted by pulsed electric energy. Food Res. Int. 2015, 77, 711–717. [Google Scholar] [CrossRef]
- Qin, B.L.; Zhang, Q.; Canovas, G.V.B. Inactivation of microorganisms by Pulsed Electric Fields of different voltage waveforms. IEEE Trans. Dielectr. Electr. Insul. 1994, 1, 1047–1057. [Google Scholar] [CrossRef]
- Canovas, G.V.B.; Tapia, M.S.; Cano, M.P. Novel Food Processing Technologies; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Brito, P.S.; Canacsinh, H.; Mendes, J.P.; Redondo, L.M.; Pereira, M.T. Comparison between monopolar and bipolar microsecond range Pulsed Electric Fields in enhancement of apple juice extraction. IEEE Trans. Plasma Sci. 2012, 40, 2348–2354. [Google Scholar] [CrossRef]
- Sobrino-López, Á.; Raybaudi-Massilia, R.; Martín-Belloso, O. High intensity pulsed electric field variables affecting Staphylococcus aureus inoculated in milk. J. Dairy Sci. 2006, 89, 3739–3748. [Google Scholar] [CrossRef] [PubMed]
- Qin, B.L.; Barbosa-Canovas, G.V.B.; Swanson, B.G.; Pedrow, P.D.; Olsen, R.G. Inactivating microorganisms using a Pulsed Electric Field continuous treatment system. IEEE Trans. Ind. Appl. 1998, 34, 43–50. [Google Scholar] [CrossRef]
- Parniakov, O.; Barba, F.J.; Grimi, N.; Lebovka, N.; Vorobiev, E. Impact of pulsed electric fields and high voltage electrical discharges on extraction of high-added value compounds from papaya peels. Food Res. Int. 2014, 65, 337–343. [Google Scholar] [CrossRef]
- Qin, B.; Zhang, Q.; Barbosa-Canovas, G.V.; Swanson, B.G.; Pedrow, P.D. Pulsed electric field treatment chamber design for liquid food pasteurization using a finite element method. Trans. ASAE 1995, 38, 557–565. [Google Scholar] [CrossRef]
- Ghoshal, G. Comprehensive review on pulsed electric field in food preservation: Gaps in current studies for potential future research. Heliyon 2023, 9, e17532. [Google Scholar] [CrossRef]
- Tamborrino, A.; Mescia, L.; Taticchi, A.; Berardi, A.; Lamacchia, C.M.; Leone, A.; Servili, M. Continuous pulsed electric field pilot plant for olive oil extraction process. Innov. Food Sci. Emerg. Technol. 2022, 82, 103192. [Google Scholar] [CrossRef]
- Schottroff, F.; Knappert, J.; Eppmann, P.; Krottenthaler, A.; Horneber, T.; McHardy, C.; Rauh, C.; Jaeger, H. Development of a Continuous Pulsed Electric Field (PEF) Vortex-Flow Chamber for Improved Treatment Homogeneity Based on Hydrodynamic Optimization. Front. Bioeng. Biotechnol. 2020, 8, 340. [Google Scholar] [CrossRef] [PubMed]
- Arshad, R.N.; Abdul Malek, Z.; Munir, A.; Ahmad, M.H.; Nawawi, Z.; Sidik, M.A.B.; Jumani, T.A.; Khan, I.; Alotabi, H.; Khan, A. An improved electroporator with continuous liquid flow and double-exponential waveform for liquid food pasteurization. IEEE Access. 2021, 9, 147732–147742. [Google Scholar] [CrossRef]
- Seshakamal, J.H.; Kameswara, L.R. Electric Field Fluid Treatment Chamber. CA Patent CA2560858C, 22 September 2006. [Google Scholar]
- Rego, D.; Costa, L.; Pereira, M.T.; Redondo, L.M. Cell Membrane Permeabilization Studies of Chlorella sp. by Pulsed Electric Fields. IEEE Trans. Plasma Sci. 2015, 43, 3483–3488. [Google Scholar] [CrossRef]
- Evrendilek, G.A.; Li, S.; Dantzer, W.R.; Zhang, Q.H. Pulsed Electric Field Processing of Beer: Microbial, Sensory, and Quality Analyses. J. Food Sci. 2004, 69, M228–M232. [Google Scholar] [CrossRef]
- Donsì, G.; Ferrari, G.; Pataro, G. Inactivation kinetics of Saccharomyces cerevisiae by pulsed electric fields in a batch treatment chamber: The effect of electric field unevenness and initial cell concentration. J. Food Eng. 2007, 78, 784–792. [Google Scholar] [CrossRef]
