The Treatment of Natural Calcium Materials Using the Supercritical Antisolvent Method for CO2 Capture Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Calcium Materials
2.3. Characterization of the Materials
2.4. Supercritical Antisolvent Precipitation
2.5. Cyclic Carbonation–Calcination Tests in a Thermogravimetric Analyzer
3. Results
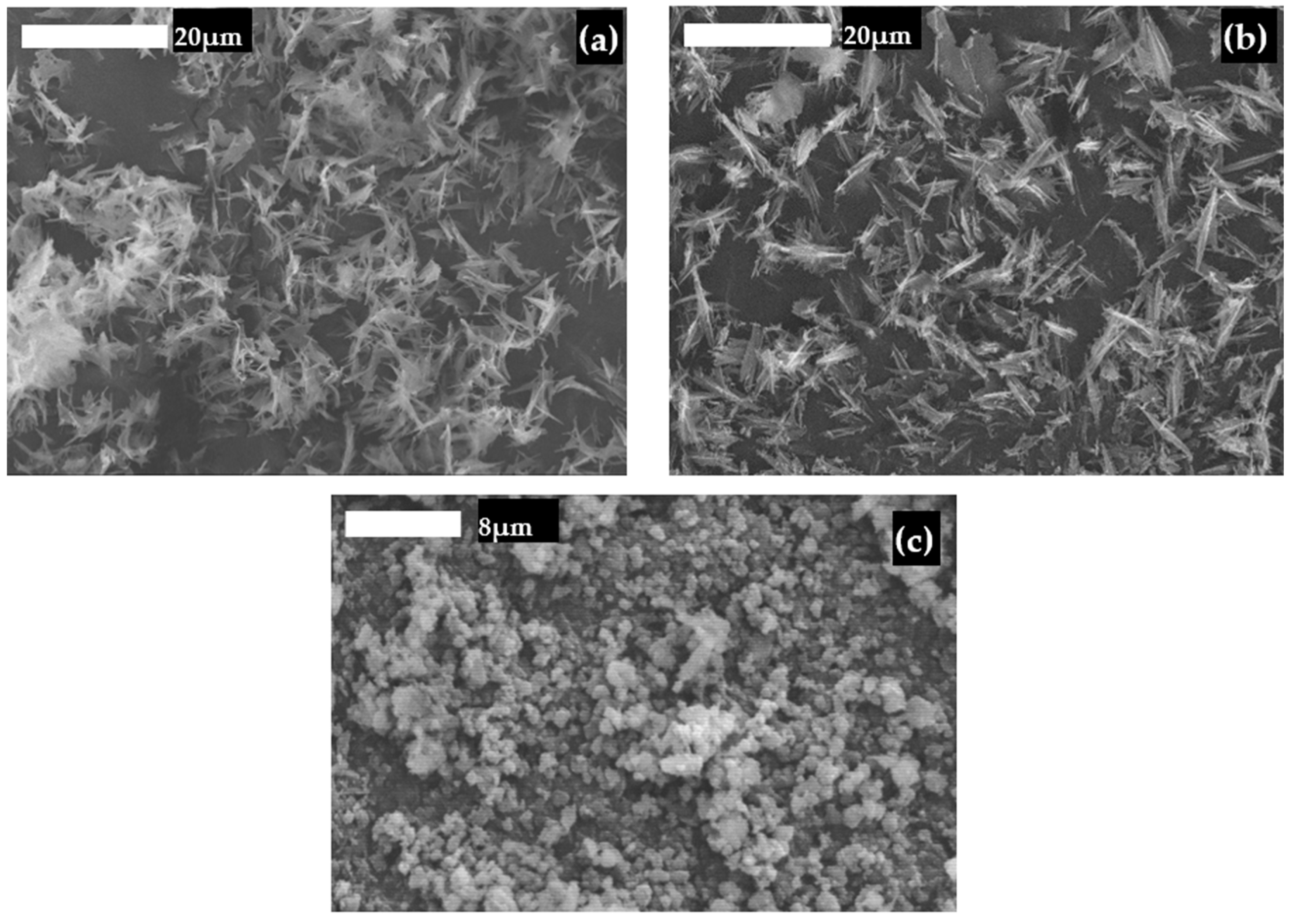
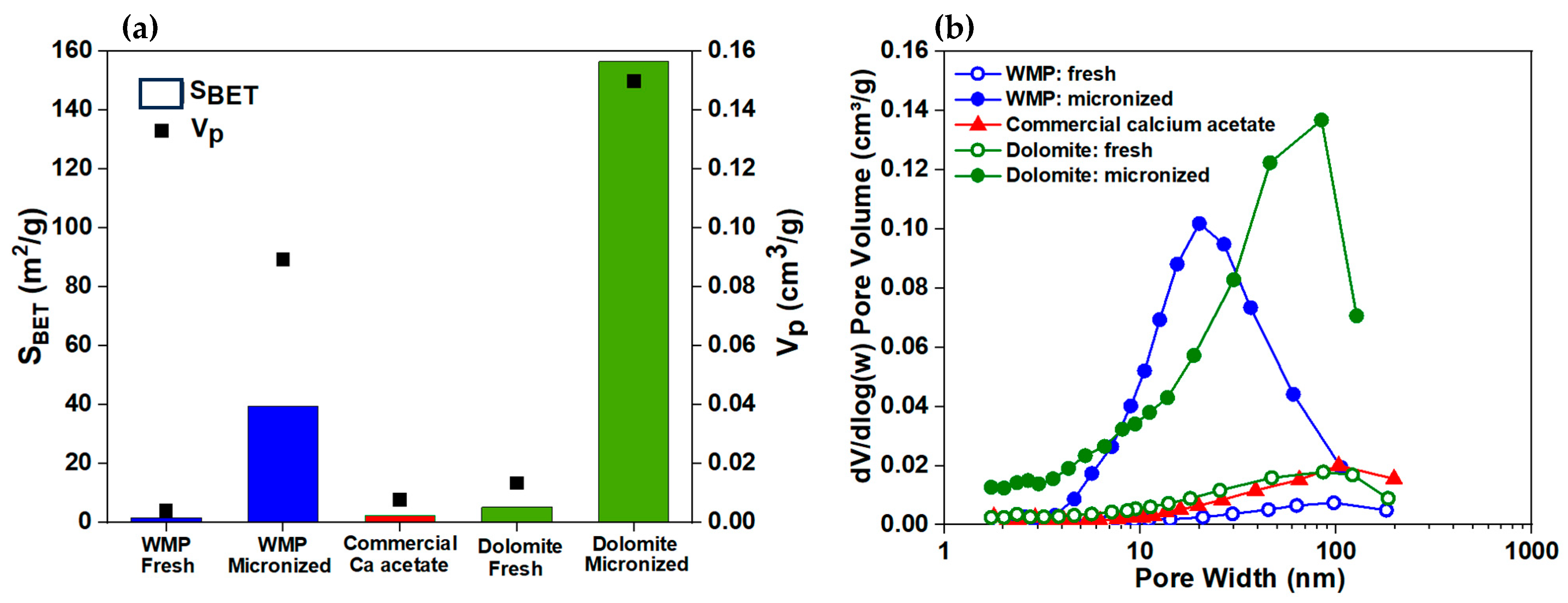
3.1. Properties of Natural Calcium Materials and Precipitated Products
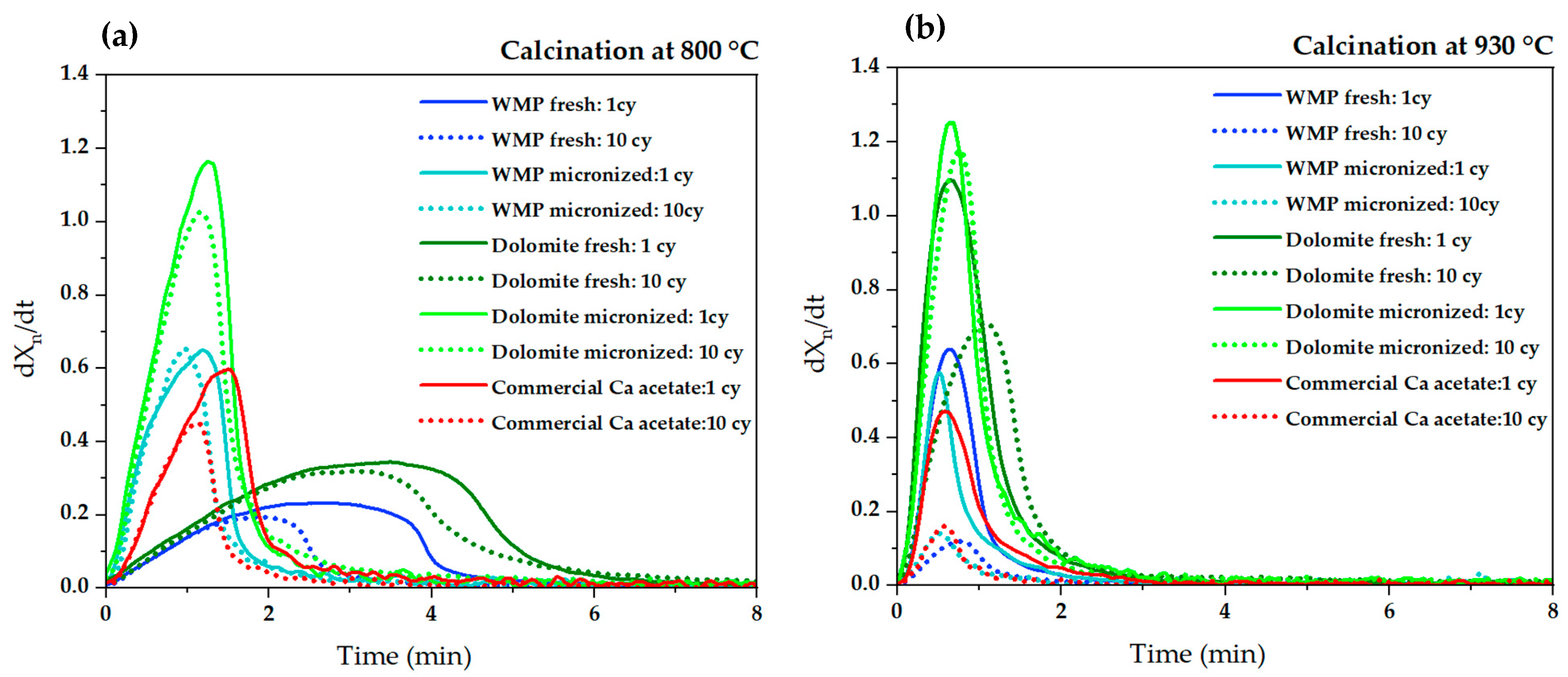
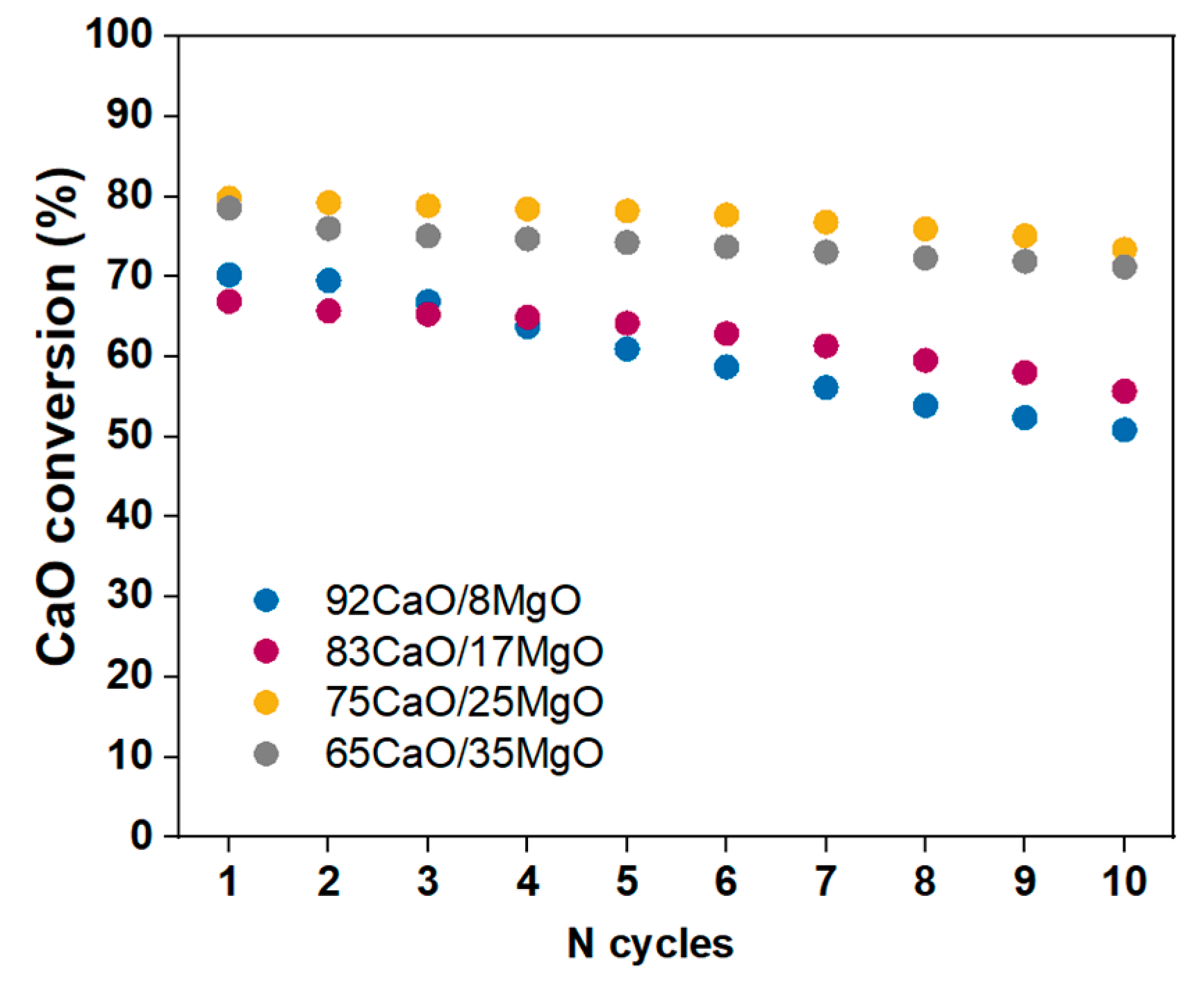
3.2. Calcium Looping Tests
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brundtland Commission. World Commission on Environment and Development; Elsevier: Amsterdam, The Netherlands, 1987. [Google Scholar]
- United Nations. Transforming Our World: The 2030 Agenda for Sustainable Development. Available online: https://sdgs.un.org/2030agenda (accessed on 22 November 2021).
- Kumar, A.; Thakur, A.K.; Gaurav, G.K.; Klemeš, J.J.; Sandhwar, V.K.; Pant, K.K.; Kumar, R. A Critical Review on Sustainable Hazardous Waste Management Strategies: A Step towards a Circular Economy. Environ. Sci. Pollut. Res. 2023, 30, 105030–105055. [Google Scholar] [CrossRef]
- Soliman, N.K.; Moustafa, A.F. Industrial Solid Waste for Heavy Metals Adsorption Features and Challenges; a Review. J. Mater. Res. Technol. 2020, 9, 10235–10253. [Google Scholar] [CrossRef]
