Supercritical Direct-Methane-to-Methanol Coupled with Gas-to-Wire for Low-Emission Offshore Processing of CO2-Rich Natural Gas: Techno-Economic and Thermodynamic Analyses
Abstract
:1. Introduction
1.1. Offshore Gas-to-Wire with CCS
1.2. Direct Methane-to-Methanol
1.3. The Present Work
2. Methods
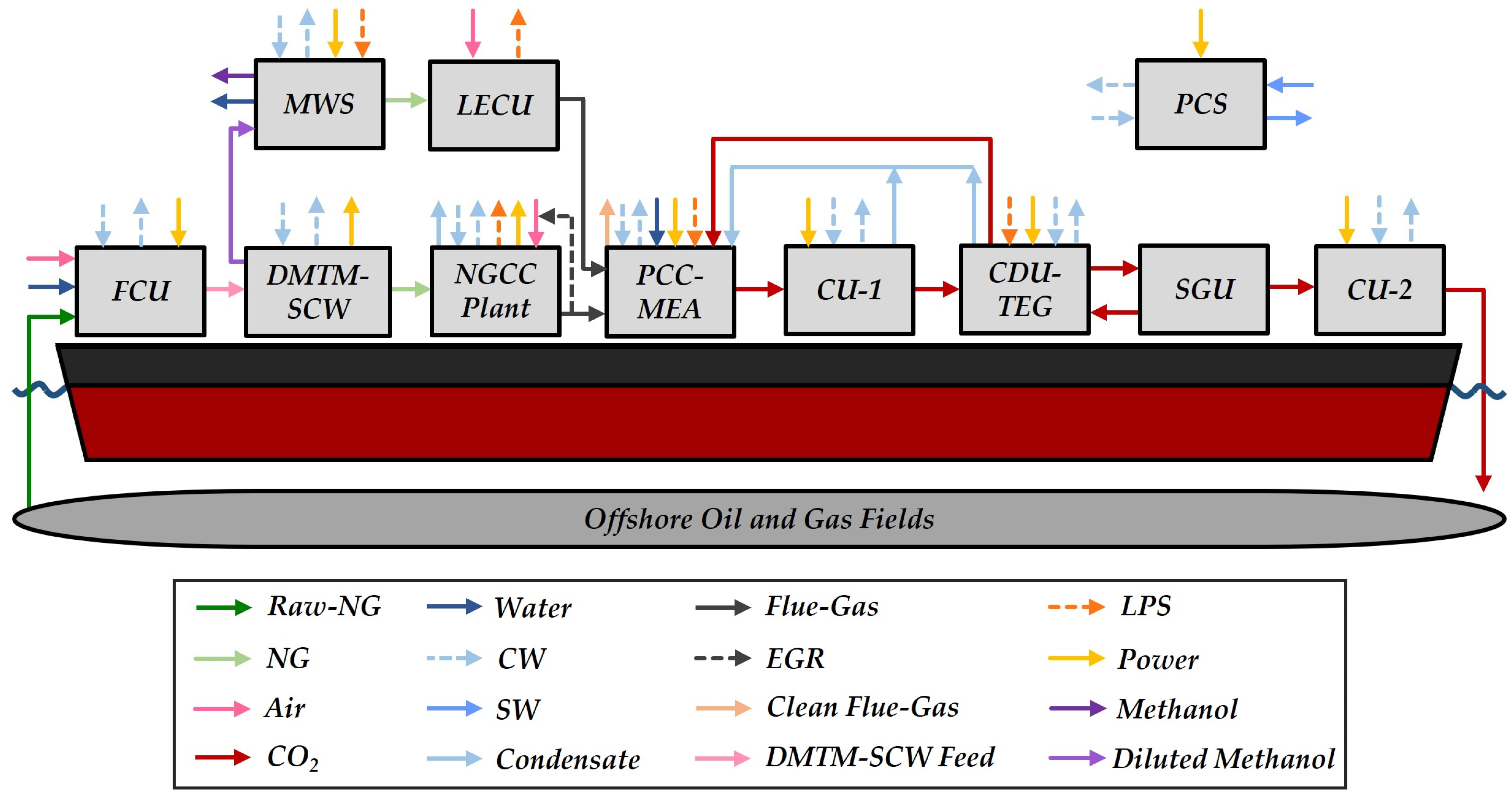
2.1. Process Structure
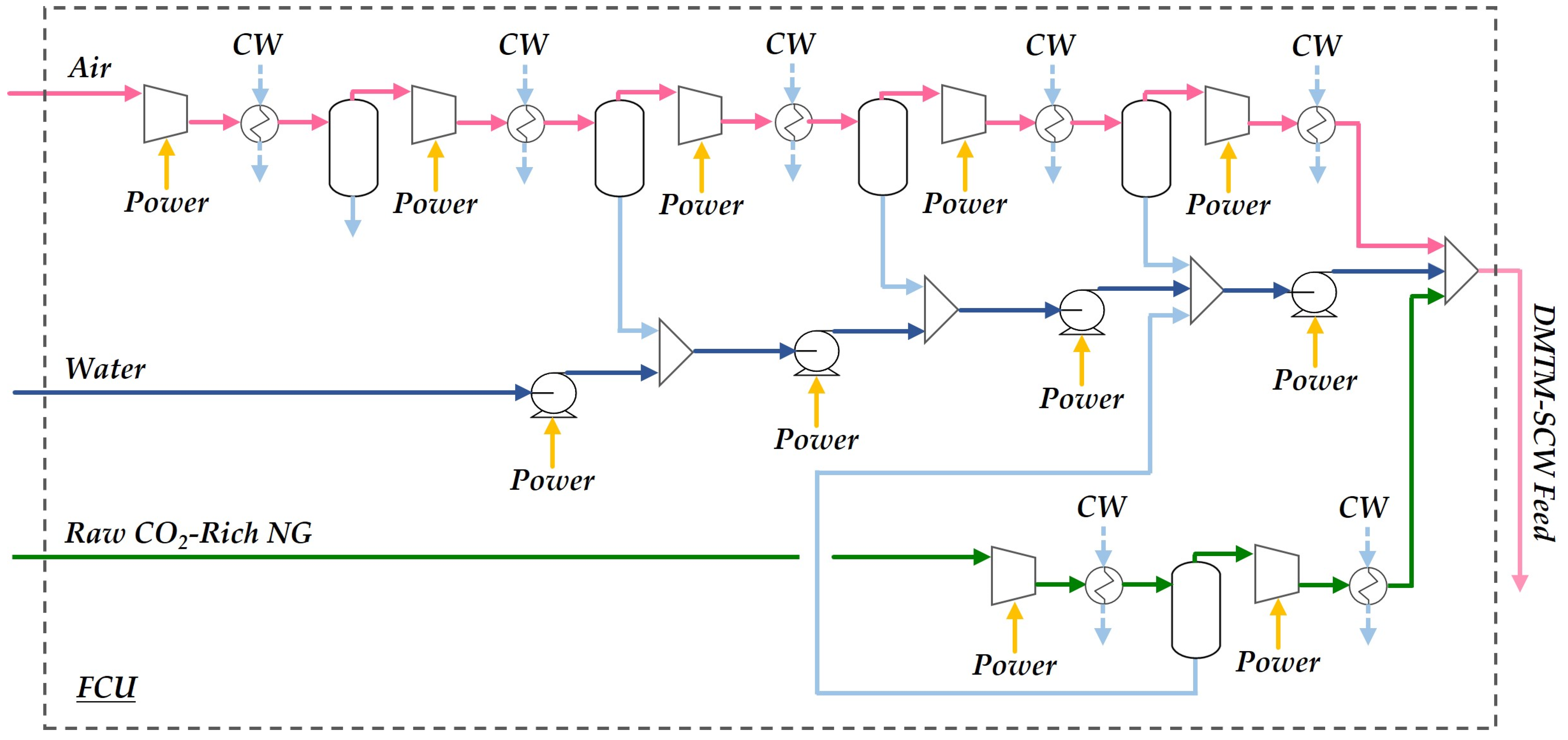
2.1.1. Feed Compression Unit
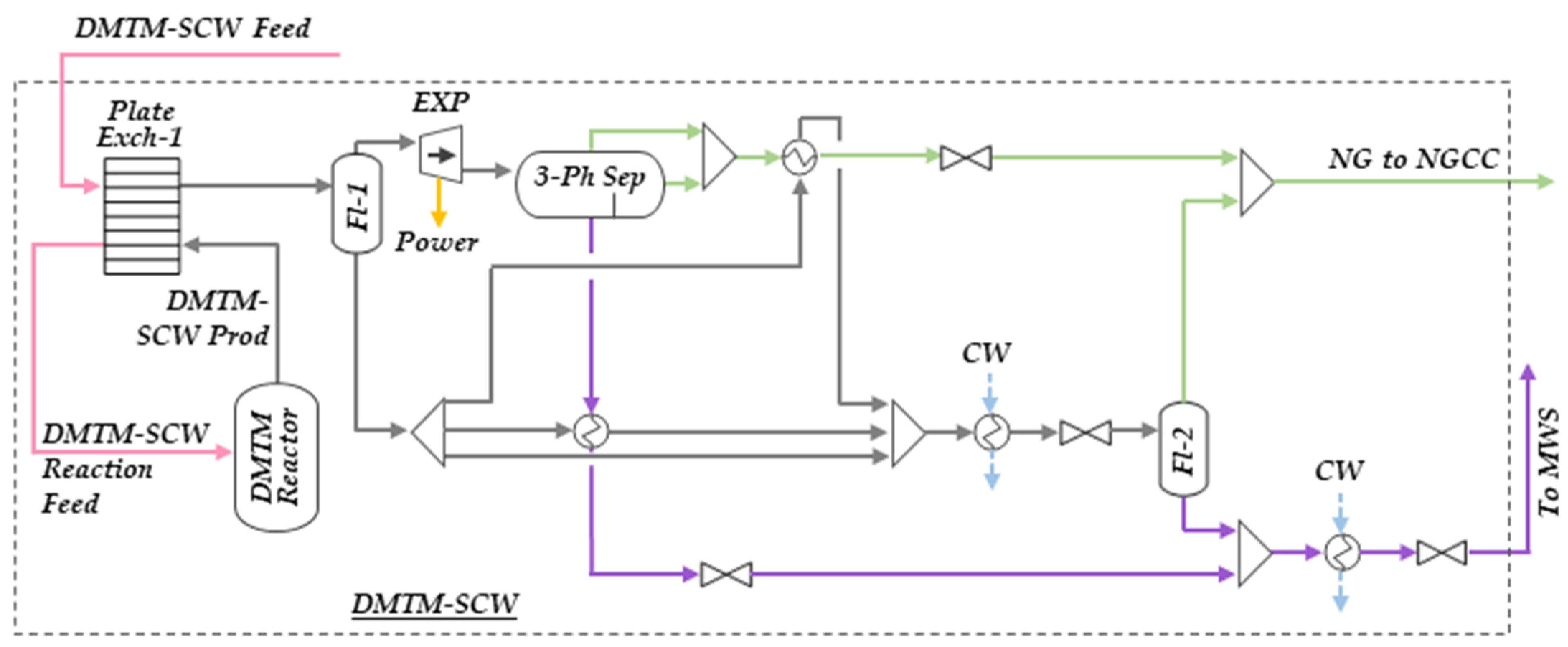
2.1.2. Direct Methane-to-Methanol in Supercritical Water (DMTM-SCW)
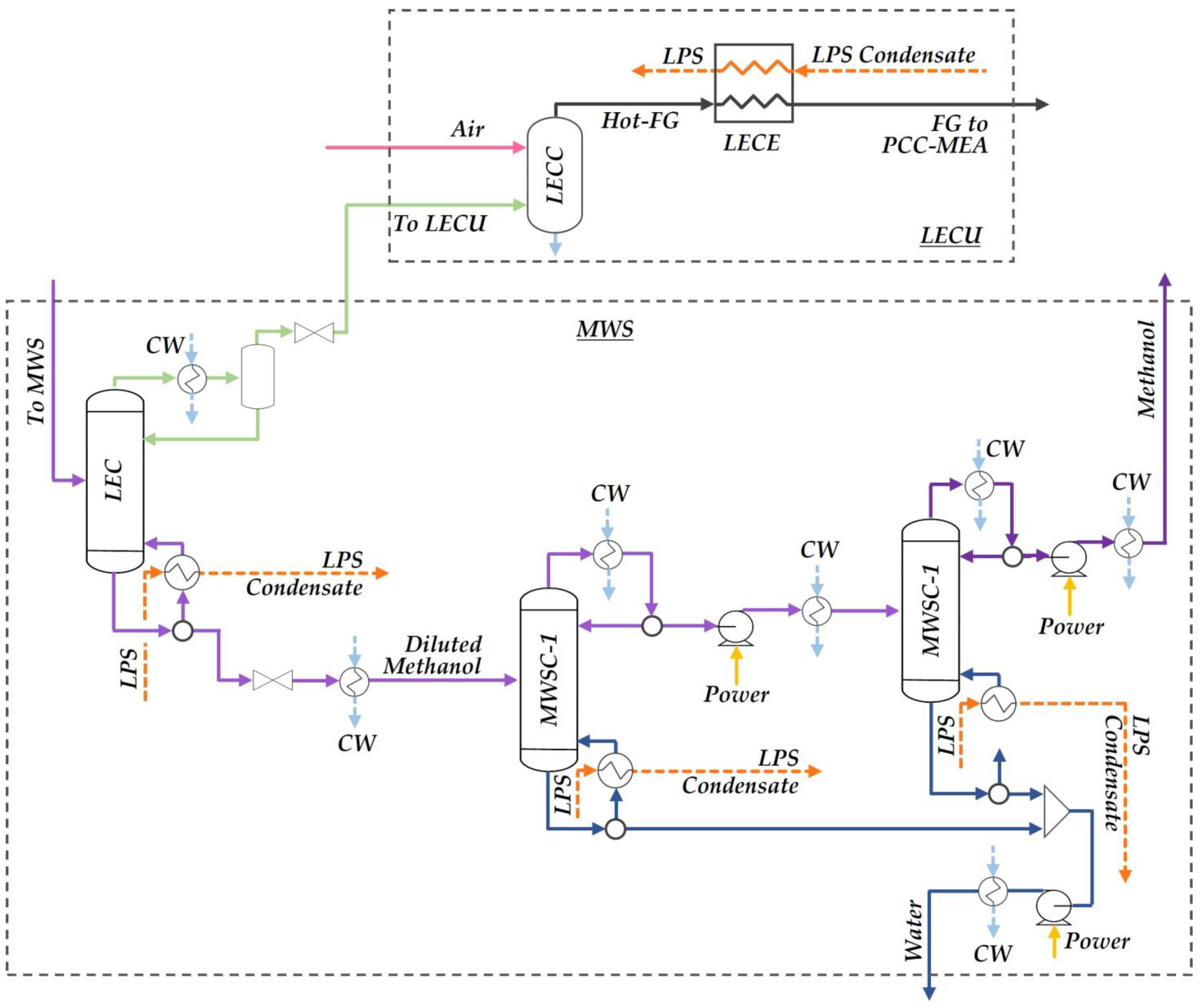
2.1.3. Methanol–Water Separation and Light-Ends Combustion
2.1.4. Natural Gas Combined Cycle Plant
2.1.5. Post-Combustion Capture with Aqueous MEA
2.1.6. CO2 Compression Units, CO2 Dehydration Unit and Stripping Gas Unit
2.1.7. Primary Cooling System
2.2. Process Economic Analysis
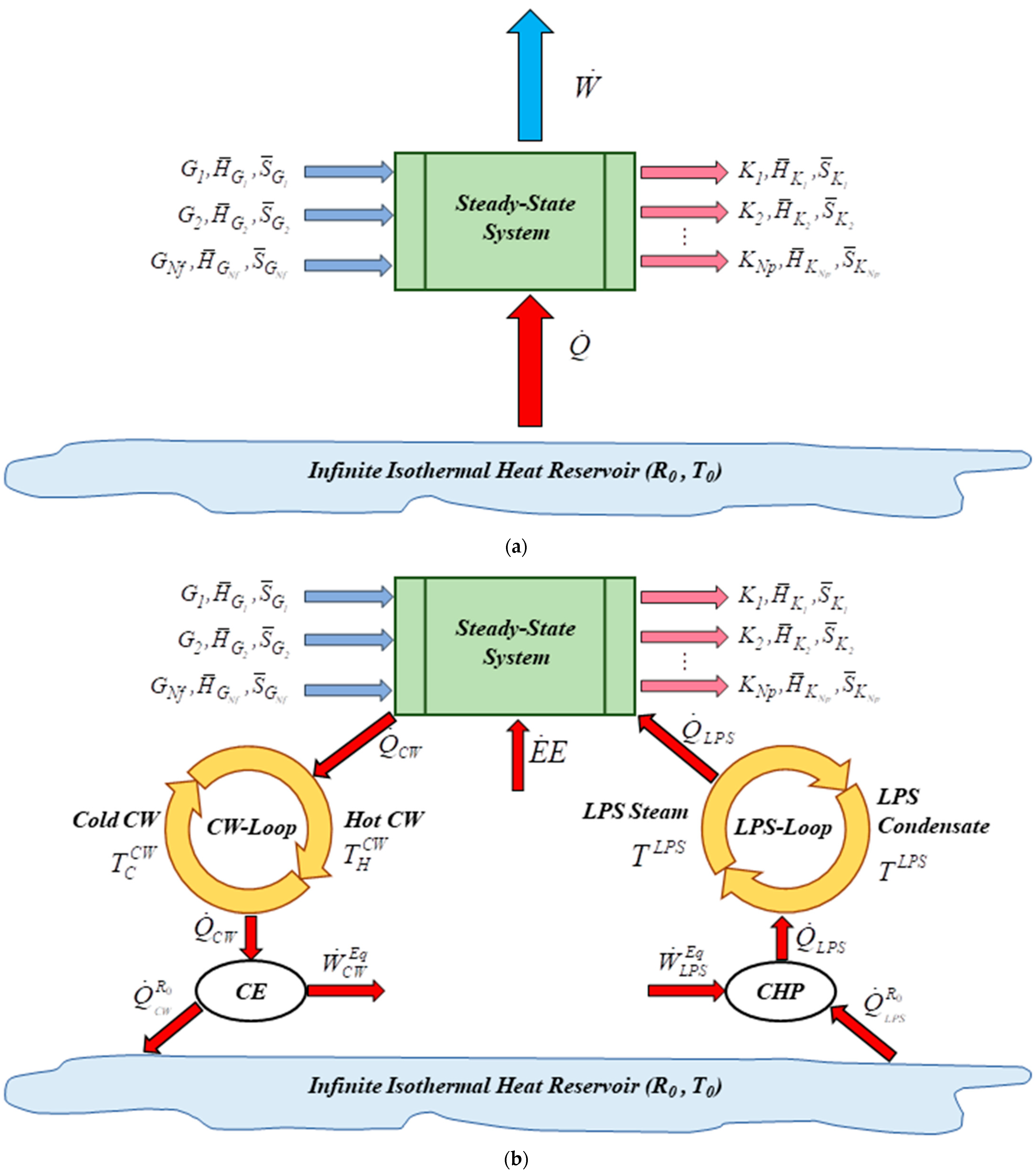
2.3. Process Thermodynamic Analysis
2.3.1. Maximum Power
2.3.2. Equivalent Power
2.3.3. Thermodynamic Efficiency
2.3.4. Lost Work
3. Results and Discussion
3.1. Technical and Economic Performance
3.2. Thermodynamic Analysis Results
3.2.1. Performance of Maximum Power, Equivalent Power and Thermodynamic Efficiency
3.2.2. Lost Work Performance
3.3. Comparison with the Literature
4. Conclusions
5. Suggestions for Future Work
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
Nomenclature
| AP, GAP | Net and gross annual profits (MMUSD/y) |
| CAP | Equipment capacity |
| CBM | Bare module cost (MMUSD) |
| COL, COM | Costs of labor and of manufacturing (MMUSD/y) |
| CRM, CUT | Costs of raw materials and of utilities (MMUSD/y) |
| DEPR | Depreciation (MMUSD/y) |
| Electricity (MW) | |
| FCI | Fixed capital investment (MMUSD) |
| Gi | Flow rate of ith feed stream (kmol/s) |
| Molar enthalpy (MJ/kmol), molar entropy (MJ/K·kmol) | |
| Ki | Flow rate of ith product stream (kmol/s) |
| Nf | Number of feed streams (inputs) |
| Np | Number of product streams (outputs) |
| NPV | Net present value (MMUSD) |
| P | Pressure (bar) |
| , W | Heat duty, power (MW) |
| REV | Revenues (MMUSD/y) |
| T | Temperature (K) |
| Greeks | |
| η | Thermodynamic efficiency (%) |
| Superscripts | |
| CW, Eq, LPS | Cooling water, equivalent, low-pressure steam |
References
- Neseli, M.A.; Ozgener, O.; Ozgener, L. Energy and exergy analysis of electricity generation from natural gas pressure reducing stations. Energy Convers. Manag. 2015, 93, 109–120. [Google Scholar] [CrossRef]