- Toepfl, S.; Volker, H.; Dietrich, K. Applications of pulsed electric fields technology for the food industry. In Pulsed Electric Fields Technology for the Food Industry; Springer: Berlin/Heidelberg, Germany, 2006; pp. 197–221. [Google Scholar]
- Kotnik, T.; Kramar, P.; Pucihar, G.; Miklavcic, D.; Tarek, M. Cell membrane electroporation-Part 1: The phenomenon. IEEE Electr. Insul. Mag. 2012, 28, 14–23. [Google Scholar] [CrossRef]
- Sugar, I.P.; Neumann, E. Stochastic model for electric field-induced membrane pores electroporation. Biophys. Chem. 1984, 19, 211–225. [Google Scholar] [CrossRef]
- Kandušer, M.; Belič, A.; Čorović, S.; Škrjanc, I. Modular Serial Flow Through device for pulsed electric field treatment of the liquid samples. Sci. Rep. 2017, 7, 8115. [Google Scholar] [CrossRef]
- Sale, A.J.H.; Hamilton, W.A. Effect of high electric fields on microorganisms. I. Killing of bacteria and yeast. Biochim. Biophys. Acta. 1967, 148, 781–788. [Google Scholar] [CrossRef]
- Saulis, G. Electroporation of Cell Membranes: The Fundamental Effects of Pulsed Electric Fields in Food Processing. Food Eng. Reviews. 2010, 2, 52–73. [Google Scholar] [CrossRef]
- Martí-Quijal, F.J.; Ramon-Mascarell, F.; Pallarés, N.; Ferrer, E.; Berrada, H.; Phimolsiripol, Y.; Barba, F.J. Extraction of Antioxidant Compounds and Pigments from Spirulina (Arthrospira platensis) Assisted by Pulsed Electric Fields and the Binary Mixture of Organic Solvents and Water. Appl. Sci. 2021, 11, 7629. [Google Scholar] [CrossRef]
- Vernon-Parry, K.D. Scanning Electron Microscopy: An Introduction. III-Vs Rev. 2000, 13, 40–44. [Google Scholar] [CrossRef]
- Sarkis, J.R.; Boussetta, N.; Blouet, C.; Tessaro, I.C.; Marczak, L.D.F.; Vorobiev, E. Effect of pulsed electric fields and high voltage electrical discharges on polyphenol and protein extraction from sesame cake. Innov. Food Sci. Emerg. Technol. 2015, 29, 170–177. [Google Scholar] [CrossRef]
- Jaeschke, D.P.; Mercali, G.D.; Marczak, L.D.F.; Müller, G.; Frey, W.; Gusbeth, C. Extraction of valuable compounds from Arthrospira platensis using pulsed electric field treatment. Bioresour. Technol. 2019, 283, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Gateau, H.; Blanckaert, V.; Veidl, B.; Burlet-Schiltz, O.; Pichereaux, C.; Gargaros, A.; Marchand, J.; Schoefs, B. Application of pulsed electric fields for the biocompatible extraction of proteins from the microalga Haematococcus pluvialis. Bioelectrochemistry 2021, 137, 107588. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Gouyo, T.; Grimi, N.; Bals, O.; Vorobiev, E. Pulsed electric field pretreatment of rapeseed green biomass (stems) to enhance pressing and extractives recovery. Bioresour. Technol. 2016, 199, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Kumari, B.; Tiwari, B.K.; Walsh, D.; Griffin, T.P.; Islam, N.; Lyng, J.G.; Brunton, N.P.; Rai, D.K. Impact of pulsed electric field pre-treatment on nutritional and polyphenolic contents and bioactivities of light and dark brewer’s spent grains. Innov. Food Sci. Emerg. Technol. 2019, 54, 200–210. [Google Scholar] [CrossRef]
- Kronbauer, M.; Shorstkii, I.; Silva, S.; Toepfl, S.; Lammerskitten, A.; Siemer, C. Pulsed electric field assisted extraction of soluble proteins from nettle leaves (Urtica dioica L.): Kinetics and optimization using temperature and specific energy. Sustain. Food Technol. 2023, 1, 886–895. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Wang, R.; Rahaman, A.; Zeng, S.X.-A.; Brennan, C.S. Combined effects of pulsed electric field and ultrasound pretreatments on mass transfer and quality of mushrooms. LWT 2021, 150, 112008. [Google Scholar] [CrossRef]