- Melo, J.F.; Junior, J.H.S.; Freire, T.B.d.M.; Rigoti, E.; Pergher, S.B.C.; Martínez-Huitle, C.A.; Castro, P.S. Industrial Waste Reuse: An Alternative Source to Reduced Graphene Oxide for Preparing Electrochemical Sensors. Electrochim. Acta 2023, 454, 142382. [Google Scholar] [CrossRef]
- Ghisman, V.; Muresan, A.C.; Buruiana, D.L.; Axente, E.R. Waste Slag Benefits for Correction of Soil Acidity. Sci. Rep. 2022, 12, 16042. [Google Scholar] [CrossRef] [PubMed]
- Buruiana, D.L.; Obreja, C.D.; Herbei, E.E.; Ghisman, V. Re-Use of Silico-Manganese Slag. Sustainability 2021, 13, 11771. [Google Scholar] [CrossRef]
- Le, V.G.; Vo, D.V.N.; Tran, H.T.; Duy Dat, N.; Luu, S.D.N.; Rahman, M.M.; Huang, Y.H.; Vu, C.T. Recovery of Magnesium from Industrial Effluent and Its Implication on Carbon Capture and Storage. ACS Sustain. Chem. Eng. 2021, 9, 6732–6740. [Google Scholar] [CrossRef]
- Gaustad, G.; Williams, E.; Leader, A. Rare Earth Metals from Secondary Sources: Review of Potential Supply from Waste and Byproducts. Resour. Conserv. Recycl. 2021, 167, 105213. [Google Scholar] [CrossRef]
- Kouzu, M.; Hidaka, J. Transesterification of Vegetable Oil into Biodiesel Catalyzed by CaO: A Review. Fuel 2012, 93, 1–12. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, G.; Huang, Y.; Zhang, H.; Yang, B.; Mao, Z. Calcium Acetate or Calcium Carbonate for Hyperphosphatemia of Hemodialysis Patients: A Meta-Analysis. PLoS ONE 2015, 10, 0121376. [Google Scholar] [CrossRef] [PubMed]
- Erans, M.; Manovic, V.; Anthony, E.J. Calcium Looping Sorbents for CO2 Capture. Appl. Energy 2016, 180, 722–742. [Google Scholar] [CrossRef]
