Recovery of Strategic Metals from Waste Printed Circuit Boards with Deep Eutectic Solvents and Ionic Liquids
Abstract
:1. Introduction
2. Experimental Design
2.1. Preparation of the Solid Material
2.2. Chemicals
2.3. Recovery Procedure
2.4. Synthesis of ILs
3. Results and Discussion
3.1. Solid WPCBs’ Content
3.2. Extraction with DESs and ILs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhu, X.-N.; Nie, C.C.; Zhang, H.; Lyu, X.-J.; Tao, Y.-J.; Quin, J.; Li, L.; Zhang, G.-W. Recovery of high-grade copper from waste printed circuit boards by mechanical grinding assisted flotation. J. Clean. Prod. 2019, 232, 1251–1256. [Google Scholar] [CrossRef]
- Zhu, X.-N.; Nie, C.-C.; Wang, S.-S.; Xie, Y.; Zhang, H.; Lyu, X.-J.; Qiu, J.; Li, L. Cleaner approach to the recycling of metals in waste printed circuit boards by magnetic and gravity separation. J. Clean. Prod. 2020, 248, 119235. [Google Scholar] [CrossRef]
- Park, Y.J.; Fray, D.J. Recovery of high purity precious metals from printed circuit boards. J. Hazard. Mater. 2009, 164, 1152–1158. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yang, M.; Shi, M.Q.; Kuang, X.; Qi, H.J.; Wang, T. Recycling waste circuit board efficiently and environmentally friendly through small-molecule assisted dissolution. Sci. Rep. 2019, 9, 17902. [Google Scholar] [CrossRef] [PubMed]
- Debnath, B.; Chowdhury, R.; Ghosh, S.K. Sustainability of metal recovery from E-waste. Front. Environ. Sci. Eng. 2018, 12, 2. [Google Scholar] [CrossRef]
- Gorewoda, T.; Eschen, M.; Charasińska, J.; Knapik, M.; Kozłowicz, S.; Anyszkiewicz, J.; Jadwiński, M.; Potempa, M.; Gawliczek, M.; Chmielarz, A.; et al. Determination of metals’ content in components mounted on printed circuit boards from end-of-life mobile phones. Recycling 2020, 5, 20. [Google Scholar] [CrossRef]
- Maurice, A.A.; Ngoc Dinh, K.; Charpentier, N.M.; Brambilla, A.; Gabriel, J.-C.P. Dismantling of printed circuit boards enabling electronic components sorting and their subsequent treatment open improved elemental sustainability opportunities. Sustainability 2021, 13, 10357. [Google Scholar] [CrossRef]
- Panda, R.; Mishra, S.; Pant, K.K.; Bhaskar, T.; Naik, S.N. A closed loop recycling strategy for sustainable recovery of group 11 metals (Cu, Au, and Ag) from waste PCBs: An amalgamation of low-temperature NH4Cl roasting, HCl leaching and cementation. Sustain. Mater. Technol. 2023, 37, e00652. [Google Scholar] [CrossRef]
- Pavόn, S.; Lorenz, T.; Fortuny, A.; Sastre, A.M.; Bertau, M. Rare earth elements recovery from secondary wastes by solid-state chlorination and selective organic leaching. Waste Manag. 2021, 122, 55–63. [Google Scholar] [CrossRef]
- Bilesan, M.R.; Makarova, I.; Wickman, B.; Repo, E. Efficient separation of precious metals from computer waste printed circuit boards by hydrocyclone and dilution gravity methods. J. Clean. Prod. 2021, 286, 125505. [Google Scholar] [CrossRef]
- Beiki, V.; Naseri, T.; Mousavi, S.M. Comprehensive characterization and environmental implications of spent telecommunication printed circuit boards: Towards a cleaner and sustainable environment. J. Environ. Manag. 2023, 325, 116482. [Google Scholar] [CrossRef] [PubMed]
- Kamberovic, Z.; Korac, M.; Ranitovic, M. Hydrometallurgical Process for extraction of metals from electronic waste—Part II: Development of the processes for the recovery of copper from Printed Circuit Boards (PCB). Metalurgija 2011, 17, 139–149. Available online: https://www.researchgate.net/publication/267985628 (accessed on 5 August 2023).