- de Freitas, V.A.; Vital, J.C.S.; Rodrigues, B.R.; Rodrigues, R. Source rock potential, main depocenters, and CO2 occurrence in the pre-salt section of Santos Basin, southeast Brazil. J. S. Am. Earth Sci. 2022, 115, 103760. [Google Scholar] [CrossRef]
- Andrei, M.; Sammarco, G. Gas to wire with carbon capture & storage: A sustainable way for on-site power generation by produced gas. In Proceedings of the Abu Dhabi International Petroleum Exhibition & Conference, Abu Dhabi, United Arab Emirates, 13–16 November 2017. [Google Scholar] [CrossRef]
- Ojijiagwo, E.; Oduoza, C.F.; Emekwuru, N. Economics of gas to wire technology applied in gas flare management. Eng. Sci. Technol. Int. J. 2016, 19, 2109–2118. [Google Scholar] [CrossRef]
- Orisaremi, K.K.; Chan, F.T.S.; Chung, N.S.H. Potential reductions in global gas flaring for determining the optimal sizing of gas-to-wire (GTW) process: An inverse DEA approach. J. Nat. Gas Sci. Eng. 2021, 93, 103995. [Google Scholar] [CrossRef]
- Watanabe, T.; Inoue, H.; Horitsugi, M.; Oya, S. Gas to Wire (GTW) system for developing “small gas field” and exploiting “associated gas”. In Proceedings of the International Oil & Gas Conference and Exhibition in China, Beijing, China, 5–7 December 2006. [Google Scholar] [CrossRef]
- Sayed, S.; Massoud, A. Minimum transmission power loss in multi-terminal HVDC systems: A general methodology for radial and mesh networks. Alex. Eng. J. 2019, 58, 115–125. [Google Scholar] [CrossRef]
- Brito, T.L.F.; Galvão, C.; Fonseca, A.F.; Costa, H.K.M.; dos Santos, E.M. A review of gas-to-wire (GtW) projects worldwide: State-of-art and developments. Energy Policy 2022, 163, 112859. [Google Scholar] [CrossRef]
- Zhou, D.; Li, P.; Liang, X.; Liu, M.; Wang, L. A long-term strategic plan of offshore CO2 transport and storage in northern South China Sea for a low-carbon development in Guangdong province, China. Int. J. Greenh. Gas Control 2018, 70, 76–87. [Google Scholar] [CrossRef]
- Roussanaly, S.; Aasen, A.; Anantharaman, R.; Danielsen, B.; Jakobsen, J.; Heme-De-Lacotte, L.; Neji, G.; Sødal, A.; Wahl, P.; Vrana, T.; et al. Offshore power generation with carbon capture and storage to decarbonise mainland electricity and offshore oil and gas installations: A techno-economic analysis. Appl. Energy 2019, 233–234, 478–494. [Google Scholar] [CrossRef]