- Blamey, J.; Anthony, E.J.; Wang, J.; Fennell, P.S. The Calcium Looping Cycle for Large-Scale CO2 Capture. Prog. Energy Combust. Sci. 2010, 36, 260. [Google Scholar] [CrossRef]
- Romano, M.C.; Spinelli, M.; Campanari, S.; Consonni, S.; Cinti, G.; Marchi, M.; Borgarello, E. The Calcium Looping Process for Low CO2 Emission Cement and Power. Energy Procedia 2013, 37, 7091. [Google Scholar] [CrossRef]
- Rodriguez, N.; Murillo, R.; Abanades, C. CO2 Capture from Cement Plants Using Oxy-fired Pre-calcination and/or Calcium Looping. Environ. Sci. Technol. 2012, 46, 2460. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Duan, L.; Sun, Z. Review on the Development of Sorbents for Calcium Looping. Energy Fuels 2020, 34, 7806–7836. [Google Scholar] [CrossRef]
- Tesio, U.; Guelpa, E.; Verda, V. Comparison of SCO2 and He Brayton Cycles Integration in a Calcium-Looping for Concentrated Solar Power. Energy 2022, 247, 123467. [Google Scholar] [CrossRef]
- Teixeira, P.; Afonso, E.; Pinheiro, C.I.C. Tailoring Waste-Derived Materials for Calcium-Looping Application in Thermochemical Energy Storage Systems. J. CO2 Util. 2022, 65, 102180. [Google Scholar] [CrossRef]
- Sun, X.; Fang, D.; Zhang, L.; Duan, F.; Sun, Y. Performance Study of Modified Calcium Magnesium Acetate (MCMA) in the Process of High Temperature CO2 Capture and the Application of Spent MCMA for Sequential SO2 Removal. Asia-Pac. J. Chem. Eng. 2017, 12, 595–604. [Google Scholar] [CrossRef]
- Teixeira, P.; Fernandes, A.; Ribeiro, F.; Pinheiro, C.I.C. Blending Wastes of Marble Powder and Dolomite Sorbents for Calcium-Looping CO2 Capture under Realistic Industrial Calcination Conditions. Materials 2021, 14, 164379. [Google Scholar] [CrossRef] [PubMed]