- Wang, H.; Zhang, S.; Li, B.; Pan, D.; Wu, Y.; Zuo, T. Recovery of waste printed circuit boards through pyrometallurgical processing: A review. Resour. Conserv. Recycl. 2017, 126, 209–218. [Google Scholar] [CrossRef]
- Li, H.; Eksteen, J.; Oraby, E. Hydrometallurgical recovery of metals from waste printed circuit boards (WPCBs): Current status and perspectives—A review. Resour. Conserv. Recycl. 2018, 139, 122–139. [Google Scholar] [CrossRef]
- Akcil, A.; Erust, C.; Gahan, C.S.; Ozgun, M.; Sahin, M.; Tuncuk, A. Precious metal recovery from waste printed circuit boards using cyanide and non-cyanide lixiviants—A review. Waste Manag. 2015, 45, 258–271. [Google Scholar] [CrossRef]
- Lu, Y.; Xu, Z. Precious metals recovery from waste printed circuit boards: A review for current status and perspective. Resour. Conserv. Recycl. 2016, 113, 28–39. [Google Scholar] [CrossRef]
- Rocchetti, L.; Amato, A.; Beolchini, F. Printed circuit board recycling: A patent review. J. Clean. Prod. 2018, 178, 814–832. [Google Scholar] [CrossRef]
- Baniasadi, M.; Vakilchap, F.; Bahaloo-Horeh, N.; Mousavi, S.M.; Farnaud, S. Advances in bioleaching as a sustainable method for metal recovery from e-waste: A review. J. Ind. Eng. Chem. 2019, 76, 75–90. [Google Scholar] [CrossRef]
- Rai, V.; Liu, D.; Xia, D.; Jayaraman, Y.; Gabriel, J.-C.P. Electrochemical approaches for the recovery of metals from electronic waste: A critical review. Recycling 2021, 6, 53. [Google Scholar] [CrossRef]
- Korf, N.; Løvik, A.N.; Figi, R.; Schreiner, C.; Kuntz, C.; Mählitz, P.M.; Rösslein, M.; Wäger, P.; Rotte, V.S. Multi-element chemical analysis of printed circuit boards—Challenges and pitfalls. Waste Manag. 2019, 92, 124–136. [Google Scholar] [CrossRef]
- Qiu, R.; Lin, M.; Ruan, J.; Fu, Y.; Hu, J.; Deng, M.; Tang, Y.; Qiu, R. Recovering full metallic resources from waste printed circuit boards: A refined review. J. Clean. Prod. 2020, 244, 118690. [Google Scholar] [CrossRef]
- Krishnan, S.; Zulkapli, N.S.; Kamyab, H.; Mat Taib, S. Current technologies for recovery of metals from industrial wastes: An overview. Env. Technol. Innov. 2021, 22, 101525. [Google Scholar] [CrossRef]
- Mishra, G.; Jha, R.; Rao, M.D.; Meshram, A.; Singh, K.K. Recovery of silver from waste printed circuit boards (WPCBs) through hydrometallurgical route: A review. Environ. Chall. 2021, 4, 100073. [Google Scholar] [CrossRef]
- Mir, S.; Dhawan, N. A comprehensive review on the recycling of discarded printed circuit boards for resource recovery. Resour. Conserv. Recyc. 2022, 178, 106027. [Google Scholar] [CrossRef]
- Wu, C.; Awasthi, A.K.; Qin, W.; Liu, W.; Yang, C. Recycling value materials from waste PCBs focus on electronic components: Technologies, obstruction and prospects. J. Environ. Chem. Eng. 2022, 10, 108516. [Google Scholar] [CrossRef]
- Zhao, W.; Xu, J.; Fei, W.; Liu, Z.; He, W.; Li, G. The reuse of electronic components from waste printed circuit boards: A critical review. Environ. Sci. Adv. 2023, 2, 196–214. [Google Scholar] [CrossRef]
- Do, M.H.; Nguyen, G.T.; Thach, U.D.; Lee, Y.; Bui, T.H. Advances in hydrometallurgical approaches for gold recovery from E-waste: A comprehensive review and perspectives. Miner. Eng. 2023, 191, 107977. [Google Scholar] [CrossRef]
- Niu, B.; Shanshan, E.; Xu, Z.; Guo, J. How to efficient and high-value recycling of electronic components mounted on waste printed circuit boards: Recent progress, challenge, and future perspectives. J. Clean. Prod. 2023, 415, 137815. [Google Scholar] [CrossRef]
