Life Cycle Assessment of Aviation Fuel Production from Steel-Industry Off-Gas
Abstract
:1. Introduction
| Years | Raw Material | Preparation Method of SAFs | Reference Sources |
|---|---|---|---|
| 2020 | Sugarcane | FTJ | Bressanin et al. [23] |
| 2021 | Lignocellulosic | ETJ | Romero-Izquierdo et al. [7] |
| 2021 | Lignocellulosic | ETJ and FTJ | Capaz et al. [22] |
| 2022 | Sugarcane | ETJ | Escalante et al. [24] |
| 2022 | Lignocellulosic | FTJ | Stigsson et al. [14] |
| 2022 | Steel-industry off-gas | FTJ | Collis et al. [19] |
| 2023 | Corn cob | ETJ | Wang et al. [25] |
| 2023 | Lignocellulosic | ETJ | Voß et al. [26] |
| 2023 | Corn stover | ETJ | Sun et al. [27] |
| 2023 | Sugarcane | FTJ | Guimarães et al. [28] |
| 2024 | Waste tires | FTJ | Rogachuk et al. [29] |
2. Methods and Simulations
2.1. Goal and System Boundary
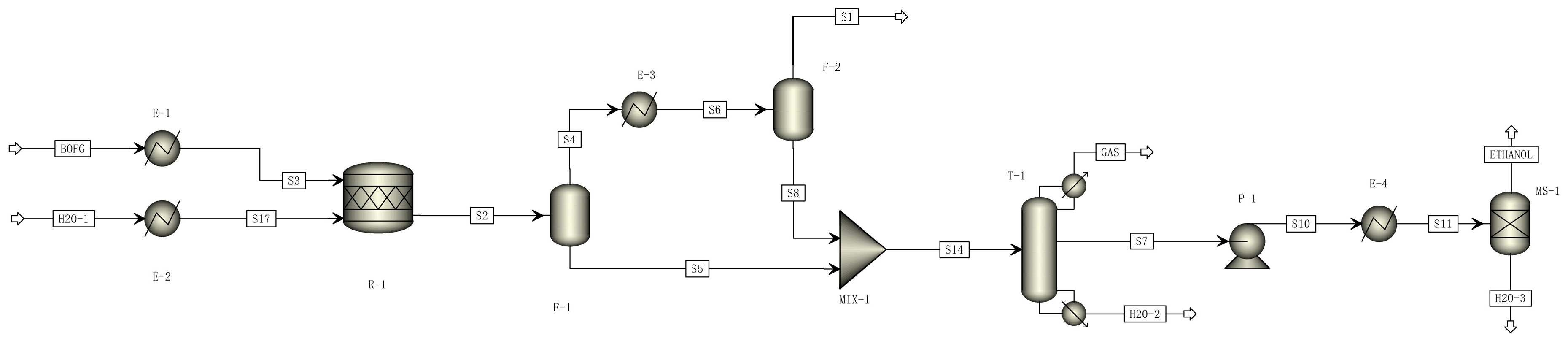
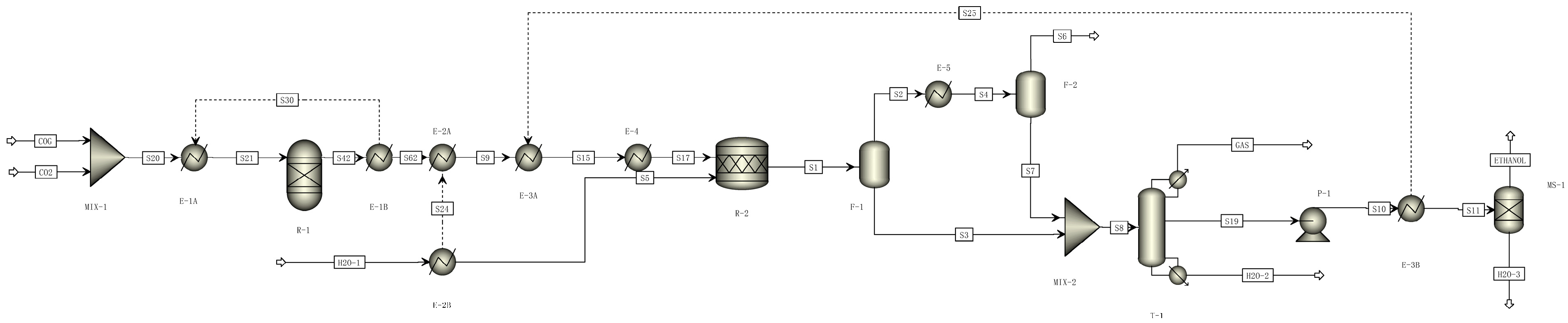
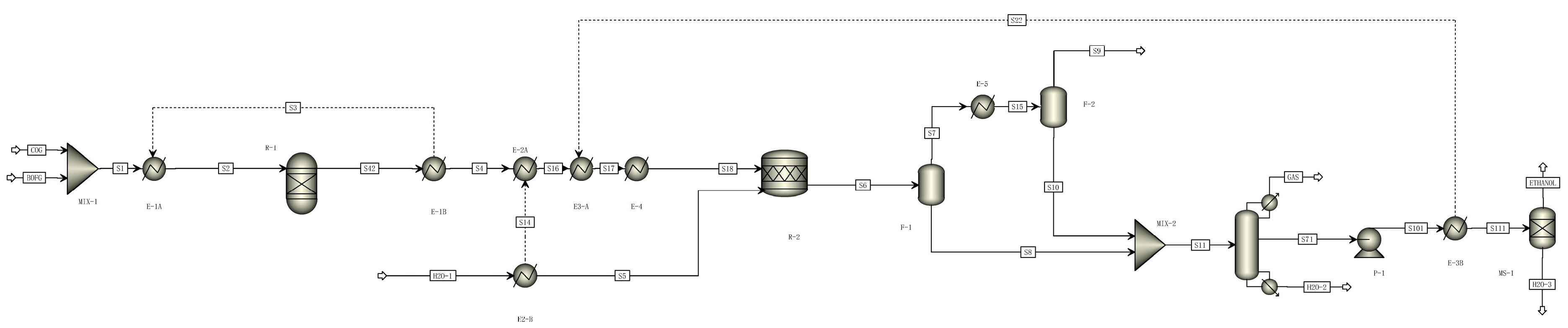
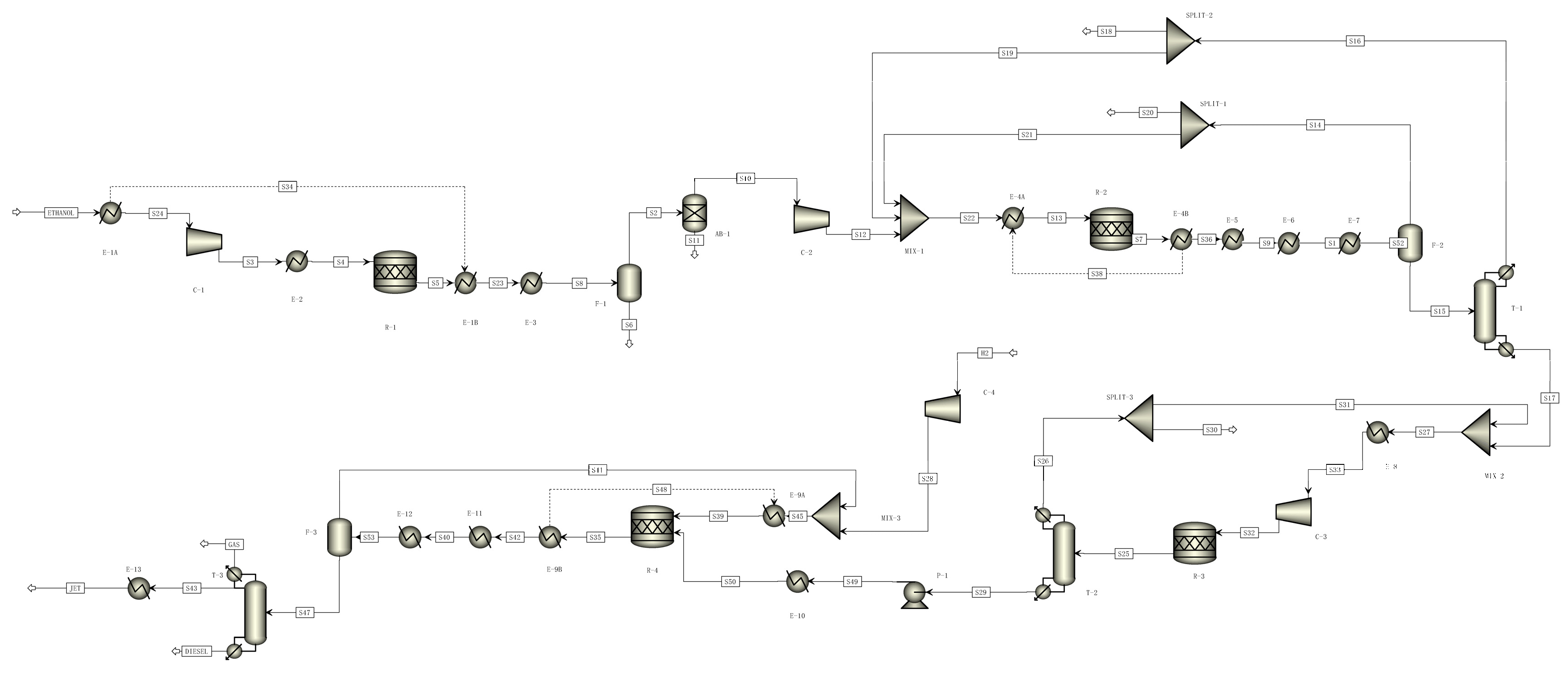
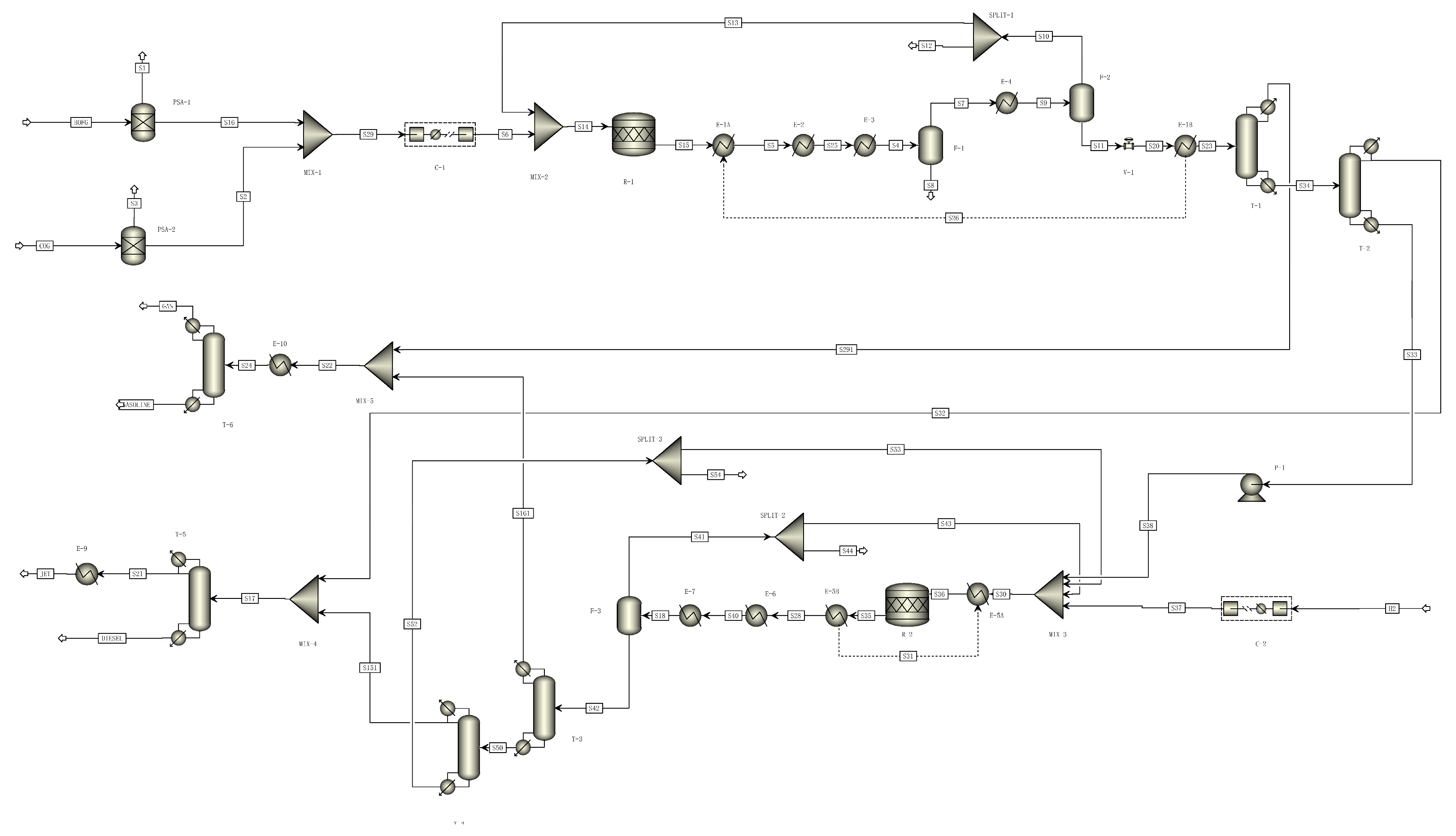
2.2. Process Description and Simulation
2.2.1. Ethanol Conversion Processes
2.2.2. Jet Fuel Production from Ethanol
2.2.3. Jet Fuel Production from Fischer–Tropsch Synthesis
- Only pure primary cracking is allowed,
- Formation of C1 and C2 is not considered,
- An equal number of fragments form between C4 and C19,
- The molar amount of C3 and C20 is half the molar amount of fragments between C4 and C19.
