Influences of Cosolvents and Antifreeze Additives Derived from Glycerol through Esterification on Fuel Properties of Biodiesel
Abstract
:1. Introduction
2. Experimental Details
2.1. Preparing the Antifreeze from Bioglycerol
2.2. Analysis of Fuel Characteristics of Blended Biodiesel
3. Results and Discussion
3.1. Effect of Irradiated UV Light and Molar Ratio on Fuel Characteristics of Glycerine Acetate
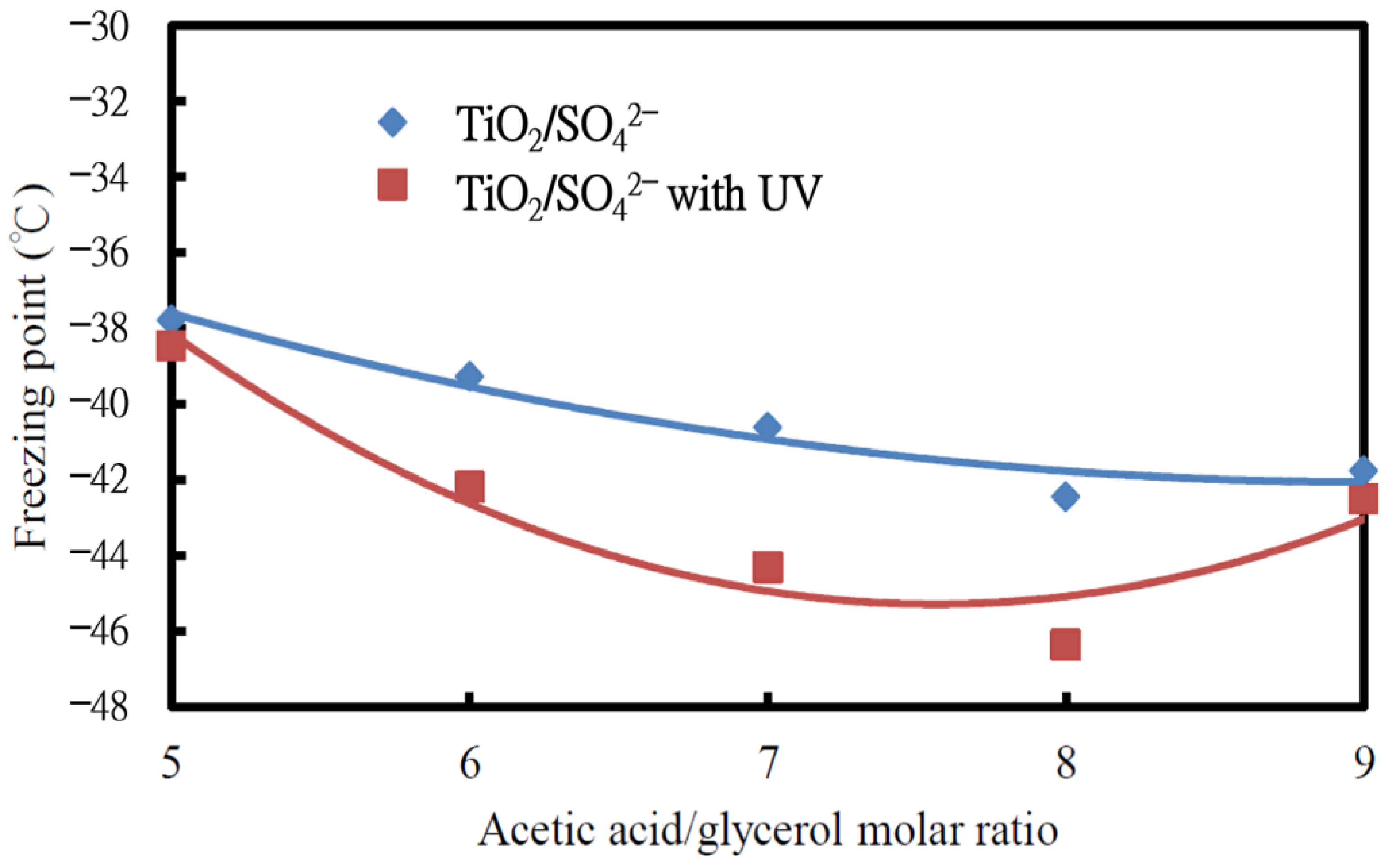
3.1.1. Effect of Irradiated UV Light and Molar Ratio on Freezing Point of Glycerine Acetate
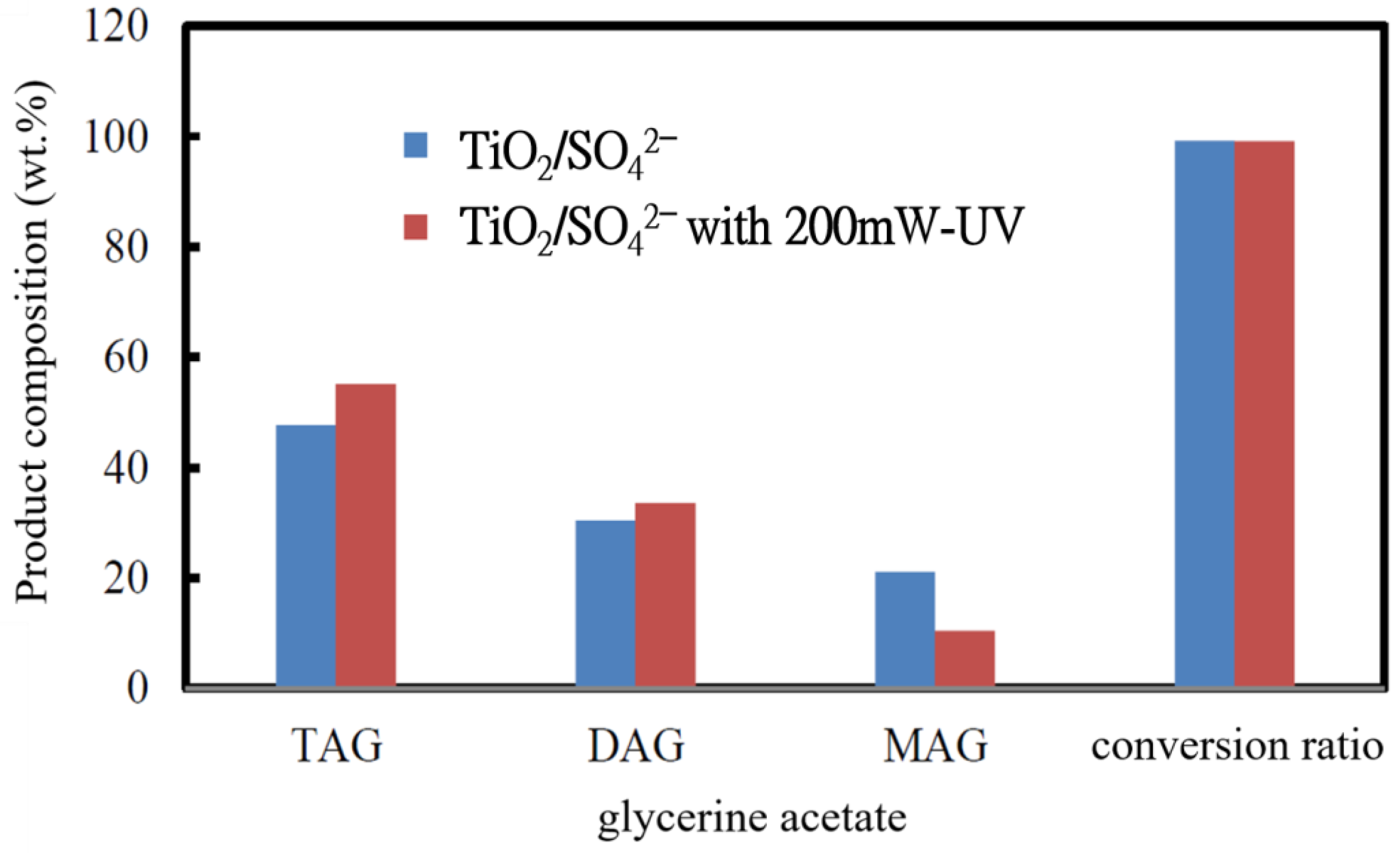