- Hau Nguyen, T.N.; Lee, M.S. Recovery of Au and Pd from the letching solution of printed circuit boards by cementation, solvent extraction, reduction, and precipitation. J. Ind. Eng. Chem. 2023, 126, 214–223. [Google Scholar] [CrossRef]
- Ly, N.H.; Joo, S.-W.; Choo, J.; Vasseghian, Y.R.; Cho, J.; Rezania, S. Sustainable cutting-edge techniques for gold valorization from electronic wastes. Chem. Eng. J. 2023, 471, 44324. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, Q.; Li, G.; Zhang, X.; Pan, D. Bromine recovery from crude bromine salts by-products of waste printed circuit boards smelting. Sep. Purif. Technol. 2023, 320, 124190. [Google Scholar] [CrossRef]
- Klose, S.; Pauliuk, S. Sector-level estimates for global future copper demand and the potential for resource efficiency. Resour. Conserv. Recycl. 2023, 193, 106941. [Google Scholar] [CrossRef]
- Li, Y.; An, H.; Gao, X.; Liu, S.; Sun, Q.; Zhao, Y. The influence of copper trade relation structure on copper price: From the perspective of industrial chain. Resour. Conserv. Recycl. 2023, 192, 106933. [Google Scholar] [CrossRef]
- Barnwal, A.; Dhawan, N. Recycling of discarded mobile printed circuit boards for extraction of gold and copper. Sustain. Mat. Technol. 2020, 25, e00164. [Google Scholar] [CrossRef]
- Hao, J.; Wang, X.; Wang, Y.; Guo, F.; Wu, Y. Study of gold leaching from pre-treated waste printed circuit boards by thiosulfate-cobalt-glycine system and separation by solvent extraction. Hydrometallurgy 2023, 221, 106141. [Google Scholar] [CrossRef]
- Dong, Z.; Jiang, T.; Xu, B.; Zhang, B.; Liu, G.; Li, Q.; Yang, Y. A systematic and comparative study of copper, nickel and cobalt-ammonia catalysed thiosulfate processes for eco-friendly and efficient gold extraction from an oxide gold concentrate. Sep. Purif. Technol. 2021, 272, 118929. [Google Scholar] [CrossRef]
- Liu, Z.; Guo, X.; Tian, Q.; Zhang, L. A systematic review of gold extraction: Fundamentals, advancements, and challenges toward alternative lixiviants. J. Hazardous Mater. 2022, 440, 129778. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Liu, C.; Yang, Y.; Zhou, J.; Guo, Y.; Xie, B. Pretreatment of refractory gold concentrate calcine using ammonium bifluoride and sulfuric acid solution. Miner. Eng. 2022, 187, 107778. [Google Scholar] [CrossRef]
- Cotty, S.R.; Kim, N.; Su, X. Electrochemically Mediated Recovery and Purification of Gold for Sustainable Mining and Electronic Waste Recycling. ACS Sustain. Chem. Eng. 2023, 11, 3975–3986. [Google Scholar] [CrossRef]
- Jha, R.; Sharma, R.; Agrawal, M.; Rao, M.D.; Singh, K.K. Exploring the pretreatment routes of waste printed circuit boards for enhanced metal recovery. Mater. Today Proc. 2023, in press. [CrossRef]
- Barrueto, Y.; Hernández, P.; Jiménez, Y.; Morales, J. Leaching of metals from printed circuit boards using ionic liquids. J. Mat. Cyc. Waste Manag. 2021, 23, 2028–2036. [Google Scholar] [CrossRef]
- Kilicarslan, A.; Nezihi Saridede, M.; Stopic, S.; Friedrich, B. Use of ionic liquid in leaching process of brass wastes for copper and zinc recovery. Int. J. Miner. Metall. Mater. 2014, 21, 138–143. [Google Scholar] [CrossRef]