2.3. Assumptions
- This study does not consider factors such as land-use change, labor, and infrastructure. (a) Land-use change refers to changes in carbon flux between terrestrial ecosystems and the atmosphere due to land-use conversion (e.g., forests, grasslands, croplands) [73]. (b) When impact indicators are associated with social, economic, and labor factors, information related to labor is required. The objective of this study is to assess the GHG emissions and energy consumption of ETJs and FTJs, so labor considerations have not been included [74]. (c) In this study, infrastructure refers to fixed assets such as equipment, facilities, and buildings. The carbon footprint of infrastructure construction is inversely related to its lifespan, meaning that a longer lifespan results in a lower average annual carbon footprint [75]. This study focuses on a short production cycle, so infrastructure considerations have not been taken into account.
- Heat loss and pressure loss were not considered during the simulation.
- The exhaust gas was combusted to generate electricity, the combustion heat release was simulated by Aspen Plus (V11), and the power generation efficiency was 35% [76].
- The LCA distribution of raw materials adopted the 50/50 method, that is, the LCA of raw materials is divided equally between producers and consumers [77].
- In this study, we assumed that 50% of the heat was derived from coal, while the remaining 50% was sourced from natural gas, each with thermal efficiencies of 80% and 90%, respectively [25].
2.4. Inventory Data
2.4.1. Background Data
2.4.2. Feedstock Data
2.4.3. Transportation Data
3. Results and Discussion
3.1. Simulation Results
3.1.1. Material Balance
3.1.2. Physical Properties of Jet Fuel
3.2. Life Cycle Assessment Results
3.2.1. Fossil Fuel Consumptions
3.2.2. GHG Emissions
3.3. Sensitivity Analysis
3.4. Uncertainty Analysis
3.5. Comparisons and Prospect
3.5.1. Comparisons
3.5.2. Prospects
4. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ETJ | Ethanol to jet fuel |
| FTJ | Fischer–Tropsch to jet fuel |
| FT | Fischer–Tropsch |
| BOFG | Basic oxygen furnace gas |
| COG | Coke oven gas |
| LCA | Life cycle assessment |
| SAF | Sustainable aviation fuel |
| WGS | Water-gas shift |
| MDR | Methane dry reforming |
| CORSIA | Carbon Offsetting and Reduction Scheme for International Aviation |
| ATJ | Alcohol-to-Jet |
| GHG | Greenhouse gas |
| VPSA | Vacuum pressure swing adsorption |
| PSA | Pressure swing adsorption |
| ASF | Anderson–Schulz–Flory |
| CHP | Combined Heat and Power |
| E-FT | Electrolysis Fischer–Tropsch |
| SMG-FT | Jet fuel produced from steel-industry off-gas |
References
- Zhang, J.; Shen, J.; Xu, L.; Zhang, Q. The CO2 emission reduction path towards carbon neutrality in the Chinese steel industry: A review. Environ. Impact Assess. Rev. 2023, 99, 107017. [Google Scholar] [CrossRef]
- Deng, L.; Adams, T.A., II. Comparison of steel manufacturing off-gas utilization methods via life cycle analysis. J. Clean. Prod. 2020, 277, 123568. [Google Scholar] [CrossRef]
- De Klerk, A.; Chauhan, G.; Halmenschlager, C.; Link, F.; Sanchez, N.M.; Gartley, B.; El-Sayed, H.E.M.; Sehdev, R.; Lehoux, R. Sustainable aviation fuel: Pathways to fully formulated synthetic jet fuel via Fischer-Tropsch synthesis. Energy Sci. Eng. 2023, 12, 394–409. [Google Scholar] [CrossRef]
- Zhang, X.; Lei, H.; Zhu, L.; Wu, J.; Chen, S. From lignocellulosic biomass to renewable cycloalkanes for jet fuels. Green Chem. 2015, 17, 4736–4747. [Google Scholar] [CrossRef]
- Doliente, S.S.; Narayan, A.; Tapia, J.F.D.; Samsatli, N.J.; Zhao, Y.; Samsatli, S. Bio-aviation Fuel: A Comprehensive Review and Analysis of the Supply Chain Components. Front. Energy Res. 2020, 8, 110. [Google Scholar] [CrossRef]
- Geleynse, S.; Brandt, K.; Garcia-Perez, M.; Wolcott, M.; Zhang, X. The Alcohol-to-Jet Conversion Pathway for Drop-In Biofuels: Techno-Economic Evaluation. ChemSusChem 2018, 11, 3728–3741. [Google Scholar] [CrossRef] [PubMed]
- Romero-Izquierdo, A.G.; Gomez-Castro, F.I.; Gutierrez-Antonio, C.; Hernandez, S.; Errico, M. Intensification of the alcohol-to-jet process to produce renewable aviation fuel. Chem. Eng. Process. Process Intensif. 2021, 160, 108270. [Google Scholar] [CrossRef]
- Tao, L.; Markham, J.N.; Haq, Z.; Biddy, M.J. Techno-economic analysis for upgrading the biomass-derived ethanol-to-jet blendstocks. Green Chem. 2017, 19, 1082–1101. [Google Scholar] [CrossRef]
- Muktham, R.; Bhargava, S.; Bankupalli, S.; Ball, A. A review on 1st and 2nd generation bioethanol production-recent progress. J. Sustain. Bioenergy Syst. 2016, 2016, 72–92. [Google Scholar] [CrossRef]
- IATA. 2015 Report on Alternative Fuels. Available online: https://www.iata.org/contentassets/462587e388e749eeb040df4dfdf02cb1/2015-report-alternative-fuels.pdf (accessed on 5 June 2023).