- Zhao, H.; Chang, Y.; Feng, S. Influence of produced natural gas on CO2-crude oil systems and the cyclic CO2 injection process. J. Nat. Gas Sci. Eng. 2016, 35, 144–151. [Google Scholar] [CrossRef]
- Monson, C.C.; Korose, C.P.; Frailey, S.M. Screening methodology for regional-scale CO2 EOR and storage using economic criteria. Energy Procedia 2014, 63, 7796–7808. [Google Scholar] [CrossRef]
- Araújo, O.d.Q.F.; Reis, A.d.C.; de Medeiros, J.L.; Nascimento, J.F.D.; Grava, W.M.; Musse, A.P.S. Comparative analysis of separation technologies for processing carbon dioxide rich natural gas in ultra-deepwater oil fields. J. Clean. Prod. 2017, 155, 12–22. [Google Scholar] [CrossRef]
- Hetland, J.; Kvamsdal, H.M.; Haugen, G.; Major, F.; Kårstad, V.; Tjellander, G. Integrating a full carbon capture scheme onto a 450 MWe NGCC electric power generation hub for offshore operations: Presenting the Sevan GTW concept. Appl. Energy 2009, 86, 2298–2307. [Google Scholar] [CrossRef]
- Hachem, J.; Schuhler, T.; Orhon, D.; Cuif-Sjostrand, M.; Zoughaib, A.; Molière, M. Exhaust gas recirculation applied to single-shaft gas turbines: An energy and exergy approach. Energy 2022, 238, 121656. [Google Scholar] [CrossRef]
- Li, H.; Ditaranto, M.; Yan, J. Carbon capture with low energy penalty: Supplementary fired natural gas combined cycles. Appl. Energy 2012, 97, 164–169. [Google Scholar] [CrossRef]
- Li, H.; Ditaranto, M.; Berstad, D. Technologies for increasing CO2 concentration in exhaust gas from natural gas-fired power production with post-combustion, amine-based CO2 capture. Energy 2011, 36, 1124–1133. [Google Scholar] [CrossRef]
- Ahmadi, M.A.; Soleimani, R.; Bahadori, A. A computational intelligence scheme for prediction equilibrium water dew point of natural gas in TEG dehydration systems. Fuel 2014, 137, 145–154. [Google Scholar] [CrossRef]
- Zakaria, Z.; Kamarudin, S.K. Direct conversion technologies of methane to methanol: An overview. Renew. Sustain. Energy Rev. 2016, 65, 250–261. [Google Scholar] [CrossRef]
- Zhang, Q.; He, D.; Li, J.; Xu, B.; Liang, Y.; Zhu, Q. Comparatively high yield methanol production from gas phase partial oxidation of methane. Appl. Catal. A Gen. 2002, 224, 201–207. [Google Scholar] [CrossRef]
- Holmen, A. Direct conversion of methane to fuels and chemicals. Catal. Today 2009, 142, 2–8. [Google Scholar] [CrossRef]