3.1.2. Effect of UV-Light Irradiation and Molar Ratio on Compositions of Glycerine Acetate
3.2. Analysis of Fuel Properties
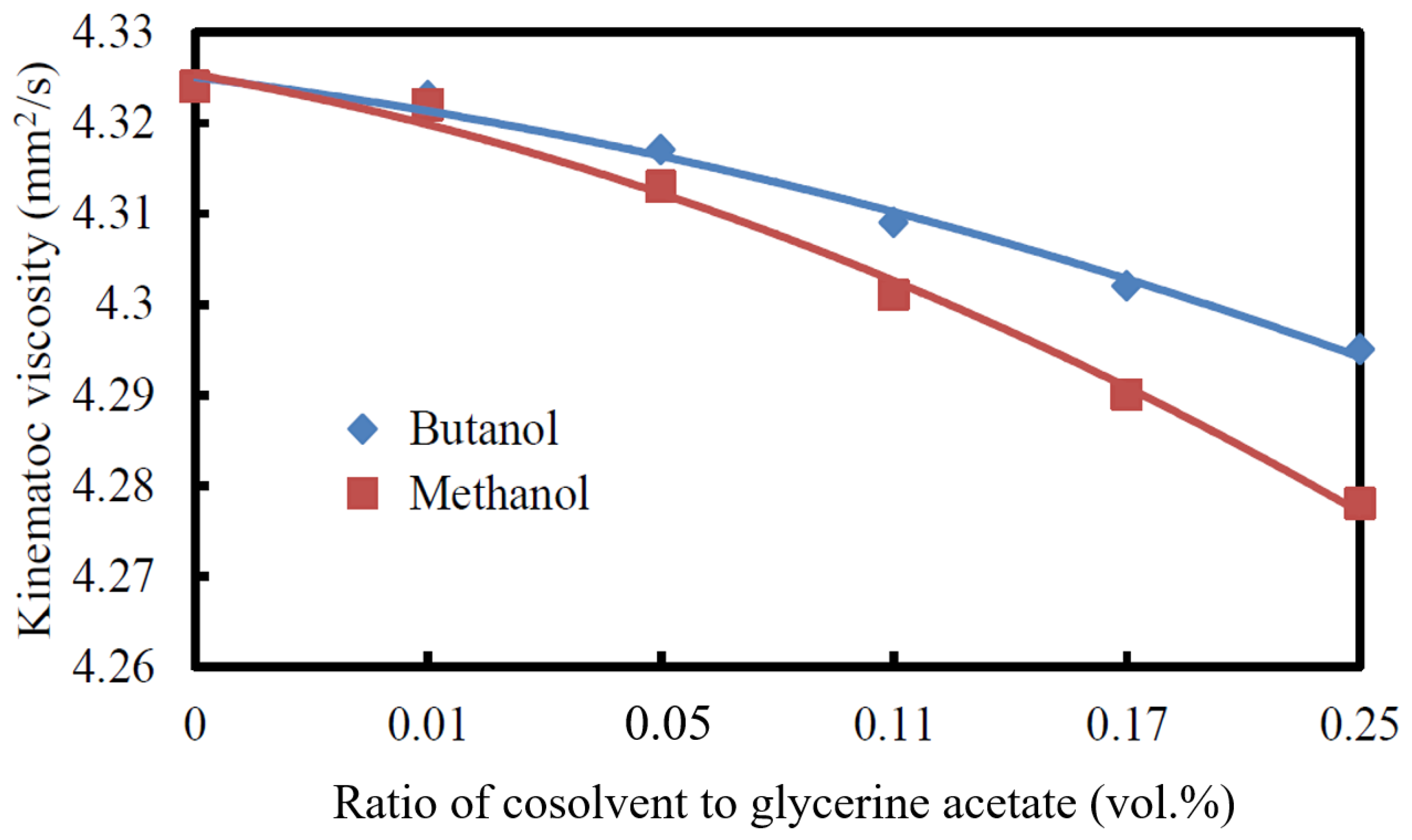
3.2.1. Kinematic Viscosity
3.2.2. Heating Value

3.2.3. Acid Value
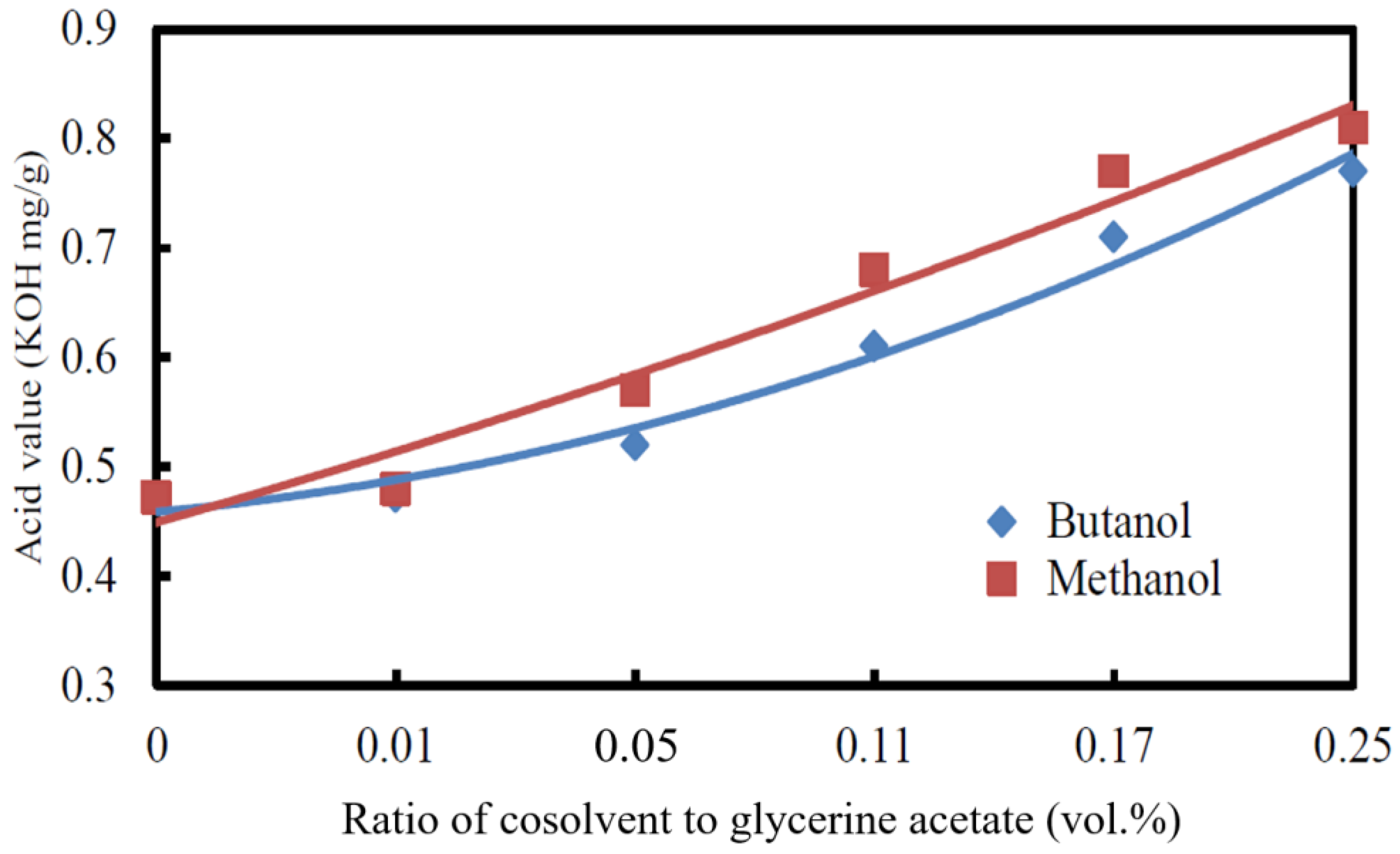