- Gholami, Z.; Tišler, Z.; Velvarská, R.; Kocík, J. Comn catalysts derived from hydrotalcite-like precursors for direct conversion of syngas to fuel range hydrocarbons. Catalysts 2020, 10, 813. [Google Scholar] [CrossRef]
- Wei, H.; Liu, W.; Chen, X.; Yang, Q.; Li, J.; Chen, H. Renewable bio-jet fuel production for aviation: A review. Fuel 2019, 254, 115599. [Google Scholar] [CrossRef]
- You, F.; Wang, B. Life cycle optimization of biomass-to-liquid supply chains with distributed–centralized processing networks. Ind. Eng. Chem. Res. 2011, 50, 10102–10127. [Google Scholar] [CrossRef]
- Stigsson, C.; Furusjö, E.; Börjesson, P. A model of an integrated hydrothermal liquefaction, gasification and Fischer-Tropsch synthesis process for converting lignocellulosic forest residues into hydrocarbons. Bioresour. Technol. 2022, 353, 126070. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez, J.M.; Ferrera-Lorenzo, N.; Luque, S.; Arenillas, A.; Menéndez, J. New process for producing methanol from coke oven gas by means of CO2 reforming. Comparison with conventional process. Fuel Process. Technol. 2013, 115, 215–221. [Google Scholar] [CrossRef]
- Graciano, J.E.; Chachuat, B.; Alves, R.M. Conversion of CO2-rich natural gas to liquid transportation fuels via trireforming and Fischer–Tropsch synthesis: Model-based assessment. Ind. Eng. Chem. Res. 2018, 57, 9964–9976. [Google Scholar] [CrossRef]
- Handler, R.M.; Shonnard, D.R.; Griffing, E.M.; Lai, A.; Palou-Rivera, I. Life cycle assessments of ethanol production via gas fermentation: Anticipated greenhouse gas emissions for cellulosic and waste gas feedstocks. Ind. Eng. Chem. Res. 2016, 55, 3253–3261. [Google Scholar] [CrossRef]
- Harmon, L.; Holladay, J. Jet Fuel Derived from Steel Mill Waste Gasses. Res. Technol. Manag. 2020, 63, 46–51. [Google Scholar] [CrossRef]
- Collis, J.; Duch, K.; Schomäcker, R. Techno-economic assessment of jet fuel production using the Fischer-Tropsch process from steel mill gas. Front. Energy Res. 2022, 10, 1049229. [Google Scholar] [CrossRef]
- Prussi, M.; Lee, U.; Wang, M.; Malina, R.; Valin, H.; Taheripour, F.; Velarde, C.; Staples, M.D.; Lonza, L.; Hileman, J.I. CORSIA: The first internationally adopted approach to calculate life-cycle GHG emissions for aviation fuels. Renew. Sustain. Energy Rev. 2021, 150, 111398. [Google Scholar] [CrossRef]
- De Jong, S.; Antonissen, K.; Hoefnagels, R.; Lonza, L.; Wang, M.; Faaij, A.; Junginger, M. Life-cycle analysis of greenhouse gas emissions from renewable jet fuel production. Biotechnol. Biofuels 2017, 10, 64. [Google Scholar] [CrossRef]
- Capaz, R.S.; Posada, J.A.; Osseweijer, P.; Seabra, J.E. The carbon footprint of alternative jet fuels produced in Brazil: Exploring different approaches. Resour. Conserv. Recycl. 2021, 166, 105260. [Google Scholar] [CrossRef]
- Bressanin, J.M.; Klein, B.C.; Chagas, M.F.; Watanabe, M.D.B.; Sampaio, I.L.d.M.; Bonomi, A.; Morais, E.R.d.; Cavalett, O. Techno-economic and environmental assessment of biomass gasification and Fischer–Tropsch synthesis integrated to sugarcane biorefineries. Energies 2020, 13, 4576. [Google Scholar] [CrossRef]
- Escalante, E.S.R.; Ramos, L.S.; Coronado, C.J.R.; de Carvalho Júnior, J.A. Evaluation of the potential feedstock for biojet fuel production: Focus in the Brazilian context. Renew. Sustain. Energy Rev. 2022, 153, 111716. [Google Scholar] [CrossRef]
- Wang, X.; Guo, L.; Lv, J.; Li, M.; Huang, S.; Wang, Y.; Ma, X. Process design, modeling and life cycle analysis of energy consumption and GHG emission for jet fuel production from bioethanol in China. J. Clean. Prod. 2023, 389, 136027. [Google Scholar] [CrossRef]
- Voß, S.; Bube, S.; Kaltschmitt, M. Aviation fuel production pathways from lignocellulosic biomass via alcohol intermediates–A technical analysis. Fuel Commun. 2023, 17, 100093. [Google Scholar] [CrossRef]
- Sun, H.; Luo, Z.; Li, S.; Xue, S.; Zhou, Q.; Wei, T.; Du, L. Comparative life cycle assessment (LCA) of biofuel production via corn stover: Fermentation to ethanol, pyrolysis to bio-oil, and gasification to jet fuel. In Biomass Conversion and Biorefinery; Springer: Berlin, Germany, 2021; Volume 13, pp. 12809–12821. [Google Scholar] [CrossRef]
- Guimarães, H.R.; Bressanin, J.M.; Motta, I.L.; Chagas, M.F.; Klein, B.C.; Bonomi, A.; Maciel Filho, R.; Watanabe, M.D.B. Decentralization of sustainable aviation fuel production in Brazil through Biomass-to-Liquids routes: A techno-economic and environmental evaluation. Energy Convers. Manag. 2023, 276, 116547. [Google Scholar] [CrossRef]
- Rogachuk, B.E.; Okolie, J.A. Comparative assessment of pyrolysis and Gasification-Fischer Tropsch for sustainable aviation fuel production from waste tires. Energy Convers. Manag. 2024, 302, 118110. [Google Scholar] [CrossRef]
- ISO 14040/44; Environmental Management—Life Cycle Assessment—Requirements and Guidelines. ISO: Geneva, Switzerland, 2006. Available online: https://pqm-online.com/assets/files/lib/std/iso_14040-2006.pdf (accessed on 10 February 2023).