- Boussetta, N.; Soichi, E.; Lanoisellé, J.L.; Vorobiev, E. Valorization of oilseed residues: Extraction of polyphenols from flaxseed hulls by pulsed electric fields. Ind. Crops Prod. 2014, 52, 347–353. [Google Scholar] [CrossRef]
- Martinchik, A.N. Nutritional value of sesame seeds. Vopr. Pitan. 2011, 80, 41–43. [Google Scholar] [PubMed]
- Kamboj, A.; Chopra, R.; Singh, R.; Saxena, V.; Prassana Kumar, G.V. Effect of pulsed electric field parameters on the alkaline extraction of valuable compounds from perilla seed meal and optimization by central composite design approach. Appl. Food Res. 2022, 2, 100240. [Google Scholar] [CrossRef]
- Pappas, V.M.; Lakka, A.; Palaiogiannis, D.; Athanasiadis, V.; Bozinou, E.; Ntourtoglou, G.; Makris, D.P.; Dourtoglou, V.G.; Lalas, S.I. Optimization of Pulsed Electric Field as Standalone “Green” Extraction Procedure for the Recovery of High Value-Added Compounds from Fresh Olive Leaves. Antioxidants 2021, 10, 1554. [Google Scholar] [CrossRef]
- Genovese, J.; Kranjc, M.; Serša, I.; Petracci, M.; Rocculi, P.; Miklavčič, D.; Mahnič-Kalamiza, S. PEF-treated plant and animal tissues: Insights by approaching with different electroporation assessment methods. Innov. Food Sci. Emerg. Technol. 2021, 74, 102872. [Google Scholar] [CrossRef]
- Saldaña, M.D.A.; Silva, E.K.; Olmos Cornejo, J.E.; Orozco Lopez, C.L. Comprehensive foodomics. In Green Processes in Foodomics: Biorefineries in the Food Industry; Elsevier: Amsterdam, The Netherlands, 2021; pp. 808–824. [Google Scholar] [CrossRef]
- Taha, A.; Casanova, F.; Šimonis, P.; Stankevič, V.; Gomaa, M.A.E.; Stirkė, A. Pulsed Electric Field: Fundamentals and Effects on the Structural and Techno-Functional Properties of Dairy and Plant Proteins. Foods 2022, 11, 1556. [Google Scholar] [CrossRef] [PubMed]
- Coustets, M.; Joubert-Durigneux, V.; Hérault, J.; Schoefs, B.; Blanckaert, V.; Garnier, J.-P.; Teissié, J. Optimization of protein electroextraction from microalgae by a flow process. Bioelectrochemistry 2015, 103, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Segovia, F.J.; Luengo, E.; Corral-Pérez, J.J.; Raso, J.; Almajano, M.P. Improvements in the aqueous extraction of polyphenols from borage (Borago officinalis L.) leaves by pulsed electric fields: Pulsed electric fields (PEF) applications. Ind. Crops Prod. 2015, 65, 390–396. [Google Scholar] [CrossRef]
- Priyadarshini, A.; Rajauria, G.; O’Donnell, C.P.; Tiwari, B.K. Emerging food processing technologies and factors impacting their industrial adoption. Crit. Rev. Food Sci. Nutr. 2019, 59, 3082–3101. [Google Scholar] [CrossRef]
- Schoenbach, K.H.; Joshi, R.P.; Stark, R.H.; Dobbs, F.C.; Beebe, S.J. Bacterial decontamination of liquids with pulsed electric fields. IEEE Trans. Dielectr. Electr. Insul. 2000, 7, 637–645. [Google Scholar] [CrossRef]
- Safi, C.; Charton, M.; Pignolet, O.; Silvestre, F.; Vaca-Garcia, C.; Pontalier, P.-Y. Influence of microalgae cell wall characteristics on protein extractability and determination of nitrogen-to-protein conversion factors. J. Appl. Phycol. 2013, 25, 523–529. [Google Scholar] [CrossRef]
- Gad, A.; Jayaram, S.H.; Pritzker, M. Performance of electrode materials during food processing by Pulsed Electric Fields. IEEE Trans. Plasma Sci. 2014, 42, 3161–3166. [Google Scholar] [CrossRef]