3.2.4. Cetane Index
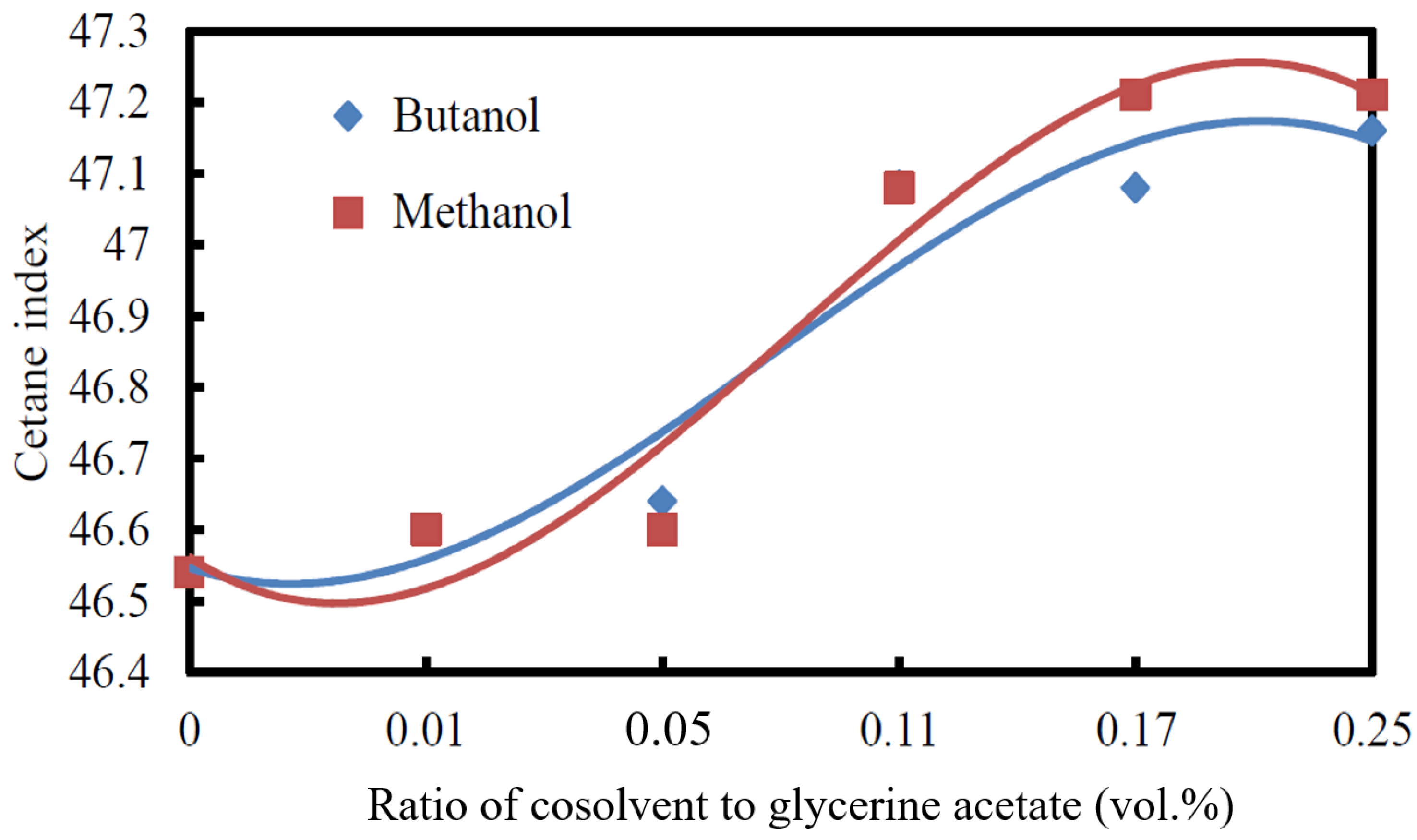
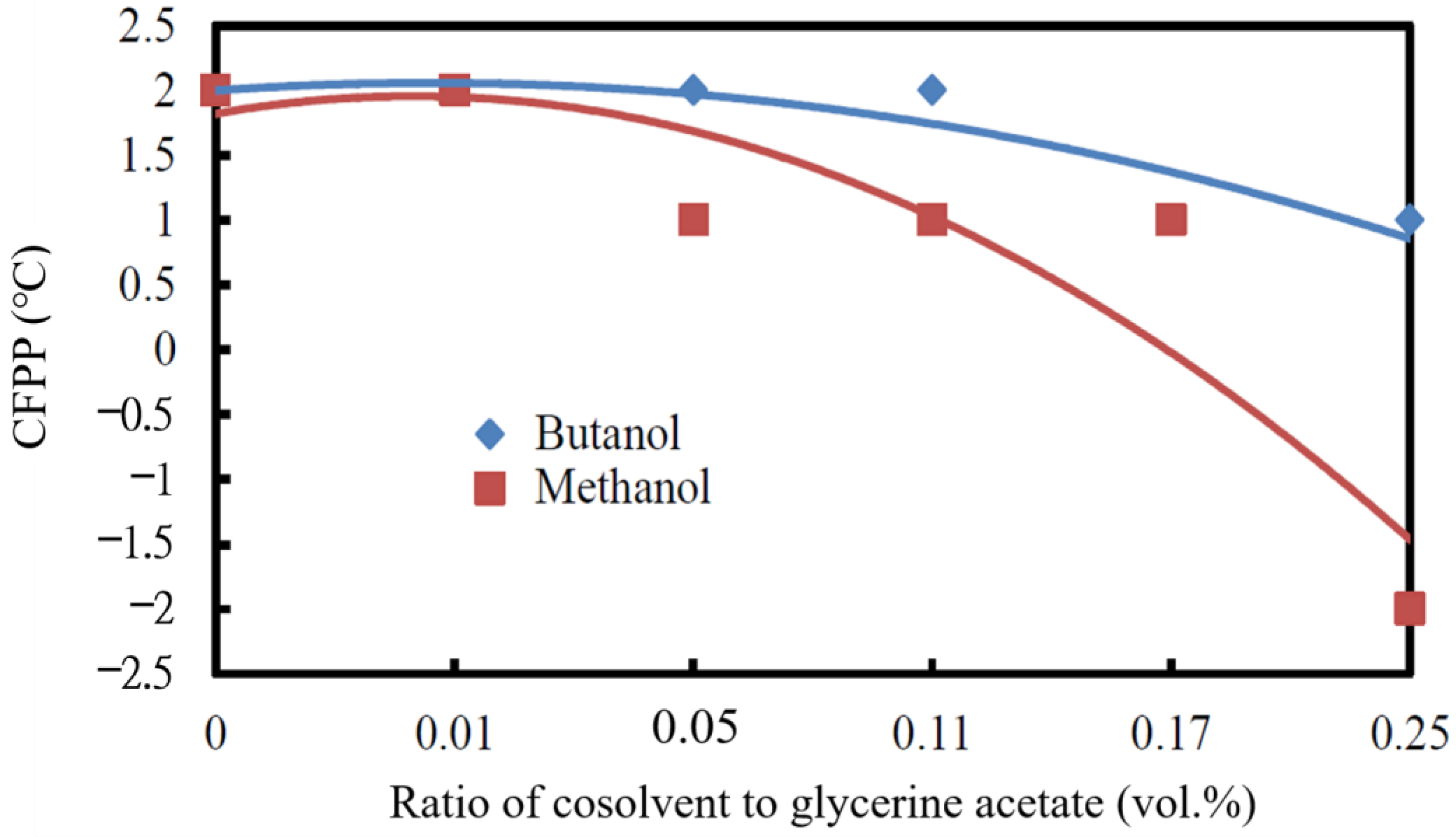
3.2.5. Cold Filter Plugging Point
4. Conclusions
- (1)
- The antifreeze of glycerine acetate, which was produced from the reactant mixture of acetic acid/glycerol at a molar ratio of 8 under UV-light irradiation, appeared to have the lowest freezing point, −46.36 °C. The UV-light irradiation on the TiO2/SO42− photocatalyst surface rendered higher diacylglycerol (DAG) and triacylglycerol (TAG) but lower monoacylglycerol (MAG) production than those without UV-light irradiation.