- De Oliveira Matias, J.C.; Devezas, T.C. Consumption dynamics of primary-energy sources: The century of alternative energies. Appl. Energy 2007, 84, 763–770. [Google Scholar] [CrossRef]
- Øvergaard, S. Issue Paper: Definition of Primary and Secondary Energy; Statistics Norway: Oslo, Norway, 2008; Available online: https://mdgs.un.org/unsd/envAccounting/londongroup/meeting13/LG13_12a.pdf (accessed on 1 March 2023).
- Wang, Y.M.; Duan, W.J.; Wang, Y.F.; Chen, Y.; Zhang, E.H.; Jia, Y.; Li, Q.G. A System for Comprehensive Utilization of Steel Industry Off-Gas to Produce Ethanol. CN211921378U. 13 November 2020. Available online: https://kns.cnki.net/kcms2/article/abstract?v=S5uBaE2M3Od0D8a7PD_8kSlI7juP0YCmOg8tyvwtjx33PMUX3VuDi8ujaFe4kizyUSK0q_W2GegEH9CrsFZ_rFz4h-7XyNQwJu_fioPJB4GxA6DSeaW6x8NtaR6LjHEtUXiW4AIIwUM=&uniplatform=NZKPT&language=CHS (accessed on 15 March 2023).
- Moreno Fernández-Villamil, J. Process and Plant Design of Ethanol Synthesis from Steel Industry Flue Gas; Universidad Polictecnica de Madrid: Madrid, Spain, 2017; Available online: https://oa.upm.es/49069/ (accessed on 25 December 2022).
- Gaddy, J.L.; Arora, D.K.; Ko, C.-W.; Phillips, J.R.; Basu, R.; Wikstrom, C.V.; Clausen, E.C. Methods for Increasing the Production of Ethanol from Microbial Fermentation; Bioengineering Resources, Inc.: Fayetteville, NC, USA, 2007. Available online: https://www.osti.gov/biblio/941118 (accessed on 2 January 2023).
- De Medeiros, E.M.; Posada, J.A.; Noorman, H.; Osseweijer, P.; Maciel Filho, R. Hydrous bioethanol production from sugarcane bagasse via energy self-sufficient gasification-fermentation hybrid route: Simulation and financial analysis. J. Clean. Prod. 2017, 168, 1625–1635. [Google Scholar] [CrossRef]
- Morales, J.Y.R.; Mendoza, J.A.B.; Torres, G.O.; Vázquez, F.d.J.S.; Rojas, A.C.; Vidal, A.F.P. Fault-tolerant control implemented to Hammerstein–Wiener model: Application to bio-ethanol dehydration. Fuel 2022, 308, 121836. [Google Scholar] [CrossRef]
- Hanchate, N.; Kulshreshtha, P.; Mathpati, C. Optimization, scale-up and cost estimation of dehydration of ethanol using temperature swing adsorption. J. Environ. Chem. Eng. 2019, 7, 102938. [Google Scholar] [CrossRef]
- Morales, J.Y.R.; López, G.L.; Martínez, V.M.A.; Vázquez, F.d.J.S.; Mendoza, J.A.B.; García, M.M. Parametric study and control of a pressure swing adsorption process to separate the water-ethanol mixture under disturbances. Sep. Purif. Technol. 2020, 236, 116214. [Google Scholar] [CrossRef]
- Petropoulou, E.G.; Carollo, C.; Pappa, G.D.; Caputo, G.; Voutsas, E.C. Sensitivity analysis and process optimization of a natural gas dehydration unit using triethylene glycol. J. Nat. Gas Sci. Eng. 2019, 71, 102982. [Google Scholar] [CrossRef]
- Britovsek, G.J.; Malinowski, R.; McGuinness, D.S.; Nobbs, J.D.; Tomov, A.K.; Wadsley, A.W.; Young, C.T. Ethylene oligomerization beyond Schulz–Flory distributions. ACS Catal. 2015, 5, 6922–6925. [Google Scholar] [CrossRef]
- Restrepo-Flórez, J.M.; Maravelias, C.T. Advanced fuels from ethanol–a superstructure optimization approach. Energy Environ. Sci. 2021, 14, 493–506. [Google Scholar] [CrossRef]
- Speight, J. Coal gasification processes for synthetic liquid fuel production. In Gasification for Synthetic Fuel Production; Elsevier: Amsterdam, The Netherlands, 2015; pp. 201–220. [Google Scholar] [CrossRef]
- Gholami, Z.; Asmawati Mohd Zabidi, N.; Gholami, F.; Ayodele, O.B.; Vakili, M. The influence of catalyst factors for sustainable production of hydrocarbons via Fischer-Tropsch synthesis. Rev. Chem. Eng. 2017, 33, 337–358. [Google Scholar] [CrossRef]
- Jia, L.; Jia, L.; Li, D.; Hou, B.; Wang, J.; Sun, Y. Silylated Co/SBA-15 catalysts for Fischer–Tropsch synthesis. J. Solid State Chem. 2011, 184, 488–493. [Google Scholar] [CrossRef]
- Kang, S.-H.; Bae, J.W.; Cheon, J.-Y.; Lee, Y.-J.; Ha, K.-S.; Jun, K.-W.; Lee, D.-H.; Kim, B.-W. Catalytic performance on iron-based Fischer–Tropsch catalyst in fixed-bed and bubbling fluidized-bed reactor. Appl. Catal. B Environ. 2011, 103, 169–180. [Google Scholar] [CrossRef]
- Van de Loosdrecht, J.; Botes, F.G.; Ciobica, I.M.; Ferreira, A.C.; Gibson, P.; Moodley, D.J.; Saib, A.M.; Visagie, J.L.; Weststrate, C.J.; Niemantsverdriet, J.W. Fischer-Tropsch Synthesis: Catalysts and Chemistry. Comprehensive Inorganic Chemistry. In Comprehensive Inorganic Chemistry II: From Elements to Applications; Elsevier: Amsterdam, The Netherlands, 2013; pp. 525–557. [Google Scholar] [CrossRef]
