Seeking Optimal Extraction Method for Augmenting Hibiscus sabdariffa Bioactive Compounds and Antioxidant Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Flower Collection and Preparation
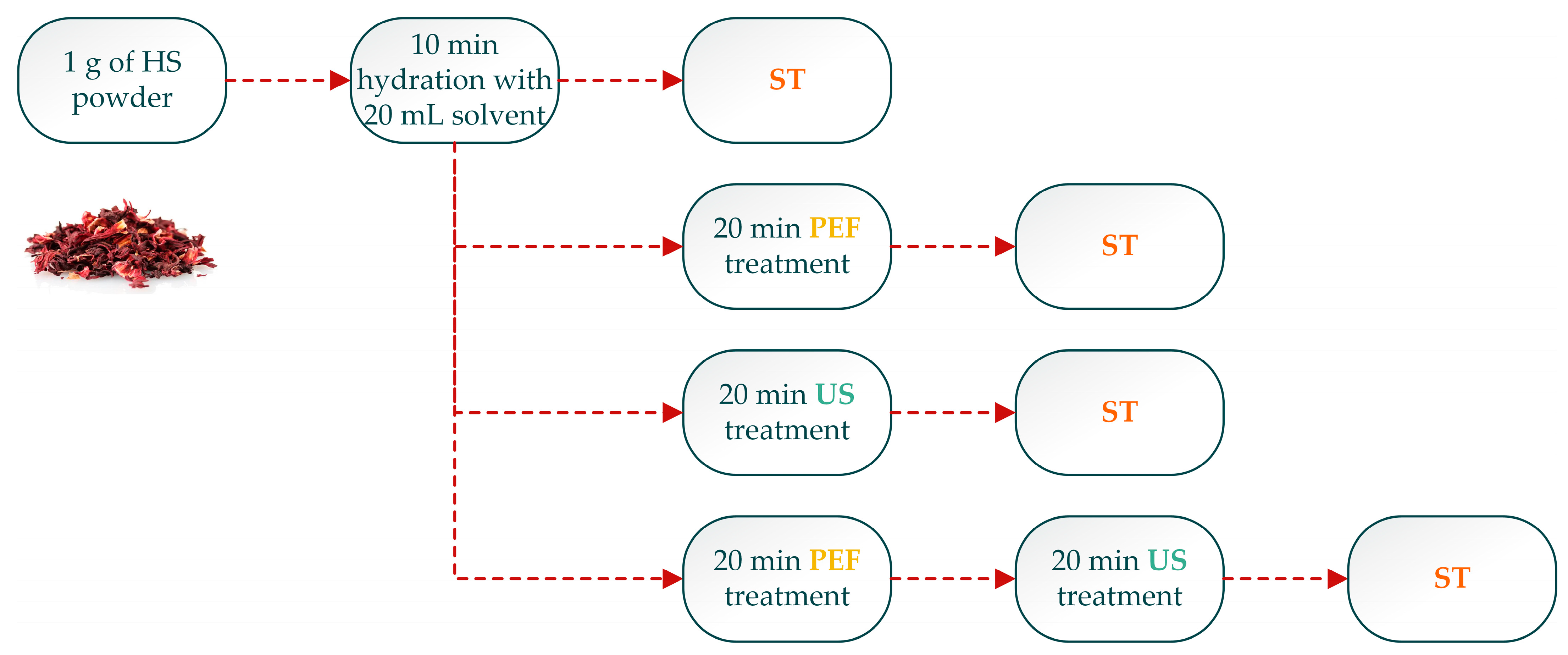