- Wstawski, S.; Emmons-Burzyńska, M.; Rzelewska-Piekut, M.; Skrzypczak, A.; Regel-Rosocka, M. Studies on copper (II) leaching from e-waste with hydrogen sulfate ionic liquids: Effect of hydrogen peroxide. Hydrometallurgy 2021, 205, 105730. [Google Scholar] [CrossRef]
- Zhang, D.-J.; Dong, L.; Li, Y.-T.; Wu, Y.; Ma, Y.-X.; Yang, B. Copper leaching from waste printed circuit boards using typical acidic ionic liquids recovery of e-wastes’ surplus value. Waste Manag. 2018, 78, 191–197. [Google Scholar] [CrossRef]
- Masilela, M.; Ndlovu, S. Extraction of Ag and Au from chloride electronic waste leach solutions using ionic liquids. J. Environ. Chem. Eng. 2019, 7, 102810. [Google Scholar] [CrossRef]
- Łukomska, A.; Wiśniewska, A.; Dąbrowski, Z.; Lach, J.; Wróbel, K.; Kolasa, D.; Domańska, U. Recovery of metals from electronic waste—Printed circuit boards by ionic liquids, DESs and organophosphorous-based acid extraction. Molecules 2022, 27, 4984. [Google Scholar] [CrossRef]
- Schaeffer, N.; Passos, H.; Billard, I.; Papaiconomou, N.; Coutinho, J.A.P. Recovery of metals from waste electrical and electronic equipment (WEEE) using unconventional solvents based on ionic liquids. Environ. Sci. Technol. 2018, 48, 859–922. [Google Scholar] [CrossRef]
- Aliakbari, R.; Marfavi, Y.; Kowsari, E.; Ramakrishna, S. Recent studies on ionic liquids in metal recovery from e-waste and secondary sources by liquid-liquid extraction and electrodeposition: A Review. Mater. Circ. Econ. 2020, 2, 10–18. [Google Scholar] [CrossRef]
- Niknam, K.; Damya, M. 1-Butyl-3-methylimidazolium hydrogen sulfate [Bmim]HSO4: An efficient reusable acidic ionic liquid for the synthesis of 1,8-dioxo-octahydroxanthenes. J. Chin. Chem. Soc. 2009, 56, 659–665. [Google Scholar] [CrossRef]
- Huang, J.; Chen, M.; Chen, H.; Chen, S.; Sun, Q. Leaching behavior of copper from waste printed circuit boards with Brønsted acidic ionic liquid. Waste Manag. 2014, 34, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Barruetoc, Y.; Hernández, P.; Jiménezeb, Y.P.; Moralesc, J. Properties and application of ionic liquids in leaching base/precious metals from e-waste. A review. Hydrometallurgy 2022, 212, 105895. [Google Scholar] [CrossRef]
- Łukomska, A.; Wiśniewska, A.; Dąbrowski, Z.; Domańska, U. Liquid-liquid extraction of cobalt(II) and zinc(II) from aqueous solutions using novel ionic liquids as extractants. J. Mol. Liq. 2020, 307, 112955. [Google Scholar] [CrossRef]
- Łukomska, A.; Wiśniewska, A.; Dąbrowski, Z.; Kolasa, D.; Luchcińska, S.; Domańska, U. Separation of cobalt, lithium and nickel from the “black mass” of waste Li-ion batteries by ionic liquids, DESs and organophosphorous-based acids extraction. J. Mol. Liq. 2021, 343, 117694. [Google Scholar] [CrossRef]
- Hartley, J.M.; Scott, S.; Rivera, R.M.; Hunt, P.; Lucio, A.J.; Bird, P.; Harris, R.; Jenkin, G.R.T. Tailoring lixiviant properties to optimise selectivity in E-waste recycling. RSC Sustain. 2023, 1, 107–116. [Google Scholar] [CrossRef]
- Feng, F.; Sun, Y.; Rui, J.; Yu, L.; Liu, J.; Zhang, N.; Zhao, M.; Wei, L.; Lu, C.; Zhao, J.; et al. Study of the “Oxidation-Complexation” coordination composite ionic liquid system for dissolving precious metal. Appl. Sci. 2020, 10, 3625. [Google Scholar] [CrossRef]
- Li, H.; Oraby, E.; Eksteen, J. Extraction of precious metals from waste printed circuit boards using cyanide-free alkaline glycine solution in the presence of an oxidant. Miner. Eng. 2022, 181, 107501. [Google Scholar] [CrossRef]