- Ma, W.; Jacobs, G.; Sparks, D.E.; Todic, B.; Bukur, D.B.; Davis, B.H. Quantitative comparison of iron and cobalt based catalysts for the Fischer-Tropsch synthesis under clean and poisoning conditions. Catal. Today 2020, 343, 125–136. [Google Scholar] [CrossRef]
- Marchese, M.; Giglio, E.; Santarelli, M.; Lanzini, A. Energy performance of Power-to-Liquid applications integrating biogas upgrading, reverse water gas shift, solid oxide electrolysis and Fischer-Tropsch technologies. Energy Convers. Manag. X 2020, 6, 100041. [Google Scholar] [CrossRef]
- Davis, B.H.; Occelli, M.L. Fischer-Tropsch Synthesis, Catalysts and Catalysis; Elsevier: Amsterdam, The Netherlands, 2006; Available online: https://shop.elsevier.com/books/fischer-tropsch-synthesis-catalysts-and-catalysis/davis/978-0-444-52221-4 (accessed on 13 January 2023).
- Jahangiri, H.; Bennett, J.; Mahjoubi, P.; Wilson, K.; Gu, S. A review of advanced catalyst development for Fischer–Tropsch synthesis of hydrocarbons from biomass derived syn-gas. Catal. Sci. Technol. 2014, 4, 2210–2229. [Google Scholar] [CrossRef]
- Perego, C. Development of a Fischer-Tropsch catalyst: From laboratory to commercial scale demonstration. Rend. Lincei 2007, 18, 305–317. [Google Scholar] [CrossRef]
- Markowitsch, C.; Lehner, M.; Maly, M. Evaluation of process structures and reactor technologies of an integrated power-to-liquid plant at a cement factory. J. CO2 Util. 2023, 70, 102449. [Google Scholar] [CrossRef]
- Li, J.; Yang, G.; Yoneyama, Y.; Vitidsant, T.; Tsubaki, N. Jet fuel synthesis via Fischer–Tropsch synthesis with varied 1-olefins as additives using Co/ZrO2–SiO2 bimodal catalyst. Fuel 2016, 171, 159–166. [Google Scholar] [CrossRef]
- Zhou, W.; Cheng, K.; Kang, J.; Zhou, C.; Subramanian, V.; Zhang, Q.; Wang, Y. New horizon in C1 chemistry: Breaking the selectivity limitation in transformation of syngas and hydrogenation of CO2 into hydrocarbon chemicals and fuels. Chem. Soc. Rev. 2019, 48, 3193–3228. [Google Scholar] [CrossRef] [PubMed]
- Spath, P.L.; Dayton, D.C. Preliminary Screening—Technical and Economic Assessment of Synthesis Gas to Fuels and Chemicals with Emphasis on the Potential for Biomass-Derived Syngas; National Renewable Energy Lab: Golden, CO, USA, 2003. [Google Scholar] [CrossRef]
- König, D.H.; Baucks, N.; Dietrich, R.-U.; Wörner, A. Simulation and evaluation of a process concept for the generation of synthetic fuel from CO2 and H2. Energy 2015, 91, 833–841. [Google Scholar] [CrossRef]
- Adelung, S.; Maier, S.; Dietrich, R.-U. Impact of the reverse water-gas shift operating conditions on the Power-to-Liquid process efficiency. Sustain. Energy Technol. Assess. 2021, 43, 100897. [Google Scholar] [CrossRef]
- Markowitsch, C.; Lehner, M.; Kitzweger, J.; Haider, W.; Ivanovici, S.; Unfried, M.; Maly, M. C2PAT-Carbon to Product Austria. In Proceedings of the ENINNOV 2022: 17. Symposium Energieinnovation-Future of Energy-Innovationen für Eine Klimaneutrale Zukunft, Online, 16–18 February 2022; pp. 284–285. Available online: https://www.nefi.at/de/news-detail/eninnov2022-17-symposium-energieinnovation (accessed on 26 July 2023).
- Ostadi, M.; Rytter, E.; Hillestad, M. Evaluation of kinetic models for Fischer–Tropsch cobalt catalysts in a plug flow reactor. Chem. Eng. Res. Des. 2016, 114, 236–246. [Google Scholar] [CrossRef]
- Wang, D.; Gu, Y.; Chen, Q.; Tang, Z. Direct conversion of syngas to alpha olefins via Fischer–Tropsch synthesis: Process development and comparative techno-economic-environmental analysis. Energy 2023, 263, 125991. [Google Scholar] [CrossRef]
- Li, M.; Zhao, W.; Xu, Y.; Zhao, Y.; Yang, K.; Tao, W.; Xiao, J. Comprehensive Life Cycle Evaluation of Jet Fuel from Biomass Gasification and Fischer–Tropsch Synthesis Based on Environmental and Economic Performances. Ind. Eng. Chem. Res. 2019, 58, 19179–19188. [Google Scholar] [CrossRef]
- Bouchy, C.; Hastoy, G.; Guillon, E.; Martens, J. Fischer-Tropsch waxes upgrading via hydrocracking and selective hydroisomerization. Oil Gas Sci. Technol. Rev. De L’ifp 2009, 64, 91–112. [Google Scholar] [CrossRef]
- Regali, F. Hydroconversion of Model Fischer-Tropsch Wax over Noble Metal/Silica-Alumina Catalysts; KTH Royal Institute of Technology: Stockholm, Sweden, 2013; Available online: https://www.diva-portal.org/smash/record.jsf?pid=diva2%3A653933&dswid=2784 (accessed on 28 July 2022).