- Moonesan, M.S.; Jayaram, S.H. Effect of pulsewidth on medium temperature rise and microbial inactivation under Pulsed Electric Field food treatment. IEEE Trans. Ind. Appl. 2013, 49, 1767–1772. [Google Scholar] [CrossRef]
- Joana Gil-Chávez, G.; Villa, J.A.; Fernando Ayala-Zavala, J.; Basilio Heredia, J.; Sepulveda, D.; Yahia, E.M.; González-Aguilar, G.A. Technologies for Extraction and Production of Bioactive Compounds to be Used as Nutraceuticals and Food Ingredients: An Overview. Compr. Rev. Food Sci. Food Saf. 2013, 12, 5–23. [Google Scholar] [CrossRef]
- Carpentieri, S.; Jambrak, A.R.; Ferrari, G.; Pataro, G. Pulsed Electric Field-Assisted Extraction of Aroma and Bioactive Compounds from Aromatic Plants and Food By-Products. Front. Nutr. 2022, 8, 792203. [Google Scholar] [CrossRef] [PubMed]
- Lajoie, L.; Fabiano-Tixier, A.-S.; Chemat, F. Water as Green Solvent: Methods of Solubilisation and Extraction of Natural Products—Past, Present and Future Solutions. Pharmaceuticals 2022, 15, 1507. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhou, J.; Castagnini, J.M.; Berrada, H.; Barba, F.J. Pulsed electric field (PEF) recovery of biomolecules from Chlorella: Extract efficiency, nutrient relative value, and algae morphology analysis. Food Chem. 2023, 404, 134615. [Google Scholar] [CrossRef]
- Ranjha, M.M.A.N.; Kanwal, R.; Shafique, B.; Arshad, R.N.; Irfan, S.; Kieliszek, M.; Kowalczewski, P.Ł.; Irfan, M.; Khalid, M.Z.; Roobab, U.; et al. A Critical Review on Pulsed Electric Field: A Novel Technology for the Extraction of Phytoconstituents. Molecules 2021, 26, 4893. [Google Scholar] [CrossRef]
- Parniakov, O.; Barba, F.J.; Grimi, N.; Marchal, L.; Jubeau, S.; Lebovka, N.; Vorobiev, E. Pulsed electric field and pH assisted selective extraction of intracellular components from microalgae Nannochloropsis. Algal Res. 2015, 8, 128–134. [Google Scholar] [CrossRef]
- Carpentieri, S.; Mazza, L.; Nutrizio, M.; Jambrak, A.R.; Ferrari, G.; Pataro, G. Pulsed electric fields- and ultrasound-assisted green extraction of valuable compounds from Origanum vulgare L. and Thymus serpyllum L. Int. J. Food Sci. Technol. 2021, 56, 4834–4842. [Google Scholar] [CrossRef]
- Toepfl, S. Pulsed electric field food processing -industrial equipment design and commercial applications. Stewart Postharvest Rev. 2012, 8, 1–7. [Google Scholar] [CrossRef]
- Timmermans, R.A.H.; Mastwijk, H.C.; Berendsen, L.B.J.M.; Nederhoff, A.L.; Matser, A.M.; Van Boekel, M.A.J.S.; Nierop Groot, M.N. Moderate intensity Pulsed Electric Fields (PEF) as alternative mild preservation technology for fruit juice. Int. J. Food Microbiol. 2019, 298, 63–73. [Google Scholar] [CrossRef]
- Li, X.; Farid, M. A review on recent development in non-conventional food sterilization technologies. J. Food Eng. 2016, 182, 33–45. [Google Scholar] [CrossRef]
- Kempkes, M. Industrial pulsed electric field systems. In Handbook of Electroporation; Miklavcic, D., Ed.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–22. [Google Scholar] [CrossRef]
- Bhat, Z.F.; Morton, J.D.; Mason, S.L.; Bekhit, A.E.-D.A. Current and future prospects for the use of pulsed electric field in the meat industry. Crit. Rev. Food Sci. Nutr. 2019, 59, 1660–1674. [Google Scholar] [CrossRef] [PubMed]
- Arshad, R.N.; Abdul-Malek, Z.; Munir, A.; Buntat, Z.; Ahmad, M.H.; Jusoh, Y.M.M.; Bekhit, A.E.-D.; Roobab, U.; Manzoor, M.F.; Aadil, R.M. Electrical systems for pulsed electric field applications in the food industry: An engineering perspective. Trends Food Sci. Technol. 2020, 104, 1–13. [Google Scholar] [CrossRef]