- Han, Y.; Yi, X.; Wang, R.; Huang, J.; Chen, M.; SUN, Z.; Sun, S.; Shu, J. Copper extraction from waste printed circuit boards by glicyne. Sep. Purif. Technol. 2020, 253, 117463. [Google Scholar] [CrossRef]
- Martinez-Ballesteros, G.; Valenzuela-Garci, J.L.; Gomez-Alvarez, A.; Encinas-Romero, M.A.; Mejia-Zamudio, F.A.; de Jesus Rosas-Durazo, A. Base Metals Extraction from Printed Circuit Boards by Pressure Acid Leaching. Minerals 2023, 13, 98. [Google Scholar] [CrossRef]
- Correa, M.M.J.; Silvas, F.P.C.; Aliprandini, P.; Tavares de Moraes, V.; Dreisinger, D.; Espinosa, D.C.R. Separation of copper from a leaching solution of printed circuit boards by using solvent extraction with D2EHPA. Braz. J. Chem. Eng. 2018, 35, 919–930. [Google Scholar] [CrossRef]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep eutectic solvents formed between choline chloride and carboxylic acids: Versatile alternatives to ionic liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef]
- Łukomska, A.; Wiśniewska, A.; Dąbrowski, Z.; Kolasa, D.; Luchcińska, S.; Lach, J.; Wróbel, K.; Domańska, U. Recovery of zinc and manganese from “black mass” of waste Zn-MnO2 alkaline batteries by solvent extraction technique with ionic liquids, DESs and organophosphorous-based acids. J. Mol. Liq. 2021, 338, 116590. [Google Scholar] [CrossRef]
- Domańska-Żelazna, U.; Wiśniewska, A.; Dąbrowski, Z. New ammonium ionic liquids, methods of synthesis and their application. Pol. Pat. Appl. 2023, 198, 444. [Google Scholar]
- Boudesocque, S.; Mohamadou, A.; Dupont, L.; Martinez, A.; Dechamps, I. Use of dicyanamide ionic liquids for extraction of metal ions. RSC Adv. 2016, 6, 107894. [Google Scholar] [CrossRef]
- Domańska-Żelazna, U.; Wiśniewska, A.; Dąbrowski, Z. The method of extraction of silver, copper, aluminium, iron and zinc from electronic waste with ionic liquids. Pol. Pat. Appl. 2023, 199, 444. [Google Scholar]


| Content of Metals in the Solid Phase | Mass of Sample | ||||
|---|---|---|---|---|---|
| Cu(II) | Ag(I) | Al(III) | Fe(II) | Zn(II) | kg |
| g/kg | mg/kg | g/kg | |||
| Primary material | |||||
| 224 | 483 | 81.9 | 79.4 | 18.1 | 1.000 |
| Primary material after thermal pre-treatment | |||||
| 335 | 721 | 122 | 119 | 27.0 | 0.670 |
| Chemical Structure | Name, Abbreviation of Name, Supplier, CAS Number | Molar Mass M/ (g mol−1) | Purity * in Mass Percent (%) |
|---|---|---|---|
 | Choline chloride ([N2OH,1,1,1][Cl]), Sigma-Aldrich, Darmstadt, Germany, CAS: 67-48-1 | 139.62 | >98 |
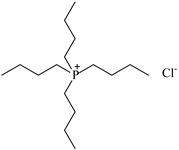 | Tetrabutylphosphonium chloride ([P4,4,4,4][Cl]), IoLiTec, Heilbronn, Germany, CAS: 2304-30-5 | 294.88 | >95 |
 | Tributyltetradecylphosphonium chloride ([P4,4,4,14][Cl]), IoLiTec, Heilbronn, Germany CAS: 81741-28-8 | 435.24 | >95 |
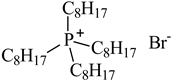 | Tetraoctylphosphonium bromide ([P8,8,8,8][Br]), IoLiTec, Heilbronn, Germany CAS: 23906-97-0 | 563.76 | >95 |
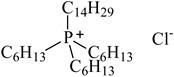 | Trihexyltetradecylphosphonium chloride, known as Cyphos IL 101, ([P6,6,6,14][Cl]), IoLiTec, Heilbronn, Germany CAS 258864-54-9 | 519.42 | >95 |
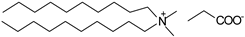 | Didecyldimethylammonium propionate ([N10,10,1,1][C2H5COO]), synthesized | 399.79 | >95 |
 | Didecyldimethylammonium hydrogensulphate ([N10,10,1,H][HSO4]), synthesized | 409.67 | >95 |
 | Didecyldimethylammonium bis(2-ethylhexyl) dihydrogen phosphate ([N10,10,1,1][H2PO4]), synthesized | 423.7 | 95 |