- Han, B.; Yang, Y.; Xu, Y.; Etim, U.; Qiao, K.; Xu, B.; Yan, Z. A review of the direct oxidation of methane to methanol. Chin. J. Catal. 2016, 35, 1206–1215. [Google Scholar] [CrossRef]
- Ravi, M.; Ranocchiari, M.; van Bokhoven, J.A. The Direct Catalytic Oxidation of Methane to Methanol—A Critical Assessment. Angew. Chem. Int. Ed. 2017, 56, 16464–16483. [Google Scholar] [CrossRef]
- Savage, P.E.; Li, R.; Santini, J.T. Methane to methanol in supercritical water. J. Supercrit. Fluids 1994, 7, 135–144. [Google Scholar] [CrossRef]
- Zhang, F.; Li, Y.; Liang, Z.; Wu, T. Energy conversion and utilization in supercritical water oxidation systems: A review. Biomass Bioenergy 2022, 156, 106322. [Google Scholar] [CrossRef]
- Lee, J.H.; Foster, N.R. Direct partial oxidation of methane to methanol in supercritical water. J. Supercrit. Fluids 1996, 9, 99–105. [Google Scholar] [CrossRef]
- Le Guennec, Y.; Privat, R.; Jaubert, J.-N. Development of the translated-consistent tc-PR and tc-RK cubic equations of state for a safe and accurate prediction of volumetric, energetic and saturation properties of pure compounds in the sub- and super-critical domains. Fluid Phase Equilib. 2016, 429, 301–312. [Google Scholar] [CrossRef]
- Le Guennec, Y.; Privat, R.; Lasala, S.; Jaubert, J.-N. On the imperative need to use a consistent α-function for the prediction of pure-compound supercritical properties with a cubic equation of state. Fluid Phase Equilib. 2017, 445, 45–53. [Google Scholar] [CrossRef]
- Milão, R.d.F.D.; de Medeiros, J.L.; Interlenghi, S.F.; Araújo, O.d.Q.F. Low-emission Gas-to-Wire with thermodynamic efficiency: Monetization of carbon dioxide rich natural gas from offshore fields. Gas Sci. Eng. 2023, 115, 205021. [Google Scholar] [CrossRef]
- Ott, J.; Gronemann, V.; Pontzen, F.; Fiedler, E.; Grossman, G.; Kersebohm, K.B.; Weiss, G.; Witte, C. Methanol—An Industrial Review by Lurgi GmbH, Air Liquide GmbH and BASF AG. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012. [Google Scholar]
- Chebbi, R.; Qasim, M.; Jabbar, N.A. Optimization of triethylene glycol dehydration of natural gas. Energy Rep. 2019, 5, 723–732. [Google Scholar] [CrossRef]
- Siemens Energy. SGT-A35 Aeroderivative Gas Turbine. Available online: https://www.siemens-energy.com/global/en/offerings/power-generation/gas-turbines/sgt-a30-a35-rb.html (accessed on 2 September 2022).