- Fahim, T.K.; Zaidul, I.S.M.; Abu Bakar, M.R.; Salim, U.M.; Awang, M.B.; Sahena, F.; Jalal, K.C.A.; Sharif, K.M.; Sohrab, M.H. Particle Formation and Micronization Using Non-Conventional Techniques—Review. Chem. Eng. Process. Process Intensif. 2014, 86, 47–52. [Google Scholar] [CrossRef]
- Franco, P.; Sacco, O.; de Marco, I.; Sannino, D.; Vaiano, V. Photocatalytic Degradation of Eriochrome Black-T Azo Dye Using Eu-Doped ZnO Prepared by Supercritical Antisolvent Precipitation Route: A Preliminary Investigation. Top. Catal. 2020, 63, 1193–1205. [Google Scholar] [CrossRef]
- Smith, P.J.; Kondrat, S.A.; Carter, J.H.; Chater, P.A.; Bartley, J.K.; Taylor, S.H.; Spencer, M.S.; Hutchings, G.J. Supercritical Antisolvent Precipitation of Amorphous Copper–Zinc Georgeite and Acetate Precursors for the Preparation of Ambient-Pressure Water-Gas-Shift Copper/Zinc Oxide Catalysts. ChemCatChem 2017, 9, 1621–1631. [Google Scholar] [CrossRef]
- Miedziak, P.J.; Tang, Z.; Davies, T.E.; Enache, D.I.; Bartley, J.K.; Carley, A.F.; Herzing, A.A.; Kiely, C.J.; Taylor, S.H.; Hutchings, G.J. Ceria Prepared Using Supercritical Antisolvent Precipitation: A Green Support for Gold-Palladium Nanoparticles for the Selective Catalytic Oxidation of Alcohols. J. Mater. Chem. 2009, 19, 8619–8627. [Google Scholar] [CrossRef]
- Reverchon, E.; della Porta, G.; di Trolio, A.; Pace, S. Supercritical Antisolvent Precipitation of Nanoparticles of Superconductor Precursors. Ind. Amp Eng. Chem. Res. 1998, 37, 952–958. [Google Scholar] [CrossRef]