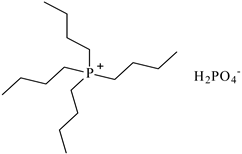 | Tetrabutylphosphonium dihydrogen phosphate ([P4,4,4,4][H2PO4]), synthesized | 356.48 | 95 |
| Name, Molecular Formula, Supplier, CAS Number | Molar Mass (g·mol−1) | Purity * in Mass Percent (%) |
|---|---|---|
| Malonic acid, C3H4O4, Sigma-Aldrich, Darmstadt, Germany, CAS 141-82-2 | 104.06 | 99.0 |
| Ethylene glycol, HOCH2CH2OH, Riedel-de-Haën, Seelze 1, Germany, CAS 107-21-1 | 62.08 | 99.0 |
| Didecyldimethylammonium chloride, [N10,10,1,1][Cl], DDACl, Alpinus Sp. z o.o., Solec Kujawski, Poland, CAS 7173-51-5 | 362.16 | 50 wt% aq. solution |
| Trichloroisocyanuric acid (TCCA), Thermo Fisher Scientific, Karlsruhe, Germany, CAS 87-90-1 | 232.41 | 99.0 |
| Glycine, C2H5NO2, Sigma-Aldrich, Heilbronn, Germany, CAS 56-40-6 | 75.07 | 99.0 |
| Pentapotassium bis(peroxymonosulphate)bis(sulphate) PHM, (2KHSO5 · KHSO4 · K2SO4), Sigma-Aldrich, Heilbronn, Germany, CAS 70693-62-8 | 614.76 | 98.0 |
| Sulphuric acid, H2SO4, Riedel-de Haën, Seelze 1, Gemany, CAS 7664-93-9 | 98.08 | 96.0 |
| Sodium chloride, NaCl, Chempur, Karlsruhe, Germany, CAS 7647-14-5 | 58.44 | 99.9 |
| Sodium sulphate, Na2SO4, Chempur, Karlsruhe, Germany, CAS 7757-82-6 | 142.04 | 99.0 |
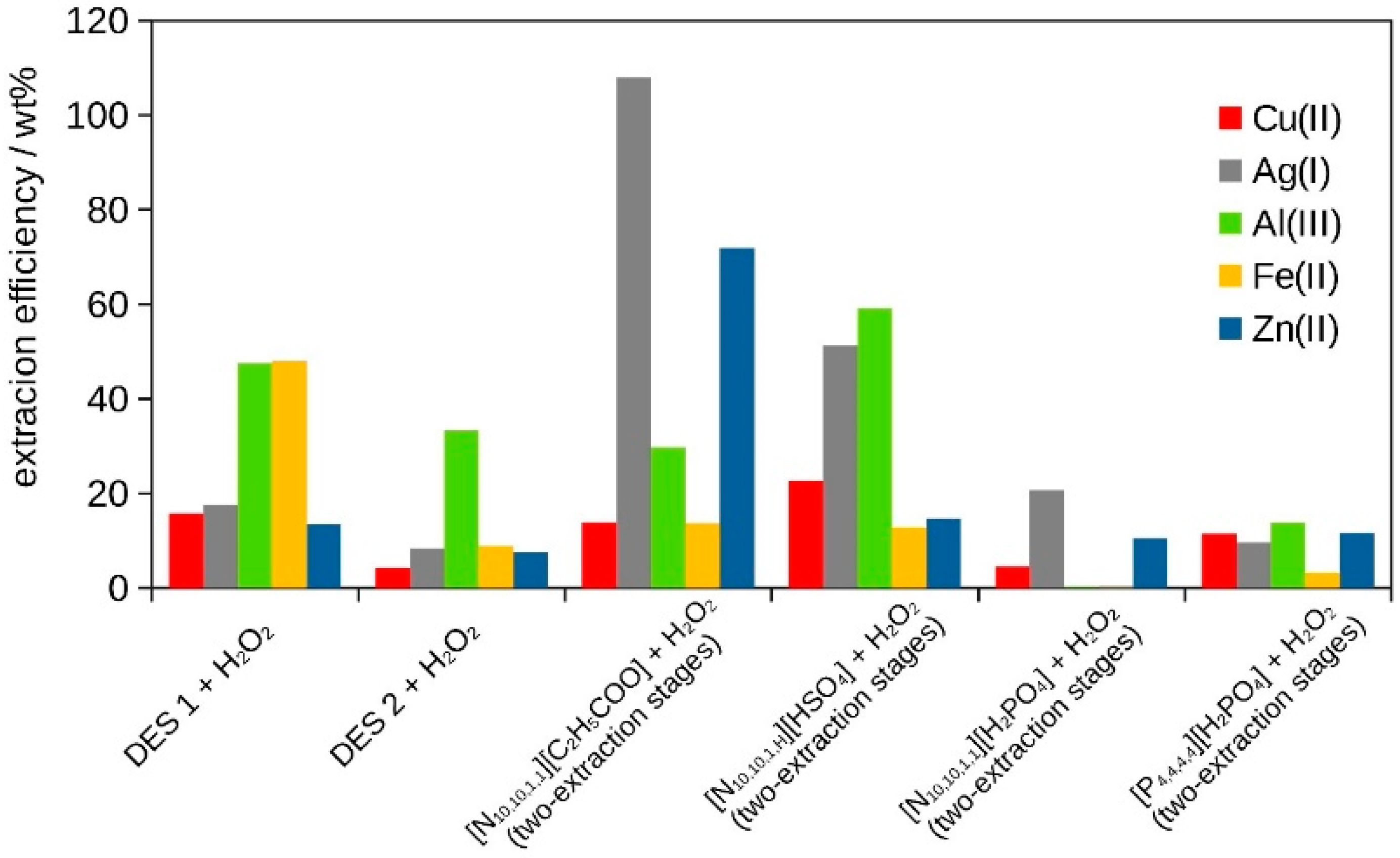
| Extracting Solvent | Ion | g0 * (mg) | gE * (mg) | E (wt%) | D | pH |
|---|---|---|---|---|---|---|
| DES 1 + H2O2 | Cu(II) | 502.50 | 78.73 | 15.7 | 0.2 | 5 |
| Ag(I) | 1.082 | 0.191 | 17.6 | 0.2 | ||
| Al(III) | 183.00 | 86.89 | 47.5 | 0.5 | ||
| Fe(II) | 178.50 | 85.46 | 47.9 | 0.5 | ||
| Zn(II) | 40.50 | 5.45 | 13.5 | 0.1 | ||
| DES 2 + H2O2 | Cu(II) | 502.50 | 21.13 | 4.2 | 0 | 5 |
| Ag(I) | 1.082 | 0.09 | 8.3 | 0 | ||
| Al(III) | 183.00 | 60.98 | 33.3 | 0.3 | ||
| Fe(II) | 178.50 | 15.76 | 8.8 | 0 | ||
| Zn(II) | 40.50 | 3.03 | 7.5 | 0 |
| Extracting Solvent | Ion | g0 * (mg) | gE * (mg) | E (wt%) | D | pH |
|---|---|---|---|---|---|---|
| [N10,10,1,1][C2H5COO] + H2O2 (single-extraction stage) | Cu(II) | 502.50 | 48.45 | 9.6 | 0 | 6 |
| Ag(I) | 1.082 | 1.096 | 100 | 1 | ||
| Al(III) | 183.00 | 37.12 | 20.3 | 0.2 | ||
| Fe(II) | 178.50 | 23.84 | 13.3 | 0.1 | ||
| Zn(II) | 40.50 | 27.23 | 67.2 | 0.7 | ||
| [N10,10,1,1][C2H5COO] + H2O2 (two-extraction stage) | Cu(II) | 502.50 | 69.45 | 13.8 | 0.1 | 6 |
| Ag(I) | 1.082 | 1.168 | 100 | 1 | ||
| Al(III) | 183.00 | 54.34 | 29.7 | 0.3 | ||
| Fe(II) | 178.50 | 24.32 | 13.6 | 0.1 | ||
| Zn(II) | 40.50 | 29.11 | 71.9 | 0.7 | ||
| [N10,10,1,H][HSO4] + H2O2 (two-extraction stage) | Cu(II) | 502.50 | 113.35 | 22.6 | 0.2 | 3–1.5 |
| Ag(I) | 1.082 | 0.555 | 51.3 | 0.5 | ||
| Al(III) | 183.00 | 108.24 | 59.1 | 0.6 | ||
| Fe(II) | 178.50 | 21.57 | 12.8 | 0.1 | ||
| Zn(II) | 40.50 | 5.93 | 14.6 | 0.1 |
| Extracting Solvent | Ion | g0 * (mg) | gE * (mg) | E (wt%) | D | pH |
|---|---|---|---|---|---|---|
| [N10,10,1,1][H2PO4] + H2O2 (single-extraction stage) | Cu(II) | 502.50 | 15.33 | 3.0 | 0 | 5.5 |
| Ag(I) | 1.082 | 0.214 | 19.8 | 0.2 | ||
| Al(III) | 183.00 | 0.58 | 0.32 | 0 | ||
| Fe(II) | 178.50 | 0.116 | 0.06 | 0 | ||
| Zn(II) | 40.50 | 2.04 | 5.0 | 0 | ||
| [N10,10,1,1][H2PO4] + H2O2 (two-extraction stage) | Cu(II) | 502.50 | 22.56 | 4.5 | 0 | 5.5 |
| Ag(I) | 1.082 | 0.224 | 20.7 | 0.2 | ||
| Al(III) | 183.00 | 0.433 | 0.24 | 0 | ||
| Fe(II) | 178.50 | 0.163 | 0.09 | 0 | ||
| Zn(II) | 40.50 | 4.25 | 10.5 | 0.1 | ||