- Sun, C.; Zhan, T.; Pfeifer, P.; Dittmeyer, R. Influence of Fischer-Tropsch synthesis (FTS) and hydrocracking (HC) conditions on the product distribution of an integrated FTS-HC process. Chem. Eng. J. 2017, 310, 272–281. [Google Scholar] [CrossRef]
- Pellegrini, L.; Locatelli, S.; Rasella, S.; Bonomi, S.; Calemma, V. Modeling of Fischer–Tropsch products hydrocracking. Chem. Eng. Sci. 2004, 59, 4781–4787. [Google Scholar] [CrossRef]
- Hense, J.; Bachmann, M.; Polte, L.; von der Aßen, N.; Jupke, A. Integrated Process Design and Life Cycle Assessment of Carbon Monoxide Provision from Basic Oxygen Furnace Gas. Chem. Ing. Tech. 2022, 94, 1524–1535. [Google Scholar] [CrossRef]
- Belloni, A. Industrial Gases Processing; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar] [CrossRef]
- Iulianelli, A.; Pirola, C.; Comazzi, A.; Galli, F.; Manenti, F.; Basile, A. Water gas shift membrane reactors. In Membrane Reactors for Energy Applications and Basic Chemical Production; Elsevier: Amsterdam, The Netherlands, 2015; pp. 3–29. [Google Scholar] [CrossRef]
- Pal, D.B.; Chand, R.; Upadhyay, S.; Mishra, P. Performance of water gas shift reaction catalysts: A review. Renew. Sustain. Energy Rev. 2018, 93, 549–565. [Google Scholar] [CrossRef]
- Kušar, H.; Hočevar, S.; Levec, J. Kinetics of the water–gas shift reaction over nanostructured copper–ceria catalysts. Appl. Catal. B Environ. 2006, 63, 194–200. [Google Scholar] [CrossRef]
- Wang, J.; Chen, X.; Du, T.; Liu, L.; Webley, P.A.; Li, G.K. Hydrogen production from low pressure coke oven gas by vacuum pressure swing adsorption. Chem. Eng. J. 2023, 472, 144920. [Google Scholar] [CrossRef]
- Brandão, M.; Heijungs, R.; Cowie, A.R. On quantifying sources of uncertainty in the carbon footprint of biofuels: Crop/feedstock, LCA modelling approach, land-use change, and GHG metrics. Biofuel Res. J. 2022, 9, 1608–1616. [Google Scholar] [CrossRef]
- Weidema, B.P. The integration of economic and social aspects in life cycle impact assessment. Int. J. Life Cycle Assess. 2006, 11, 89–96. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Rincón, L.; Vilariño, V.; Pérez, G.; Castell, A. Life cycle assessment (LCA) and life cycle energy analysis (LCEA) of buildings and the building sector: A review. Renew. Sustain. Energy Rev. 2014, 29, 394–416. [Google Scholar] [CrossRef]
- Cudjoe, D.; Han, M.S.; Nandiwardhana, A.P. Electricity generation using biogas from organic fraction of municipal solid waste generated in provinces of China: Techno-economic and environmental impact analysis. Fuel Process. Technol. 2020, 203, 106381. [Google Scholar] [CrossRef]
- Schrijvers, D.L.; Loubet, P.; Sonnemann, G. Critical review of guidelines against a systematic framework with regard to consistency on allocation procedures for recycling in LCA. Int. J. Life Cycle Assess. 2016, 21, 994–1008. [Google Scholar] [CrossRef]
- Ren, L.; Zhou, S.; Ou, X. Life-cycle energy consumption and greenhouse-gas emissions of hydrogen supply chains for fuel-cell vehicles in China. Energy 2020, 209, 118482. [Google Scholar] [CrossRef]
- Jun, L.; Peiwang, Z.; Hui, W.; Xiaolong, Q. Flexible Modification Technology and Application Prospect of Thermal Power Unit Based on High Temperature Molten Salt Heat Storage. South Energy Constr. 2021, 8, 63–70. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, L.; Hang, B.; Gong, C. Simulation of the Performance of a Centrifugal Chiller. J. Refrig. 2023, 44, 74–80. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, H.; Liang, J.; Zhang, Z. Performance research on R410a two-stage refrigeration cycle. Cryog. Supercond. 2022, 50, 52–57. [Google Scholar] [CrossRef]
- Shen, B.J.; Zhang, Y.B.; Guan, P. Analysis of Operating Condition and Control Plan about Ethylene Glycol Refrigeration System. J. Refrig. Technol. 2016, 1, 50–53. Available online: https://kns.cnki.net/kcms2/article/abstract?v=S5uBaE2M3OfnV2lxhiWVr8YH7JEH5n9_54tzE6oZ3KiEda6zrbpTYSVewnOM0JPRIig3X6WJgNm7fLCtXVliRE-VzzdA4dTXVkYFALwU3GyBl2oFvGdYXdNhHZciH9GDsKqo9EPuDQ0=&uniplatform=NZKPT&language=CHS (accessed on 10 February 2023).