- Puértolas, E.; Barba, F. Electrotechnologies applied to valorization of by-products from food industry: Main findings, energy and economic cost of their industrialization. Food Bioprod. Process. 2016, 100, 172–184. [Google Scholar] [CrossRef]
- Toepfl, S.; Kinsella, J.; Parniakov, O. Industrial Scale Equipment, Patents, and Commercial Applications in Pulsed Electric Fields to Obtain Healthier and Sustainable Food for Tomorrow; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar] [CrossRef]
- Jaeger, H.; Balasa, A.; Knorr, D. Food industry applications for pulsed electric fields. In Electrotechnologies for Extraction from Food Plants and Biomaterials; Food Engineering Series; Springer: New York, NY, USA, 2009; pp. 181–216. [Google Scholar] [CrossRef]
- Mohammed, M.; Eiss, A.A. Pulsed electric fields for food processing technology. In Structure and Function of Food Engineering; Eissa, A.A., Ed.; Intech: Rijeka, Croatia, 2012; pp. 275–306. [Google Scholar] [CrossRef]
- Morren, J.; Roodenburg, B.; de Haan, S.W.H. Electrochemical reactions and electrode corrosion in pulsed electric field (PEF) treatment chambers. Innov. Food Sci. Emerg. Technol. 2003, 4, 285–295. [Google Scholar] [CrossRef]
- Pataro, G.; Barca, G.M.J.; Donsì, G.; Ferrari, G. On the modeling of electrochemical phenomena at the electrode-solution interface in a PEF treatment chamber: Methodological approach to describe the phenomenon of metal release. J. Food Eng. 2015, 165, 34–44. [Google Scholar] [CrossRef]
- Samaranayake, C.P.; Sastry, S.K.; Zhang, H. Pulsed Ohmic Heating–A Novel Technique for Minimization of Electrochemical Reactions During Processing. J. Food Sci. 2005, 70, e460–e465. [Google Scholar] [CrossRef]
- Kotnik, T.; Miklavčič, D.; Mir, L.M. Cell membrane electropermeabilization by symmetrical bipolar rectangular pulses: Part II. Reduced electrolytic contamination. Bioelectrochemistry 2001, 54, 91–95. [Google Scholar] [CrossRef]
- Gad, A.; Jayaram, S.H. Effect of electric pulse parameters on releasing metallic particles from stainless steel electrodes during PEF processing of milk. IEEE Trans. Ind. Appl. 2013, 50, 1402–1409. [Google Scholar] [CrossRef]
- Bocker, R.; Silva, E.K. Pulsed electric field assisted extraction of natural food pigments and colorings from plant matrices. Food Chem. X 2022, 15, 100398. [Google Scholar] [CrossRef] [PubMed]
- Picart-Palmade, L.; Cunault, C.; Chevalier-Lucia, D.; Belleville, M.-P.; Marchesseau, S. Potentialities and Limits of Some Non-thermal Technologies to Improve Sustainability of Food Processing. Front. Nutr. 2019, 5, 130. [Google Scholar] [CrossRef] [PubMed]
- Martínez, J.M.; Delso, C.; Álvarez, I.; Raso, J. Pulsed Electric Field-assisted extraction of valuable compounds from microorganisms. Compr. Rev. Food Sci. Food Saf. 2020, 19, 530–552. [Google Scholar] [CrossRef] [PubMed]
- Schottroff, F.; Gratz, M.; Krottenthaler, A.; Johnson, N.B.; Bédard, M.F.; Jaeger, H. Pulsed electric field preservation of liquid whey protein formulations—Influence of process parameters, pH, and protein content on the inactivation of Listeria innocua and the retention of bioactive ingredients. J. Food Eng. 2019, 243, 142–152. [Google Scholar] [CrossRef]