- Følgesvold, E.; Skjefstad, H.; Riboldi, L.; Nord, L. Combined heat and power plant on offshore oil and gas installations. J. Power Technol. 2017, 97, 117–126. [Google Scholar]
- GE. Aeroderivative LM2500 Gas Turbine (50Hz): Fact Sheet. 2019. Available online: https://www.ge.com/content/dam/gepower/global/en_US/documents/gas/gas-turbines/aero-products-specs/lm2500-50hz-fact-sheet-product-specifications.pdf. (accessed on 2 September 2022).
- Kloster, P. Energy Optimization on Offshore Installations with Emphasis on Offshore Combined Cycle Plants. In Proceedings of the SPE Offshore Europe Oil and Gas Conference and Exhibition, Aberdeen, UK, 7–10 September 1999; Society of Petroleum Engineers: Aberdeen, UK, 1999. [Google Scholar] [CrossRef]
- GE. LM2500+G4 Marine Gas Turbine. 2021. Available online: https://www.geaviation.com/sites/default/files/datasheet-lm2500plusg4.pdf (accessed on 2 September 2022).
- Oh, S.-Y.; Binns, M.; Cho, H.; Kim, J.-K. Energy minimization of MEA-based CO2 capture process. Appl. Energy 2016, 169, 353–362. [Google Scholar] [CrossRef]
- Araújo, O.d.Q.F.; de Medeiros, J.L. Carbon capture and storage technologies: Present scenario and drivers of innovation. Curr. Opin. Chem. Eng. 2017, 17, 22–34. [Google Scholar] [CrossRef]
- Liu, G.; Zhu, L.; Hong, J.; Liu, H. Technical, Economical, and Environmental Performance Assessment of an Improved Triethylene Glycol Dehydration Process for Shale Gas. ACS Omega 2022, 7, 1861–1873. [Google Scholar] [CrossRef] [PubMed]
- Turton, R.; Bailie, R.C.; Whiting, W.B.; Shaeiwitz, J.A. Analysis, Synthesis and Design of Chemical Processes, 4th ed.; Prentice Hall: Columbia, MI, USA, 2012. [Google Scholar]
- Towering Skills. 2023. Available online: https://toweringskills.com/financial-analysis/cost-indices/ (accessed on 20 September 2023).
- Windén, B.; Chen, M.; Okamoto, N.; Kim, D.K.; McCaig, E.; Shenoi, A.; Wilson, P. Investigation of offshore thermal power plant with carbon capture as an alternative to carbon dioxide transport. Ocean Eng. 2014, 76, 152–162. [Google Scholar] [CrossRef]
- EIA. U.S. Energy Information Administration. Natural Gas. 2023. Available online: https://www.eia.gov/dnav/ng/hist/rngwhhdm.htm (accessed on 20 September 2023).
- CHEMANALYST. 2023. Available online: https://www.chemanalyst.com/Pricing-data/methanol-1 (accessed on 20 September 2023).
- Statista. Petroleum & Refinery. 2023. Available online: https://www.statista.com/statistics/262861/uk-brent-crude-oil-monthly-price-development/ (accessed on 20 September 2023).
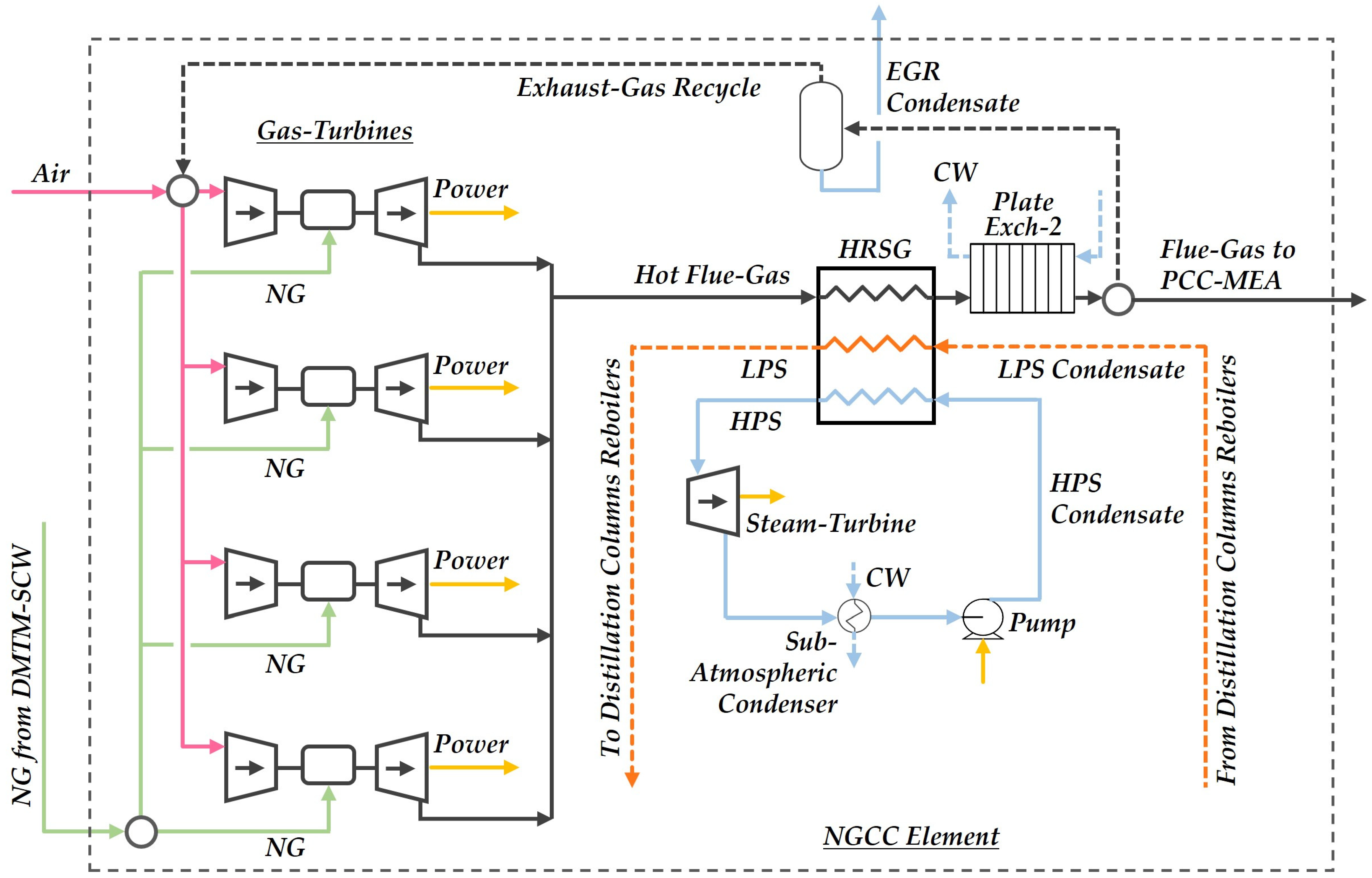