- Bounaceur, R.; Lape, N.; Roizard, D.; Vallieres, C.; Favre, E. Membrane processes for post-combustion carbon dioxide capture: A parametric study. Energy 2006, 31, 2556–2570. [Google Scholar] [CrossRef]
- Zhao, L.; Ou, X.; Chang, S. Life-cycle greenhouse gas emission and energy use of bioethanol produced from corn stover in China: Current perspectives and future prospectives. Energy 2016, 115, 303–313. [Google Scholar] [CrossRef]
- Jiao, J.; Li, J.; Bai, Y. Uncertainty analysis in the life cycle assessment of cassava ethanol in China. J. Clean. Prod. 2019, 206, 438–451. [Google Scholar] [CrossRef]
- Yu, S.; Tao, J. Economic, energy and environmental evaluations of biomass-based fuel ethanol projects based on life cycle assessment and simulation. Appl. Energy 2009, 86, S178–S188. [Google Scholar] [CrossRef]
- Tu, Q.; McDonnell, B.E. Monte Carlo analysis of life cycle energy consumption and greenhouse gas (GHG) emission for biodiesel production from trap grease. J. Clean. Prod. 2016, 112, 2674–2683. [Google Scholar] [CrossRef]
- Lyu, H.; Zhang, J.; Zhai, Z.; Feng, Y.; Geng, Z. Life cycle assessment for bioethanol production from whole plant cassava by integrated process. J. Clean. Prod. 2020, 269, 121902. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, C.; Tian, H.; Li, L.; Wang, X.; Qiu, T. Environmental and techno-economic analyses of bio-jet fuel produced from jatropha and castor oilseeds in China. Int. J. Life Cycle Assess. 2021, 26, 1071–1084. [Google Scholar] [CrossRef]
- Fagerström, A.; Grahn, D.; Lundberg, S.; Poulikidou, S.; Rydberg, T.; Lewrén, A.; Martin, M.; Anderson, S.; Hansson, J.; Hjort, A. Large Scale Bio Electro Jet Fuel Production Integration at CHP-Plant in Östersund, Sweden; DIVA: Brixe, Italy, 2021; Available online: https://www.diva-portal.org/smash/record.jsf?pid=diva2%3A1552218&dswid=-1895 (accessed on 10 February 2024).
- Lai, Y.Y.; Karakaya, E.; Björklund, A. Employing a socio-technical system Approach in prospective life cycle assessment: A case of large-scale Swedish sustainable aviation fuels. Front. Sustain. 2022, 3, 912676. [Google Scholar] [CrossRef]
- Zhang, J.; Yoo, E.; Davison, B.H.; Liu, D.; Schaidle, J.A.; Tao, L.; Li, Z. Towards cost-competitive middle distillate fuels from ethanol within a market-flexible biorefinery concept. Green Chem. 2021, 23, 9534–9548. [Google Scholar] [CrossRef]
- Aghbashlo, M.; Hosseinzadeh-Bandbafha, H.; Shahbeik, H.; Tabatabaei, M. The role of sustainability assessment tools in realizing bioenergy and bioproduct systems. Biofuel Res. J. 2022, 9, 1697–1706. [Google Scholar] [CrossRef]







| Component (mol%) | BOFG | COG |
|---|---|---|
| H2 | 1 | 56.7 |
| CO | 55 | 7 |
| CO2 | 18 | 3 |
| CH4 | - | 26 |
| N2 | 26 | 7.3 |
| Transportation Modes | Fuel Mix | Fuel Economy (km/L) |
|---|---|---|
| Heavy Heavy-Duty Truck | Diesel (72%), Gasoline (28%) | 1.2 |
| Medium Heavy-Duty Truck | Diesel (72%), Gasoline (28%) | 3.27 |
| Description of Data | Unit | BOFG/ETJ | COG/ETJ | (COG + BOFG)/ETJ | BOFG/FTJ | COG/FTJ | (COG + BOFG)/FTJ |
|---|---|---|---|---|---|---|---|
| Input | |||||||
| BOFG | g | 464.5 | 0 | 112.2 | 611.9 | 0 | 201.9 |
| COG | g | 0 | 65.2 | 78.43 | 0 | 74.94 | 141.1 |
| H2O | g | 72.75 | 0 | 7.6 | 129 | 0 | 0 |
| CO2 | g | 0 | 84.5 | 0 | 0 | 56.83 | 0 |
| Electricity | KJ | 164.3 | 128.4 | 94.33 | 352.6 | 313.4 | 303 |
| Coal | KJ | 746.6 | 560.4 | 504.5 | 2078 | 106.8 | 106.8 |
| Natural gas | KJ | 663.6 | 1130 | 904.4 | 1848 | 687.5 | 94.94 |
| Output | |||||||
| Jet fuel | MJ | 1 | 1 | 1 | 1 | 1 | 1 |
| Gasoline yield | KJ | 0 | 0 | 0 | 161 | 158.8 | 160 |
| Diesel yield | KJ | 398.3 | 398.3 | 398.3 | 593.5 | 593.1 | 593 |
| Electricity yield | KJ | 125 | 273.5 | 556.3 | 122.8 | 153 | 1145 |
| Low-pressure steam | KJ | 156.8 | 156.8 | 156.8 | 1354 | 946.1 | 745.1 |
| CO2, direct 1 | g | 387.4 | 81.07 | 134.1 | 502.6 | 29.34 | 282.6 |
| Property | Unit | Properties for Blendstock [8] | ETJ | FTJ |
|---|---|---|---|---|
| Net heating value | MJ/kg | 42.8 | 44.03 | 44.12 |
| Viscosity | cP | 8 max | 2.27 | 1.46 |
| Flash point | °C | 38 min | 82.15 | 46.16 |
| boiling point | °C | 150–300 | 250 | 216 |
| Mass Density (15 °C) | kg/m3 | 730–770 | 756.9 | 741.1 |
| Variable Name | Distribution | Unit | Parameters |
|---|---|---|---|
| Raw Material LCA | Triangular | % | Min: 45 Max: 55 Mode: 50 |
| GHG emissions per unit of MJ electrical energy | Triangular | g CO2e/MJ Electricity | Min: 183.85 Max: 224.71 Mode: 204.28 |
| CHP electric efficiency | Triangular | % | Min: 31.5 Max: 38.5 Mode: 35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, L.; Wang, X.; Yang, W.; Lv, J. Life Cycle Assessment of Aviation Fuel Production from Steel-Industry Off-Gas. Processes 2024, 12, 579. https://doi.org/10.3390/pr12030579
Guo L, Wang X, Yang W, Lv J. Life Cycle Assessment of Aviation Fuel Production from Steel-Industry Off-Gas. Processes. 2024; 12(3):579. https://doi.org/10.3390/pr12030579
Chicago/Turabian StyleGuo, Lin, Xiao Wang, Weili Yang, and Jing Lv. 2024. "Life Cycle Assessment of Aviation Fuel Production from Steel-Industry Off-Gas" Processes 12, no. 3: 579. https://doi.org/10.3390/pr12030579