- (2)
- The lowest cold filter plugging point (CFPP) was observed when the mixture of 0.25 vol.% methanol/antifreeze of glycerine acetate was added to the biodiesel, which decreased from 3 °C of the neat biodiesel to −2 °C of the blended biodiesel. The CFPP of the blended biodiesel only decreased to 1 °C if the cosolvent butanol was used instead.
- (3)
- The kinematic viscosity and CFPP decreased while the cetane index and acid value increased with the increase in the volumetric ratio of cosolvent/glycerine acetate added to the biodiesel.
- (4)
- The increase in the volumetric ratio of cosolvent/glycerine acetate increased the heating value of butanol while decreasing that of methanol for the blended biodiesel.
- (5)
- The cosolvent butanol in the blended biodiesel caused a higher CFPP, kinematic viscosity, and heating value but a lower acid value and cetane index than the cosolvent methanol.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Phung Hai, T.A.; Tessman, M.; Neelakantan, N.; Samoylov, A.A.; Ito, Y.; Rajput, B.S.; Pourahmady, N.; Burkart, M.D. Renewable Polyurethanes from Sustainable Biological Precursors. Biomacromolecules 2021, 22, 1770–1794. [Google Scholar] [CrossRef]
- Koul, R.; Kumar, N.; Singh, R.C. A review on the production and physicochemical properties of renewable diesel and its comparison with biodiesel. Energy Sources A Recovery Util. Environ. Eff. 2021, 43, 2235–2255. [Google Scholar] [CrossRef]
- Babamohammadi, S.; Yusoff, R.; Aroua, M.K.; Borhani, T.N. Mass transfer coefficients of carbon dioxide in aqueous blends of monoethanolamine and glycerol using wetted-wall column. J. Environ. Chem. Eng. 2021, 9, 106618. [Google Scholar] [CrossRef]
- Dhabhai, R.; Koranian, P.; Huang, Q.; Scheibelhoffer, D.S.B.; Dalai, A.K. Purification of glycerol and its conversion to value-added chemicals: A review. Sep. Sci. Technol. 2023, 58, 1383–1402. [Google Scholar] [CrossRef]
- Ioannidou, G.; Loukia Yfanti, V.; Lemonidou, A.A. Optimization of reaction conditions for hydrodeoxygenation of bio-glycerol towards green propylene over molybdenum-based catalyst. Catal. Today 2023, 423, 113902. [Google Scholar] [CrossRef]
- Lima, P.J.M.; da Silva, R.M.; Neto, C.A.C.G.; Gomes e Silva, N.C.; Souza, J.E.d.S.; Nunes, Y.L.; Sousa dos Santos, J.C. An overview on the conversion of glycerol to value-added industrial products via chemical and biochemical routes. Biotechnol. Appl. Biochem. 2022, 69, 2794–2818. [Google Scholar] [CrossRef]
- Karawek, A.; Kittipoom, K.; Tansuthepverawongse, L.; Kitjanukit, N.; Neamsung, W.; Lertthanaphol, N.; Chanthara, P.; Ratchahat, S.; Phadungbut, P.; Kim-Lohsoontorn, P.; et al. The Photocatalytic Conversion of Carbon Dioxide to Fuels Using Titanium Dioxide Nanosheets/Graphene Oxide Heterostructure as Photocatalyst. Nanomaterials 2023, 13, 320. [Google Scholar] [CrossRef] [PubMed]
- Nivetha, N.; Thangamani, A.; Velmathi, S. Sulfated Titania (TiO2-SO42−) as an Efficient Catalyst for Organic Synthesis: Overarching Review from 2000 to 2021. ChemistrySelect 2022, 7, e202104505. [Google Scholar] [CrossRef]
- Jin, R.; Li, G.; Sharma, S.; Li, Y.; Du, X. Toward Active-Site Tailoring in Heterogeneous Catalysis by Atomically Precise Metal Nanoclusters with Crystallographic Structures. Chem. Rev. 2021, 121, 567–648. [Google Scholar] [CrossRef]
- Mujtaba, M.A.; Kalam, M.A.; Masjuki, H.H.; Gul, M.; Soudagar, M.E.M.; Ong, H.C.; Ahmed, W.; Atabani, A.E.; Razzaq, L.; Yusoff, M. Comparative study of nanoparticles and alcoholic fuel additives-biodiesel-diesel blend for performance and emission improvements. Fuel 2020, 279, 118434. [Google Scholar] [CrossRef]
- Al-Rabiah, A.A.; Al Darwish, R.K.; Alqahtani, A.E.; Chaves, D.M.; da Silva, M.J. Production of Biofuel Additives Using Catalytic Bioglycerol Etherification: Kinetic Modelling and Reactive Distillation Design. Catalysts 2022, 12, 1332. [Google Scholar] [CrossRef]