| [P4,4,4,4][H2PO4] + H2O2 (single-extraction stage) | Cu(II) | 502.50 | 30.15 | 6.0 | 0 | 6 |
| Ag(I) | 1.082 | 0.089 | 8.2 | 0 | ||
| Al(III) | 183.00 | 17.78 | 9.7 | 0 | ||
| Fe(II) | 178.50 | 5.22 | 2.9 | 0 | ||
| Zn(II) | 40.50 | 3.94 | 9.7 | 0 | ||
| [P4,4,4,4][H2PO4] + H2O2 (two-extraction stage) | Cu(II) | 502.50 | 57.17 | 11.4 | 0.1 | 6 |
| Ag(I) | 1.082 | 0.104 | 9.6 | 0 | ||
| Al(III) | 183.00 | 25.31 | 13.8 | 0.1 | ||
| Fe(II) | 178.50 | 5.72 | 3.2 | |||
| Zn(II) | 40.50 | 4.68 | 11.6 | 0.1 |
| Extracting Solvent | Ion | g0 * (mg) | gE * (mg) | E (wt%) | D | pH |
|---|---|---|---|---|---|---|
| [P4,4,4,4][Cl] + TCCA (4 g) (single-extraction stage) | Cu(II) | 502.50 | 267.6 | 53.2 | 0.5 | 2 |
| Ag(I) | 1.082 | 1.10 | 100 | 1 | ||
| Al(III) | 183.00 | 92.0 | 50.3 | 0.5 | ||
| Fe(II) | 178.50 | 62.4 | 35.0 | 0.3 | ||
| Zn(II) | 40.50 | 10.5 | 25.9 | 0.3 | ||
| [P4,4,4,4][Cl] + TCCA (8 g) (two-extraction stage) | Cu(II) | 502.50 | 346.4 | 68.9 | 0.7 | 2 |
| Ag(I) | 1.082 | 1.08 | 100 | 1 | ||
| Al(III) | 183.00 | 148.7 | 81.3 | 0.8 | ||
| Fe(II) | 178.50 | 45.4 | 25.4 | 0.2 | ||
| Zn(II) | 40.50 | 17.7 | 43.7 | 0.4 | ||
| [P4,4,4,4][Cl] + TCCA (12 g) (single-extraction stage) | Cu(II) | 502.50 | 190.1 | 37.8 | 0.4 | 2 |
| Ag(I) | 1.082 | 1.122 | 100 | 1 | ||
| Al(III) | 183.00 | 80.79 | 44.1 | 0.4 | ||
| Fe(II) | 178.50 | 29.24 | 16.4 | 0.2 | ||
| Zn(II) | 40.50 | 4.69 | 11.6 | 0.1 | ||
| [P4,4,4,14][Cl] + TCCA (8 g) (two-extraction stage) | Cu(II) | 502.50 | 197.1 | 39.2 | 0.4 | 2 |
| Ag(I) | 1.082 | 1.09 | 100 | 1 | ||
| Al(III) | 183.00 | 150.6 | 82.3 | 0.8 | ||
| Fe(II) | 178.50 | 29.57 | 16.6 | 0.2 | ||
| Zn(II) | 40.50 | 8.74 | 21.6 | 0.2 | ||
| [P8,8,8,8][Br] + TCCA (8 g) (single-extraction stage) | Cu(II) | 502.50 | 124.08 | 24.7 | 0.2 | 3 |
| Ag(I) | 1.082 | 0.284 | 26.2 | 0.3 | ||
| Al(III) | 183.00 | 54.85 | 30.0 | 0.3 | ||
| Fe(II) | 178.50 | 12.40 | 6.9 | 0 | ||
| Zn(II) | 40.50 | 1.79 | 4.4 | 0 | ||
| [P8,8,8,8][Br] + TCCA (8 g) (two-extraction stage) | Cu(II) | 502.50 | 154.5 | 30.7 | 0.3 | 3 |
| Ag(I) | 1.082 | 0.36 | 33.3 | 0.3 | ||
| Al(III) | 183.00 | 62.81 | 34.3 | 0.3 | ||
| Fe(II) | 178.50 | 13.73 | 7.7 | 0 | ||
| Zn(II) | 40.50 | 2.16 | 5.3 | 0 | ||
| [P6,6,6,14][Cl] + TCCA (4 g) (organic phase after the demineralization)(single-extraction stage) | Cu(II) | 502.50 | 100.7 | 20.0 | 0.2 | 2 |
| Ag(I) | 1.082 | 0.30 | 27.7 | 0.3 | ||
| Al(III) | 183.00 | 66.18 | 36.2 | 0.4 | ||
| Fe(II) | 178.50 | 72.12 | 40.4 | 0.4 | ||
| Zn(II) | 40.50 | 7.54 | 18.6 | 0.2 | ||
| [N10,10,1,1][C2H5COO] + TCCA (4 g) (two-extraction stage) | Cu(II) | 502.50 | 147.6 | 29.4 | 0.3 | 2 |
| Ag(I) | 1.082 | 0.12 | 11.1 | 0.1 | ||
| Al(III) | 183.00 | 38.11 | 20.8 | 0.2 | ||
| Fe(II) | 178.50 | 8.96 | 5.0 | 0 | ||
| Zn(II) | 40.50 | 3.76 | 9.3 | 0 | ||
| [N10,10,1,1][C2H5COO] + TCCA (8 g), (two-extraction stage) | Cu(II) | 502.50 | 251.6 | 50.1 | 0.5 | 2 |
| Ag(I) | 1.082 | 1.08 | 100 | 1 | ||
| Al(III) | 183.00 | 96.05 | 52.5 | 0.5 | ||
| Fe(II) | 178.50 | 19.6 | 11.0 | 0.1 | ||
| Zn(II) | 40.50 | 13.0 | 32.1 | 0.3 | ||
| [N10,10,1,1][C2H5COO] + TCCA (12 g), (single-extraction stage) | Cu(II) | 502.50 | 133.4 | 26.5 | 0.3 | 2 |
| Ag(I) | 1.082 | 0.347 | 32.1 | 0.3 | ||
| Al(III) | 183.00 | 80.58 | 44.0 | 0.4 | ||
| Fe(II) | 178.50 | 15.72 | 8.8 | 0 | ||
| Zn(II) | 40.50 | 8.40 | 20.7 | 0.2 | ||