- Martínez-Beamonte, R.; Ripalda, M.; Herrero-Continente, T.; Barranquero, C.; Dávalos, A.; López de las Hazas, M.C.; Álvarez-Lanzarote, I.; Sánchez-Gimeno, A.C.; Raso, J.; Arnal, C.; et al. Pulsed electric field increases the extraction yield of extra virgin olive oil without loss of its biological properties. Front. Nutr. 2022, 9, 1065543. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, M.F.; Zeng, X.A.; Rahaman, A.; Siddeeg, A.; Aadil, R.M.; Ahmed, Z.; Li, J.; Niu, D. Combined impact of pulsed electric field and ultrasound on bioactive compounds and FT-IR analysis of almond extract. J. Food Sci. Technol. 2019, 56, 2355–2364. [Google Scholar] [CrossRef]
- Steinbruch, E.; Wise, J.; Levkov, K.; Chemodanov, A.; Israel, A.; Livney, Y.D.; Golberg, A. Enzymatic cell wall degradation combined with pulsed electric fields increases yields of water-soluble-protein extraction from the green marine macroalga Ulva sp. Innov. Food Sci. Emerg. Technol. 2023, 84, 103231. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, M.; Berrada, H.; Zhu, Z.; Grimi, N.; Barba, F.J. Pulsed electric fields (PEF), pressurized liquid extraction (PLE) and combined PEF + PLE process evaluation: Effects on Spirulina microstructure, biomolecules recovery and Triple TOF-LC-MS-MS polyphenol composition. Innov. Food Sci. Emerg. Technol. 2022, 77, 102989. [Google Scholar] [CrossRef]


| S.No | Plant Wastes | Protein (%) * | Protein (g/100 g) ** | Ref. |
|---|---|---|---|---|
| 1 | Sesame Press Cake | 35–50 | X | [43] |
| 2 | Papaya Seeds | 27.3–28.3 | X | [44] |
| 3 | Olive pomace | 4.51 (Crude) | X | [45] |
| 4 | Flaxseed hulls | 17.45–19.14 | X | [46] |
| 5 | Tomato wastes | [47] | ||
| (i) Pomace | 15.1–22.7 | X | ||
| (ii) Peels | 5.7–20 | X | ||
| (iii) Seeds | 16.6–39.3 | X | ||
| 6 | Brewer’s spent grains | 26–30 | X | [48] |
| 7 | Cabbage leaf residues | 17.0–17.2 (Crude protein on DM*) | X | [49] |
| 8 | Potato peels | 13.02 (dry powder) | X | [50] |
| 9 | Apple pomace | 4 | X | [51] |
| 10 | Orange peel waste | 7.7 | X | [52] |
| 11 | Rapeseed meal | 36 | X | [53] |
| 12 | Pumpkin peel | 1.65 | X | [54] |
| 13 | Ripe Banana peel | 5.5–7.87 (Crude) | X | [55] |
| 14 | Pomegranate peel | 4.90–8.97 | X | [56] |
| 15 | Inodorus melon peel | 34.90 | X | [57] |
| 16 | Sweet potato peel | [58] | ||
| (i) Primary peel | 6.40–6.49 | X | ||
| (ii) Blanched peel | 8.11–8.20 | X | ||
| 17 | Pumpkin seeds | ~35 (Crude) | X | [59] |
| 18 | Wheat Bran | 13.2–18.4 | X | [60] |
| 19 | Rice bran | 10–16 | X | [61] |
| 20 | Outer scales of onion | 2.64 | X | [62] |
| 21 | Almond Husk | X | 3.27 (Crude) | [63] |
| 22 | Brewer dry grain | X | 19.96 (Crude) | [63] |
| Mushroom Species | Protein Content (%) | Nutritional Properties | Medicinal Properties | Reference |
|---|---|---|---|---|
| Agaricus Bisporus | 29.64–39.84 |
|
| [65,81] |
| Pleurotus: Pleurotus ostreatus Pleurotus eryngii Pleurotus sajor-caju Pleurotus giganteus | 7 11 37.4 17.7 |
|
| [82,83,84] |
| Morchella esculenta | 32.7 (DW)* |
|
| [85,86] |
| Lentinula edodes | 15–23 |
| Medicinally used for the following diseases
| [87,88,89] |
| Auricularia heimuer | 10.62 |
|
| [90] |
| Volvariella bombycina Fruit Body Mycelia | 28.30 25.50 |
|
| [91] |
| Enoki mushrooms: Flammulina filiformis, Flammulina velutipes | 5 |
|
| [92,93] |
| Tremella fuciformis | 9.63 |
|
| [94,95,96] |
| Hypsizygus tessellatus, | 33.9 |
|
| [97,98] |
| Stropharia rugosoannulata, | 30−50 |
| Essential Properties:
| [99,100] |
| Cyclocybe aegerita | 37.6 |
| Essential Properties:
| [101,102,103] |
| Hericium erinaceus | Dried powder, 20; Mycelia, 42 |
| Essential Properties:
| [104,105] |
| Phallus Indusiatus | 4.813 |
|
| [106] |
| Protein Source | Process Parameters | Outcome | Essential Findings | Reference |