- Matkala, B.; Boggala, S.; Basavaraju, S.; Sarma Akella, V.S.; Aytam, H.P. Influence of sulphonation on Al-MCM-41 catalyst for effective bio-glycerol conversion to Solketal. Microporous Mesoporous Mater. 2024, 363, 112830. [Google Scholar] [CrossRef]
- Hájek, M.; Vávra, A.; Mück, J. Butanol as a co-solvent for transesterification of rapeseed oil by methanol under homogeneous and heterogeneous catalyst. Fuel 2020, 277, 118239. [Google Scholar] [CrossRef]
- Yaman, H.; Yesilyurt, M.K.; Shah, R.M.R.A.; Soyhan, H.S. Effects of compression ratio on thermodynamic and sustainability parameters of a diesel engine fueled with methanol/diesel fuel blends containing 1-pentanol as a co-solvent. Fuel 2024, 357, 129929. [Google Scholar] [CrossRef]
- Perry, R.H.; Green, D.W. Perry’s Chemical Engineer’s Handbook, 9th ed.; McGraw Hill: New York, NY, USA, 2018. [Google Scholar]
- Uni-Onward Corp. TiO2 Property Manual; Uni-Onward Corp: New Taipei City, Taiwan, 2020. [Google Scholar]
- ASTM D975-23; Standard Specification for Diesel Fuel. ASTM International: West Conshohocken, PA, USA, 2023. Available online: https://cdn.standards.iteh.ai/samples/116070/362c5db72e9d452da0eddf0c5a056dc0/ASTM-D975-23.pdf (accessed on 7 April 2023).
- Lin, C.-Y.; Wu, X.-E. Determination of Cetane Number from Fatty Acid Compositions and Structures of Biodiesel. Processes 2022, 10, 1502. [Google Scholar] [CrossRef]
- Aktas, A.B.; Ozen, B.; Alamprese, C. Effects of processing parameters on chemical and physical properties of enzymatically interesterified beef tallow–corn oil blends. J. Food Process. Preserv. 2021, 45, e14587. [Google Scholar] [CrossRef]
- Wang, X.; Ma, D.; Liu, Y.; Wang, Y.; Qiu, C.; Wang, Y. Physical properties of oleogels fabricated by the combination of diacylglycerols and monoacylglycerols. J. Am. Oil Chem. Soc. 2022, 99, 1007–1018. [Google Scholar] [CrossRef]
- Oliveira, L.; Pereira, M.; Pacheli Heitman, A.; Filho, J.; Oliveira, C.; Ziolek, M. Niobium: The Focus on Catalytic Application in the Conversion of Biomass and Biomass Derivatives. Molecules 2023, 28, 1527. [Google Scholar] [CrossRef]
- Tonutti, L.G.; Dalla Costa, B.O.; Decolatti, H.P.; Mendow, G.; Querini, C.A. Determination of kinetic constants for glycerol acetylation by particle swarm optimization algorithm. Chem. Eng. J. 2021, 424, 130408. [Google Scholar] [CrossRef]
- Lin, C.Y.; Chen, Y.C. Effects of heterogeneous sulfated acid photocatalysts and irradiation of ultraviolet light on the chemical conversion and characteristics of antifreeze from bioglycerol. Processes 2024, 12, 383. [Google Scholar] [CrossRef]
- Ahmed, M.T.; Farooqui, S.A.; Hsu, S.H.; Daeun, L.; Chaerun, S.K. Valorizing Glycerol into Valuable Chemicals through Photocatalytic Processes Utilizing Innovative Nano-Photocatalysts. In Solar Light-to-Hydrogenated Organic Conversion: Heterogeneous Photocatalysts; Springer Nature: Singapore, 2024; pp. 149–234. [Google Scholar]
- Khan, Z.; Javed, F.; Shamair, Z.; Hafeez, A.; Fazal, T.; Aslam, A.; Zimmerman, W.B.; Rehman, F. Current developments in esterification reaction: A review on process and parameters. J. Ind. Eng. Chem. 2021, 103, 80–101. [Google Scholar] [CrossRef]
- Arora, I.; Chawla, H.; Chandra, A.; Sagadevan, S.; Garg, S. Advances in the strategies for enhancing the photocatalytic activity of TiO2: Conversion from UV-light active to visible-light active photocatalyst. Inorg. Chem. Commun. 2022, 143, 109700. [Google Scholar] [CrossRef]