| [N10,10,1,1][H2PO4] + TCCA(4 g) (single-extraction stage) | Cu(II) | 502.50 | 199.8 | 39.8 | 0.4 | 2 |
| Ag(I) | 1.082 | 1.14 | 100 | 1 | ||
| Al(III) | 183.00 | 98.8 | 54.0 | 0.5 | ||
| Fe(II) | 178.50 | 23.1 | 12.9 | 0.1 | ||
| Zn(II) | 40.50 | 8.3 | 20.5 | 0.2 | ||
| [N10,10,1,H][HSO4] + TCCA (8 g) (two-extraction stage) | Cu(II) | 502.50 | 118.8 | 23.6 | 0.2 | 1.5 |
| Ag(I) | 1.082 | 0.417 | 38.6 | 0.4 | ||
| Al(III) | 183.00 | 151.8 | 83.0 | 0.8 | ||
| Fe(II) | 178.50 | 72.75 | 40.8 | 0.4 | ||
| Zn(II) | 40.50 | 8.28 | 20.5 | 0.2 |
| Extracting Solvent | Ion | g0 * (mg) | gE * (mg) | E (wt%) | D | pH |
|---|---|---|---|---|---|---|
| [P4,4,4,4][Cl] + glycine (4 g) | Cu(II) | 502.50 | 25.88 | 5.1 | 0 | 6 |
| Ag(I) | 1.082 | 0.086 | 8.0 | 0 | ||
| Al(III) | 183.00 | 1.48 | 0.8 | 0 | ||
| Fe(II) | 178.50 | 1.87 | 1.0 | 0 | ||
| Zn(II) | 40.50 | 1.40 | 3.4 | 0 | ||
| [P4,4,4,4][Cl] + glycine (12 g) | Cu(II) | 502.50 | 48.40 | 9.6 | 0 | 6 |
| Ag(I) | 1.082 | 1.09 | 100 | 1 | ||
| Al(III) | 183.00 | 12.80 | 7.0 | 0 | ||
| Fe(II) | 178.50 | 9.84 | 5.5 | 0 | ||
| Zn(II) | 40.50 | 14.69 | 36.3 | 0.4 | ||
| [P8,8,8,8][Br] + glycine (4 g) | Cu(II) | 502.50 | 35.28 | 7.02 | 0 | 6 |
| Ag(I) | 1.082 | 1.072 | 100 | 1 | ||
| Al(III) | 183.00 | 17.22 | 9.41 | 0 | ||
| Fe(II) | 178.50 | 8.79 | 4.92 | 0 | ||
| Zn(II) | 40.50 | 2.832 | 6.99 | 0 | ||
| [N10,10,1,1][C2H5COO] + glycine (12 g) | Cu(II) | 502.50 | 25.52 | 5.1 | 0 | 6 |
| Ag(I) | 1.082 | 1.16 | 100 | 1 | ||
| Al(III) | 183.00 | 54.80 | 29.9 | 0.3 | ||
| Fe(II) | 178.50 | 7.32 | 4.1 | 0 | ||
| Zn(II) | 40.50 | 13.24 | 32.7 | 0.3 |
| Extracting Solvent | Ion | g0 * (mg) | gE * (mg) | E (wt%) | D | pH |
|---|---|---|---|---|---|---|
| [P4,4,4,4][Cl] + PHM (4g) (2 phases) | Cu(II) | 502.50 | 62.3 | 12.4 | 0.1 | 3 |
| Ag(I) | 1.082 | 0.07 | 6.5 | 0 | ||
| Al(III) | 183.00 | 8.52 | 4.6 | 0 | ||
| Fe(II) | 178.50 | 18.28 | 10.2 | 0.1 | ||
| Zn(II) | 40.50 | 3.58 | 8.8 | 0 | ||
| [P4,4,4,4][Cl] + PHM (8g) (2 phases) | Cu(II) | 502.50 | 120.5 | 24.0 | 0.2 | 3 |
| Ag(I) | 1.082 | 0.136 | 12.6 | 0.1 | ||
| Al(III) | 183.00 | 12.47 | 6.8 | 0 | ||
| Fe(II) | 178.50 | 16.81 | 9.4 | 0 | ||
| Zn(II) | 40.50 | 5.73 | 14.1 | 0.1 | ||
| [P4,4,4,4][Cl] + PHM (16g) (2 phases) | Cu(II) | 502.50 | 106.5 | 21.2 | 0.2 | 3 |
| Ag(I) | 1.082 | 0.770 | 71.2 | 0.7 | ||
| Al(III) | 183.00 | 41.29 | 22.6 | 0.2 | ||
| Fe(II) | 178.50 | 23.29 | 13.0 | 0.1 | ||
| Zn(II) | 40.50 | 3.56 | 8.8 | 0 | ||
| [P4,4,4,14][Cl] + PHM (4g) (2 phases) | Cu(II) | 502.50 | 19.81 | 3.9 | 0 | 3 |
| Ag(I) | 1.082 | 0.206 | 19.1 | 0.2 | ||
| Al(III) | 183.00 | 28.18 | 15.4 | 0.1 | ||
| Fe(II) | 178.50 | 47.64 | 26.7 | 0.3 | ||
| Zn(II) | 40.50 | 19.19 | 47.4 | 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domańska, U.; Wiśniewska, A.; Dąbrowski, Z. Recovery of Strategic Metals from Waste Printed Circuit Boards with Deep Eutectic Solvents and Ionic Liquids. Processes 2024, 12, 530. https://doi.org/10.3390/pr12030530
Domańska U, Wiśniewska A, Dąbrowski Z. Recovery of Strategic Metals from Waste Printed Circuit Boards with Deep Eutectic Solvents and Ionic Liquids. Processes. 2024; 12(3):530. https://doi.org/10.3390/pr12030530
Chicago/Turabian StyleDomańska, Urszula, Anna Wiśniewska, and Zbigniew Dąbrowski. 2024. "Recovery of Strategic Metals from Waste Printed Circuit Boards with Deep Eutectic Solvents and Ionic Liquids" Processes 12, no. 3: 530. https://doi.org/10.3390/pr12030530