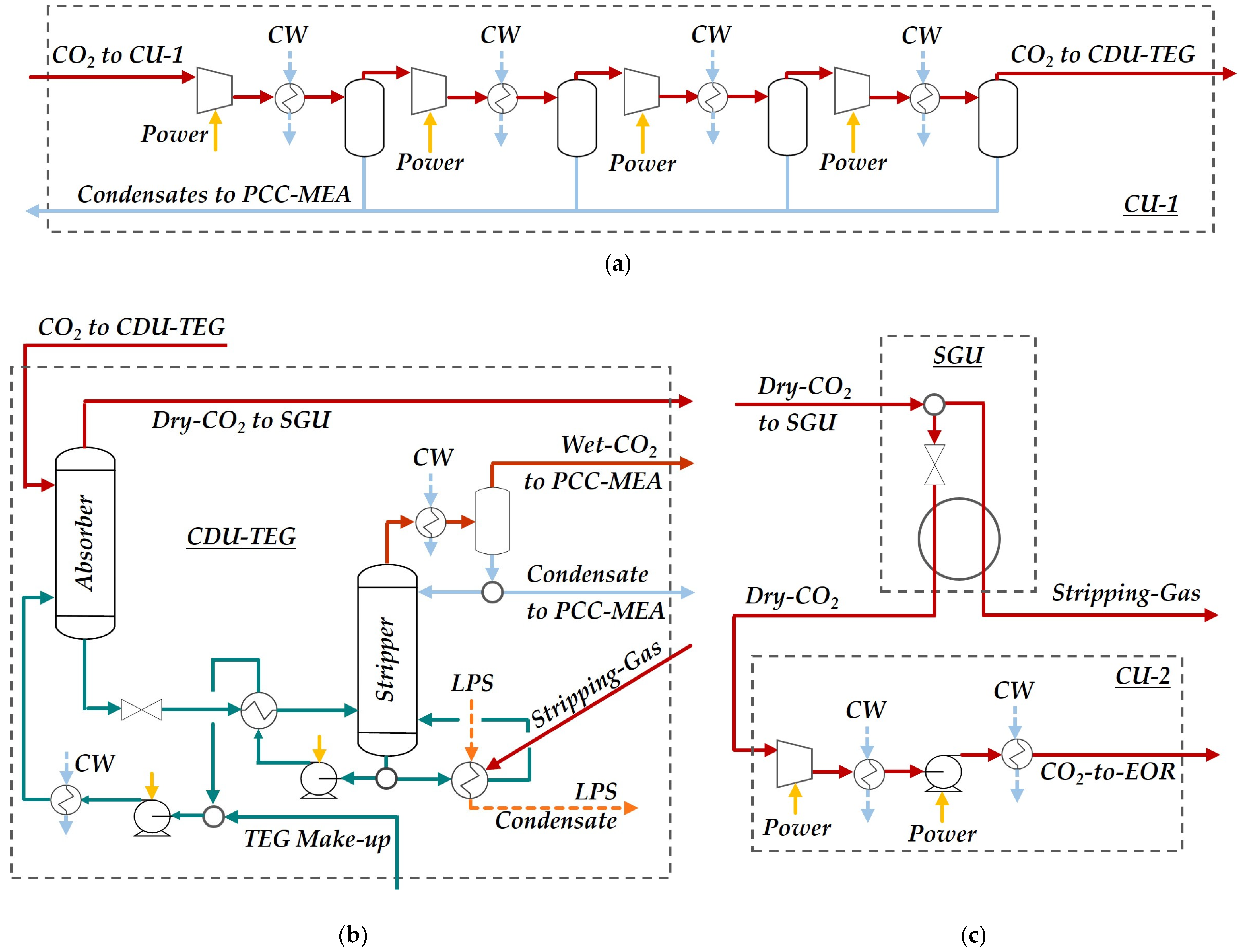
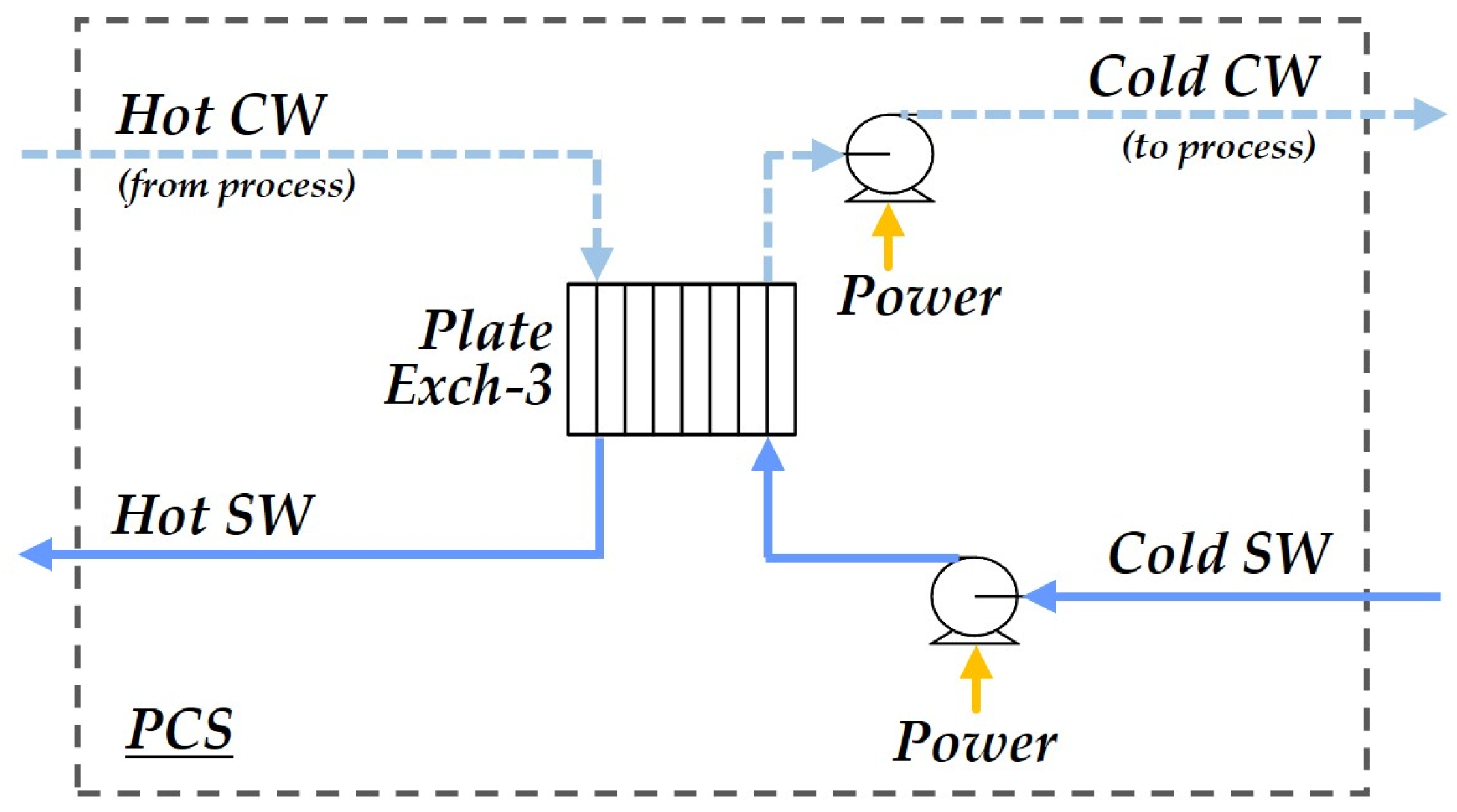


| Item | Description | Assumption |
|---|---|---|
| A1 | Thermodynamic Models | Gas, Liquid and Supercritical Streams: Peng–Robinson–Twu Equation of State [27,28]; Rankine Cycle and CW: ASME Steam Table; MWS: NRTL + Peng–Robinson Equation of State (NRTL-PR); PCC-MEA: HYSYS Acid Gas Package; CDU-TEG: HYSYS Glycol Package |
| A2 | Raw CO2-Rich NG | 6.5 MMSm3/d; T = 40 °C; P = 25 bar; CH4 = 49.82%mol, CO2 = 43.84%mol, C2H6 = 2.99%mol, C3H8 = 1.99%mol, iC4H10 = 0.3%mol, C4H10 = 0.2%mol, iC5H12 = 0.2%mol, C5H12 = 0.1%mol, C6H14 = 0.1%mol, C7H16 = 0.05%mol, C8H18 = 0.03%mol, C9H20 = 0.01%mol, C10H22 = 0.01%mol, H2O = 0.36%mol [2,29] |
| A3 | Air | T = 25 °C; P = 1.013bar; N2 = 77.49%mol; O2 = 20.60%mol; H2O = 1.91%mol [29] |
| A4 | Water | T = 35 °C; P = 1.013bar; H2O = 100%mol |
| A5 | DMTM Reaction (DMTM-SCW) | T = 410 °C; P = 250 bar; DMTM-SCW Reaction Feed: CH4/H2O mol ratio = 1; Conversions: O2 = 100%; CH4 = 4.5% [26]; Selectivity: CO = 50%; CO2 = 22%; CH3OH = 28% [26] |
| A6 | Methanol–Water Separation System (MWS) | Light-Ends Column (LEC): StagesTheoretical = 20; PTop = 7 bar; TTop = 35 °C; TReboiler = 139.6 °C; MWSC-1: StagesTheoretical = 20; PTop = 1.013 bar; TTop = 72.7 °C; TReboile r = 100.8 °C; MWSC-2: StagesTheoretical = 20; PTop = 1.013 bar; TTop = 64.3 °C; TReboiler = 100.8 °C; Methanol Purity: ≥99.85%w/w (Grade AA) [30] |
| A7 | PCC-MEA | Absorber: StagesTheoretical = 40; PTop = 1.013 bar; TInlet-Top = 40 °C; Capture ≈ 90% [29]; Stripper: StagesTheoretical = 10; PTop = 1.013 bar; TTop = 35 °C; TReboiler = 102.6 °C [29]; Lean MEA: H2O = 64.75%w/w, MEA = 30.44%w/w, CO2 = 4.81%w/w [29]; Capture Ratio: CR = 13.7 kgSolvent/kgCO2; Stripping Heat Ratio: HR = 223.5kJ/molCO2 [29] |
| A8 | CO2 Dehydration Unit (CDU-TEG) | Absorber: StagesTheoretical = 15; P = 50 bar; TInlet = 35 °C; Lean Solvent: TEG = 98.5%w/w; Stripper: StagesTheoretical = 10; PTop = 1.013 bar; TTop = 35 °C; TReboiler = 138.9 °C [31] |
| A9 | Gas Turbine | Aero-Derivative Siemens SGT-A35; EfficiencyLHV = 33.8% [32]; PInlet = 25 bar; TFlue-Gas = 484 °C [32] |
| A10 | Steam Turbine | HPS: PInlet = 24 bar; POutlet = 0.12 bar; TInlet = 459 °C; Outlet Quality = 95.0% |
| A11 | Compressors | Stage Compression Ratio ≈ 3.0 [29]; Intercoolers: TGas-Outlet = 35 °C; ΔTApproach = 5 °C; ΔP = 0.5 bar |
| A12 | Adiabatic Efficiencies | ηPumps = ηCompressors = ηSteam-Turbine = 75% [29]; Gas Turbines: ηAir-Compressor = 87.3%, ηExpander = 86.2% |