|---|---|---|---|---|
| Agaricus Bisporus | 38.4 kV/cm, Pulse width of 2 µS at both 400 Hz and 800 Hz frequencies Inlet Temperature: 20 °C | Protein extraction of 40.8% | * Significant increase in protein yield when combined with mild heating * Protein concentration increased with an increase in the electric field, temperature, and treatment time | [119] |
| Agaricus Bisporus | 2.5 kV/cm, Specific energy of 50 kJ/kg, and 6 h of extraction time | Maximum protein recovery of 140.72 +/− 15.14 mg BSA/g DM* | * PEF increased the recovery of proteins | [143] |
| Papaya peels | 13.3 kV/cm, 400 pulses, 40 kV applied voltage, treatment time of 2720 secs. | Protein concentration of 20 mg/L | * Two stages of PEF + supplementary aqueous extraction resulted in good recovery of bioactive compounds including proteins | [165] |
| Rapeseed stems and leaves | 20 kV/cm 200 pulses, 40 kV applied voltage, pulse duration of 10 μs | Protein extraction of up to 80% | * PEF treatment increased the yield of proteins. * Protein contents changed during plant development | [4] |
| Rapeseed green biomass (stems) | 8 kV/cm, Pulse duration of 10 μS, pressure of 10 bar, maximum applied voltage of 40 kV, frequency of 0.5 Hz, 200 pulses | 0.14 g BSA/100 g DM* | * Protein content increased by about 2 times compared with the untreated sample and 50 pulse applications | [186] |
| Sesame cake | 13.3 kV/cm Pulse duration of 10 μs Frequency of 0.5 Hz (2 s between pulses or discharges) 0–700 pulses Temperature of 40 °C Variable treatment time between 1 and 7 ms Energy inputs between 0 and 291 kJ/kg | Between 374 and 2001 mg BSA/100 g DM*. | * Protein contents increased with energy inputs until 83 kJ/kg was reached. * PEF improved the protein extraction yield. | [183] |
| Tomato wastes | 5 kV/cm Treatment time of 1.5 ms 200 pulses | 145.1 mg/100 g | * Protein concentration increased with increasing the electric field strength and time | [158] |
| Rice bran | 2.3 kV/cm Treatment time of 25 min 250 pulses per minute | 20.71–22.8% | * Improved the extraction yield of rice bran protein * Improved oil’s holding and emulsifying properties * Increased foaming ability and stability * Enhanced in vitro digestibility | [145] |
| Brewers spent grains (BSG) Light Dark | 2.8 kV/cm 3000 pulses 20 μs pulse-width | 20.31 ± 0.01 (%DWe*) 23.78 ± 0.16 (%DWe*) | * Increased yield of proteins from dark BSG compared with light BSG * Light BSG exhibited higher antimicrobial activity than untreated ones | [187] |
| Nettle leaves | 3 kV/cm Specific energy of 10–24 kJ/kg Temperature between 70 and 78°C Extraction time of 5 min | >60% | PEF specific energy input, extraction temperature, and particle size had high impacts on solid liquid extraction kinetics | [188] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramaswamy, R.; Krishnan, S.B.; Leong, S.S.J. Pulsed Electric Field Technology for Recovery of Proteins from Waste Plant Resources and Deformed Mushrooms: A Review. Processes 2024, 12, 342. https://doi.org/10.3390/pr12020342
Ramaswamy R, Krishnan SB, Leong SSJ. Pulsed Electric Field Technology for Recovery of Proteins from Waste Plant Resources and Deformed Mushrooms: A Review. Processes. 2024; 12(2):342. https://doi.org/10.3390/pr12020342
Chicago/Turabian StyleRamaswamy, Ramya, Sivaneasan Bala Krishnan, and Susanna Su Jan Leong. 2024. "Pulsed Electric Field Technology for Recovery of Proteins from Waste Plant Resources and Deformed Mushrooms: A Review" Processes 12, no. 2: 342. https://doi.org/10.3390/pr12020342