- Reverchon, E.; de Marco, I.; della Porta, G. Tailoring of Nano- and Micro-Particles of Some Superconductor Precursors by Supercritical Antisolvent Precipitation. J. Supercrit. Fluids 2002, 23, 81–87. [Google Scholar] [CrossRef]
- Nobre, L.C.S.; Santos, S.; Palavra, A.M.F.; Calvete, M.J.F.; de Castro, C.A.N.; Nobre, B.P. Supercritical Antisolvent Precipitation of Calcium Acetate from Eggshells. J. Supercrit. Fluids 2020, 163, 104862. [Google Scholar] [CrossRef]
- Cardoso, M.A.T.; Monteiro, G.A.; Cardoso, J.P.; Prazeres, T.J.V.; Figueiredo, J.M.F.; Martinho, J.M.G.; Cabral, J.M.S.; Palavra, A.M.F. Supercritical Antisolvent Micronization of Minocycline Hydrochloride. J. Supercrit. Fluids 2008, 44, 238–244. [Google Scholar] [CrossRef]
- Teixeira, P.; Bacariza, C.; Mohamed, I.; Pinheiro, C.I.C. Improved Performance of Modified CaO-Al2O3based Pellets for CO2 capture under Realistic Ca-Looping Conditions. J. CO2 Util. 2022, 61, 102007. [Google Scholar] [CrossRef]
- Stanmore, B.R.; Gilot, P. Review-Calcination and Carbonation of Limestone during Thermal Cycling for CO2 Sequestration. Fuel Process. Technol. 2005, 86, 1707–1743. [Google Scholar] [CrossRef]
- Silcox, G.D.; Kramlich, J.C.; Pershing, D.W. A Mathematical Model for the Flash Calcination of Dispersed CaC03 and Ca(OH)2, Particles. Ind. Eng. Chem. Res. 1989, 28, 155–160. [Google Scholar] [CrossRef]