- lliev, S. A Comparison of Ethanol, Methanol, and Butanol Blending with Gasoline and Its Effect on Engine Performance and Emissions Using Engine Simulation. Processes 2021, 9, 1322. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Lin, K.-H.; Yang, H. Effects of Surfactant Characteristics on Fuel Properties of Emulsions of Alternative Engine Fuel through the Phase Inversion Method. Processes 2023, 11, 1864. [Google Scholar] [CrossRef]
- Ortiz Lechuga, E.G.; Rodríguez Zúñiga, M.; Arévalo Niño, K. Efficiency Evaluation on the Influence of Washing Methods for Biodiesel Produced from High Free Fatty Acid Waste Vegetable Oils through Selected Quality Parameters. Energies 2020, 13, 6328. [Google Scholar] [CrossRef]
- Remucal, C.K.; Salhi, E.; Walpen, N.; von Gunten, U. Molecular-Level Transformation of Dissolved Organic Matter during Oxidation by Ozone and Hydroxyl Radical. Environ. Sci. Technol. 2020, 54, 10351–10360. [Google Scholar] [CrossRef]
- Zhen, X.; Wang, Y.; Liu, D. Bio-butanol as a new generation of clean alternative fuel for SI (spark ignition) and CI (compression ignition) engines. Renew. Energy 2020, 147, 2494–2521. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Lin, K.-H. Comparison of the Engine Performance of Soybean Oil Biodiesel Emulsions Prepared by Phase Inversion Temperature and Mechanical Homogenization Methods. Processes 2023, 11, 907. [Google Scholar] [CrossRef]
- Ning, L.; Duan, Q.; Chen, Z.; Kou, H.; Liu, B.; Yang, B.; Zeng, K. A comparative study on the combustion and emissions of a non-road common rail diesel engine fueled with primary alcohol fuels (methanol, ethanol, and n-butanol)/diesel dual fuel. Fuel 2020, 266, 117034. [Google Scholar] [CrossRef]
- Laza, T.; Bereczky, Á. Basic fuel properties of rapeseed oil-higher alcohols blends. Fuel 2011, 90, 803–810. [Google Scholar] [CrossRef]
| Physical Properties | Glycerol |
|---|---|
| Chemical formula | C3H8O3 |
| Boiling point (°C) | 290 |
| Melting point (°C) | 17.9 |
| Density (g/cm3) | 1.26 |
| Absolute viscosity (Pa·s) | 1.5 |
| Flash point (°C) | 160 |
| Physical Properties | TiO2 |
|---|---|
| Average surface area (m2/g) | 124.7 |
| Specific primary particle size (nm) | 50.5 |
| pH value in 4% dispersion | 3.5–4.5 |
| TiO2 content (%) | >99.5 |
| Al2O3 content (%) | <0.3 |
| HCL content (%) | <0.3 |
| Physical Properties | Triacylglycerols (TAGs) | Diacylglycerols (DAGs) | Monoacylglycerols (MAGs) |
|---|---|---|---|
| Molecular weight (g/mol) | 218.78 | 176.17 | 134.13 |
| Boiling point (°C) | 260 | 280 | 258 |
| Flash point (°C) | 142 | 140 | 145 |
| Freezing point (°C) | −78 | −30 | 18 |
| Specific gravity (at 25 °C) | 1.158 | 1.187 | 1.21 |
| Physical Properties | Butanol | Methanol | Glycerine Acetate |
|---|---|---|---|
| Specific gravity (at 15 °C) | 0.81 | 0.79 | 1.16 |
| Kinematic viscosity (mm2/s, at 40 °C) | 2.2 | 0.58 | 5.15 |
| Flash point (°C) | 35 | 12 | 140 |
| Heating value (MJ/kg) | 34.47 | 13.93 | 17.02 |
| Physical Properties | Neat Biodiesel | Biodiesel with 5 vol.% Glycerine Acetate |
|---|---|---|
| Specific gravity (at 15 °C) | 0.876 | 0.882 |
| Kinematic viscosity (mm2/s, at 40 °C) | 4.281 | 4.290 |
| Heating value (MJ/kg) | 40.81 | 39.62 |
| Acid value (mg KOH/g) | 0.382 | 0.375 |
| CFPP (°C) | 3 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-Y.; Chen, Y.-C. Influences of Cosolvents and Antifreeze Additives Derived from Glycerol through Esterification on Fuel Properties of Biodiesel. Processes 2024, 12, 419. https://doi.org/10.3390/pr12020419
Lin C-Y, Chen Y-C. Influences of Cosolvents and Antifreeze Additives Derived from Glycerol through Esterification on Fuel Properties of Biodiesel. Processes. 2024; 12(2):419. https://doi.org/10.3390/pr12020419
Chicago/Turabian StyleLin, Cherng-Yuan, and Yun-Chih Chen. 2024. "Influences of Cosolvents and Antifreeze Additives Derived from Glycerol through Esterification on Fuel Properties of Biodiesel" Processes 12, no. 2: 419. https://doi.org/10.3390/pr12020419