| A13 | HRSG | ∆PFlue-Gas = 0.025 bar; ∆PSteam = 0.05 bar; ΔTApproach = 25 °C [33] |
| A14 | Exchangers | ΔTApproach = 10 °C (gas–gas, liq–liq); ΔTApproach = 5 °C (gas–liq); ΔP = 0.5 bar [29] |
| A15 | Low-Pressure Steam (LPS) | TLPS = 154.6 °C; PLPS = 5.4 bar |
| A16 | Steam Production | Priority: LPSMWS + LPSPCC-MEA + LPSCDU-TEG; Surplus: HPSRankine-Cycle |
| A17 | Cooling Water (CW) | CW: TInlet = 30 °C; TOutlet = 45 °C; PInlet = 4 bar; POutlet = 3.5 bar [29] |
| A18 | Sea Water | SW: TInlet = 25 °C (Tropical Latitudes); TOutlet = 35 °C; ΔPPump = 1.5 bar |
| A19 | CO2-to-EOR | T = 35 °C; P = 300 bar; Purity: CO2 ≥ 99.9%mol [29] |
| Item | Description | Assumption |
|---|---|---|
| B1 | Operation Lifetime | 50 y |
| B2 | Construction Time | 2y (40%FCI + 60%FCI) |
| B3 | Operation | 8400 h/y |
| B4 | i | 10% |
| B5 | DEPR (MMUSD/y) | 10%FCI |
| B6 | ITR | 34% |
| B7 | N° of Operators | 30 [40] |
| B8 | Labor Cost | 89,100 USD/(y.operator) [29] |
| B9 | CEPCI | 816 (2022) [41] |
| B10 | FCI GT (Siemens SGT-A35) | 13.5 MMUSD [32] |
| B11 | FCI Subsea HVDC Cables | 1970 USD/(MW·km) [42] |
| B12 | FCI Rig Hull | 246 MMUSD [42] |
| B13 | NG Value | 2.58 USD/MMBTU [43] |
| B14 | MEA Price | 2 USD/kg [29] |
| B15 | TEG Price | 2.5 USD/kg [44] |
| B16 | Electricity Price | 0.0821 USD/kWh [43] |
| B17 | Methanol Price | 536 USD/t [44] |
| B18 | Oil Price | 80 USD/bbl [45] |
| B19 | EOR Yield | 1.5 bblOil/tCO2 [29] |
| DMTM-GTW-CCU | System | Utilities Demand | |||
|---|---|---|---|---|---|
| CW (t/h) | LPS (t/h) | Power (MW) | |||
| Gross Power (MW) | 564.5 | FCU | 3052.5 | - | 35.34 |
| Power Demand (MW) | 107.4 | DMTM-SCW | 32.4 | - | - |
| Net Power (MW) | 457.1 | MWS | 2511.7 | 68.1 | 0.002 |
| Methanol Production (t/h) | 2.2 | LECU | - | - | - |
| CO2 Flue Gas (t/h) (PCC-MEA Feed) | 554.3 | NGCC Plant | 24,175.7 | - | 0.04 |
| CO2 Emissions (t/h) (Atmosphere) | 63.0 | PCC-MEA | 32,445.0 | 1189.0 | 0.35 |
| CO2 Injected (t/h) (EOR) | 491.3 | CU-1 | 3976.6 | - | 50.46 |
| CDU-TEG | 40.3 | 1.9 | 0.01 | ||
| SGU | - | - | - | ||
| CU-2 | 2304.0 | - | 12.84 | ||
| PCS | - | - | 8.32 | ||
| Total | 68,538.2 | 1259.0 | 107.4 | ||
| CDU-TEG Results | Economic Results | ||||
| PCC-MEA Results | CO2 Inlet (ppm-mol H2O) | 2731.7 | FCI (MMUSD) | 1544.4 | |
| Flue Gas Feed (%molCO2) | 16.7 | CO2 Outlet (ppm-mol H2O) | 158.1 | COL (MMUSD/y) | 2.7 |
| Clean Flue Gas (%molCO2) | 2.1 | Capture-Ratio (kgTEG/kgH2O) | 5.7 | CUT (MMUSD/y) | 0 * |
| CO2 to CU-1 (%molCO2) | 92.6 | Lean-Solvent (t/h) | 3.3 | CRM (MMUSD/y) | 135.5 |
| Capture Ratio (kgSolvent/kgCO2) | 13.7 | Absorber: TTop (°C)/TBottom (°C) | 36.5/35.6 | REV (MMUSD/y) | 820.0 |