- Ruhaimi, A.; Aziz, M.; Jalil, A. Magnesium oxide-based adsorbents for carbon dioxide capture: Current progress and future opportunities. J. CO2 Util. 2021, 43, 101357. [Google Scholar] [CrossRef]
- Valverde, J.M.; Sanchez-Jimenez, P.E.; Perez-Maqueda, L.A. Ca-Looping for Post-combustion CO2 Capture: A Comparative Analysis on the Performances of Dolomite and Limestone. Appl. Energy 2015, 138, 202–215. [Google Scholar] [CrossRef]





| Oxide Content wt. (%) | SiO2 | CaO | MgO | Al2O3 | Fe2O3 | K2O | CO2 |
|---|---|---|---|---|---|---|---|
| WMP | 1.50 | 52.2 | 0.45 | 0.23 | 0.11 | 0.12 | 44.0 |
| Dolomite | 0.09 | 34.8 | 17.1 | 0.02 | 0.01 | n.d. | 46.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nobre, L.C.S.; Teixeira, P.; Pinheiro, C.I.C.; Palavra, A.M.F.; Calvete, M.J.F.; Nieto de Castro, C.A.; Nobre, B.P. The Treatment of Natural Calcium Materials Using the Supercritical Antisolvent Method for CO2 Capture Applications. Processes 2024, 12, 425. https://doi.org/10.3390/pr12030425
Nobre LCS, Teixeira P, Pinheiro CIC, Palavra AMF, Calvete MJF, Nieto de Castro CA, Nobre BP. The Treatment of Natural Calcium Materials Using the Supercritical Antisolvent Method for CO2 Capture Applications. Processes. 2024; 12(3):425. https://doi.org/10.3390/pr12030425
Chicago/Turabian StyleNobre, Luís C. S., Paula Teixeira, Carla I. C. Pinheiro, António M. F. Palavra, Mário J. F. Calvete, Carlos A. Nieto de Castro, and Beatriz P. Nobre. 2024. "The Treatment of Natural Calcium Materials Using the Supercritical Antisolvent Method for CO2 Capture Applications" Processes 12, no. 3: 425. https://doi.org/10.3390/pr12030425