| CO2Captured (tCO2/h) | 492.3 | Stripper: TFeed (°C)/TTop (°C)/TBottom (°C) | 78/35/139 | NPV50years (MMUSD) | 1020.9 |
| Lean Solvent (t/h) | 6740.0 | Reboiler Duty (MW) | 1.1 | Payback (y) | 10 |
| Absorber: TTop (°C)/TBottom (°C) | 61.8/61.2 | ||||
| Stripper: TFeed (°C)/TTop (°C)/TBottom (°C) | 83/35/103 | ||||
| Heat Ratio (kJ/molCO2) | 224 | ||||
| Reboiler Duty (MW) | 694 | ||||
| Second Law Analysis | Lost Work Validation | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| System | (MW) | (MW) | (MW) | (MW) | (MW) | (MW) * | (MW) # | Divergence (%) | |
| FCU | −16.45 | 35.34 | 2.13 | - | 33.21 | 49.54 | 16.76 | 16.79 | −0.20 |
| DMTM-SCW | 47.34 | 11.60 | 0.02 | - | 11.62 | 24.55 | 35.72 | 35.71 | 0.02 |
| MWS | −0.01 | 0.002 | 1.75 | 12.05 | 10.30 | 0.12 | 10.29 | 10.35 | −0.62 |
| LECU | 0.22 | - | - | 0.04 | 0.04 | 16.79 | 0.1819 | 0.1818 | 0.04 |
| NGCC Plant | 1686.34 | 552.82 | 16.87 | 222.58 | 792.27 | 46.98 | 894.07 | 893.49 | 0.06 |
| PCC-MEA | −28.23 | 0.35 | 22.64 | 210.24 | 187.95 | 15.02 | 159.72 | 158.98 | 0.46 |
| CU-1 | −28.19 | 50.46 | 2.78 | - | 47.69 | 59.12 | 19.49 | 19.54 | −0.23 |
| CDU-TEG | −0.003 | 0.01 | 0.03 | 0.33 | 0.31 | 1.11 | 0.307 | 0.309 | −0.37 |
| SGU | 0.40 | - | - | - | 0.00 | 0.00 | 0.40 | 0.40 | 0.00 |
| CU-2 | −4.82 | 12.84 | 1.61 | - | 11.23 | 42.95 | 6.41 | 6.43 | −0.40 |
| PCS | 23.99 | −8.32 | - | - | −8.32 | −34.69 | 32.31 | 32.49 | −0.53 |
| Sum Crosscheck | 1175.66 | 1174.67 | 0.08 | ||||||
| Overall | 1633.07 | 457.09 | - | - | 457.09 | 27.99 | 1175.98 | 1181.54 | −0.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reis, A.d.C.; Araújo, O.d.Q.F.; de Medeiros, J.L. Supercritical Direct-Methane-to-Methanol Coupled with Gas-to-Wire for Low-Emission Offshore Processing of CO2-Rich Natural Gas: Techno-Economic and Thermodynamic Analyses. Processes 2024, 12, 374. https://doi.org/10.3390/pr12020374
Reis AdC, Araújo OdQF, de Medeiros JL. Supercritical Direct-Methane-to-Methanol Coupled with Gas-to-Wire for Low-Emission Offshore Processing of CO2-Rich Natural Gas: Techno-Economic and Thermodynamic Analyses. Processes. 2024; 12(2):374. https://doi.org/10.3390/pr12020374
Chicago/Turabian StyleReis, Alessandra de Carvalho, Ofélia de Queiroz Fernandes Araújo, and José Luiz de Medeiros. 2024. "Supercritical Direct-Methane-to-Methanol Coupled with Gas-to-Wire for Low-Emission Offshore Processing of CO2-Rich Natural Gas: Techno-Economic and Thermodynamic Analyses" Processes 12, no. 2: 374. https://doi.org/10.3390/pr12020374