2.3. Extraction Procedure
2.4. Response Surface Methodology (RSM) Optimization of Extraction and Experiment Design
2.5. HPLC-Based Analysis of the Pelargonin Chloride Content and the Other Polyphenolic Compounds
2.6. Analyses of Extracts
2.7. Statistical Analysis
3. Results and Discussion
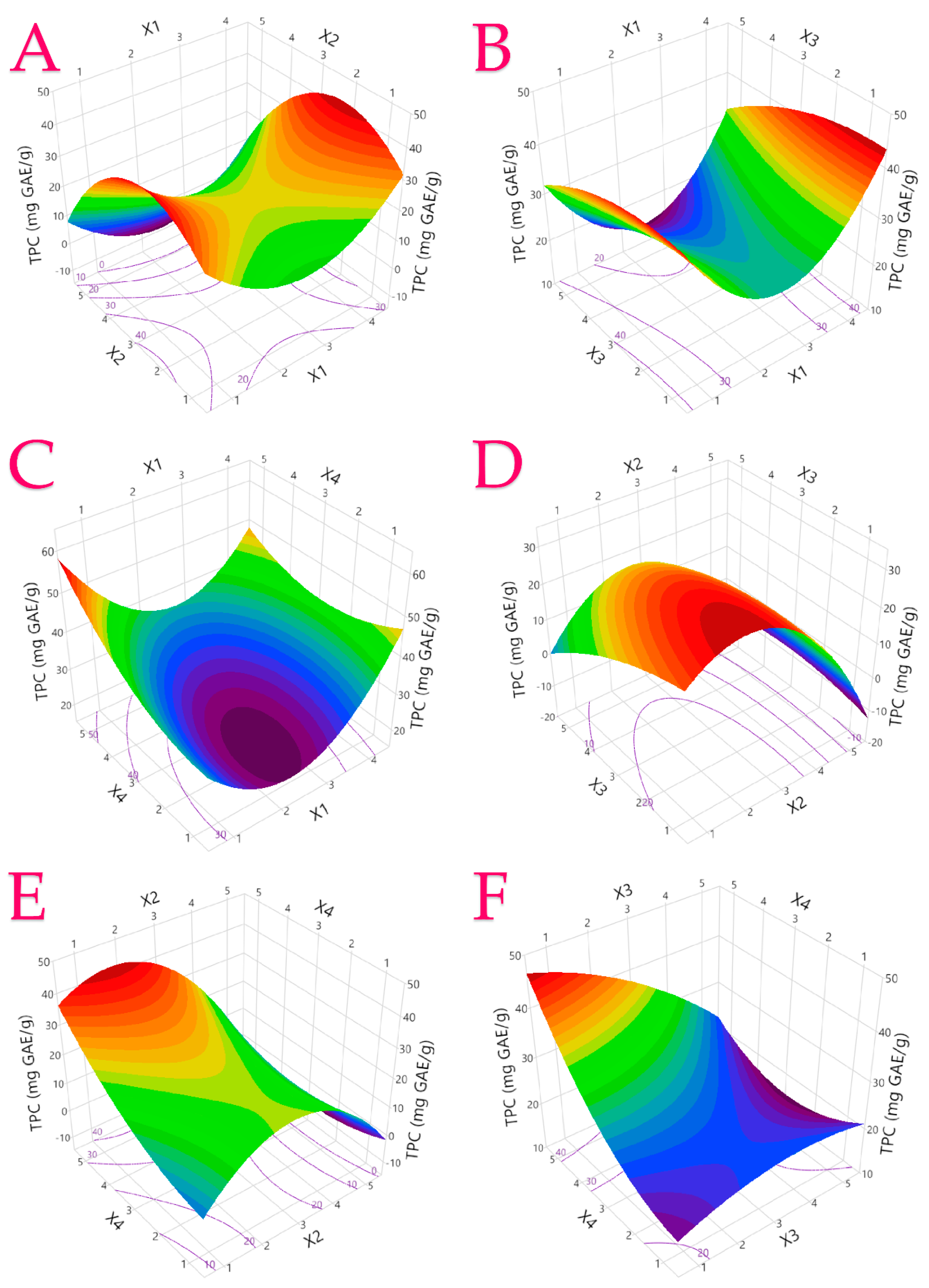
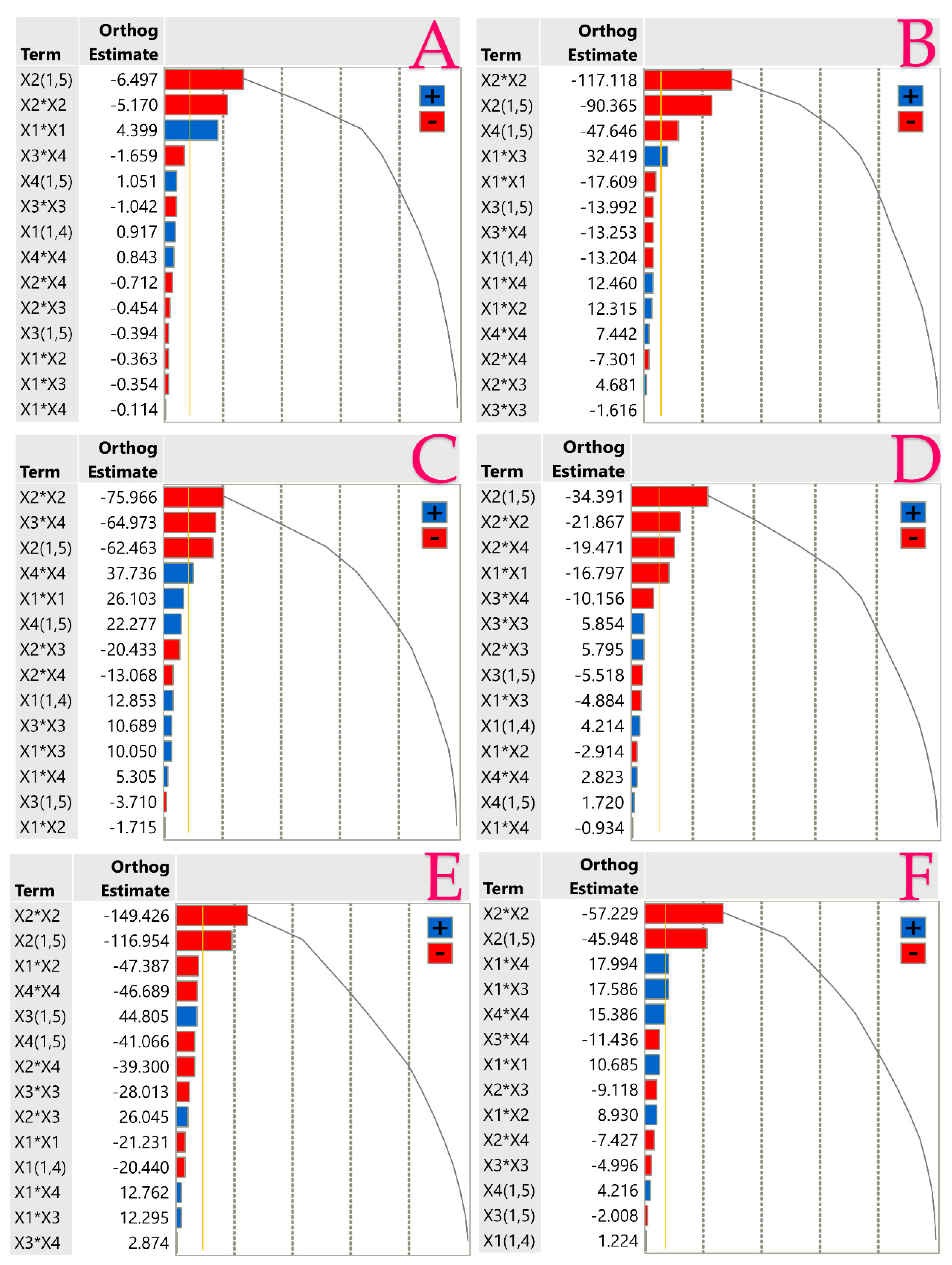
3.1. Extraction Optimization
3.2. Analysis of the Extracts
3.2.1. TPC and TAC of the Extracts
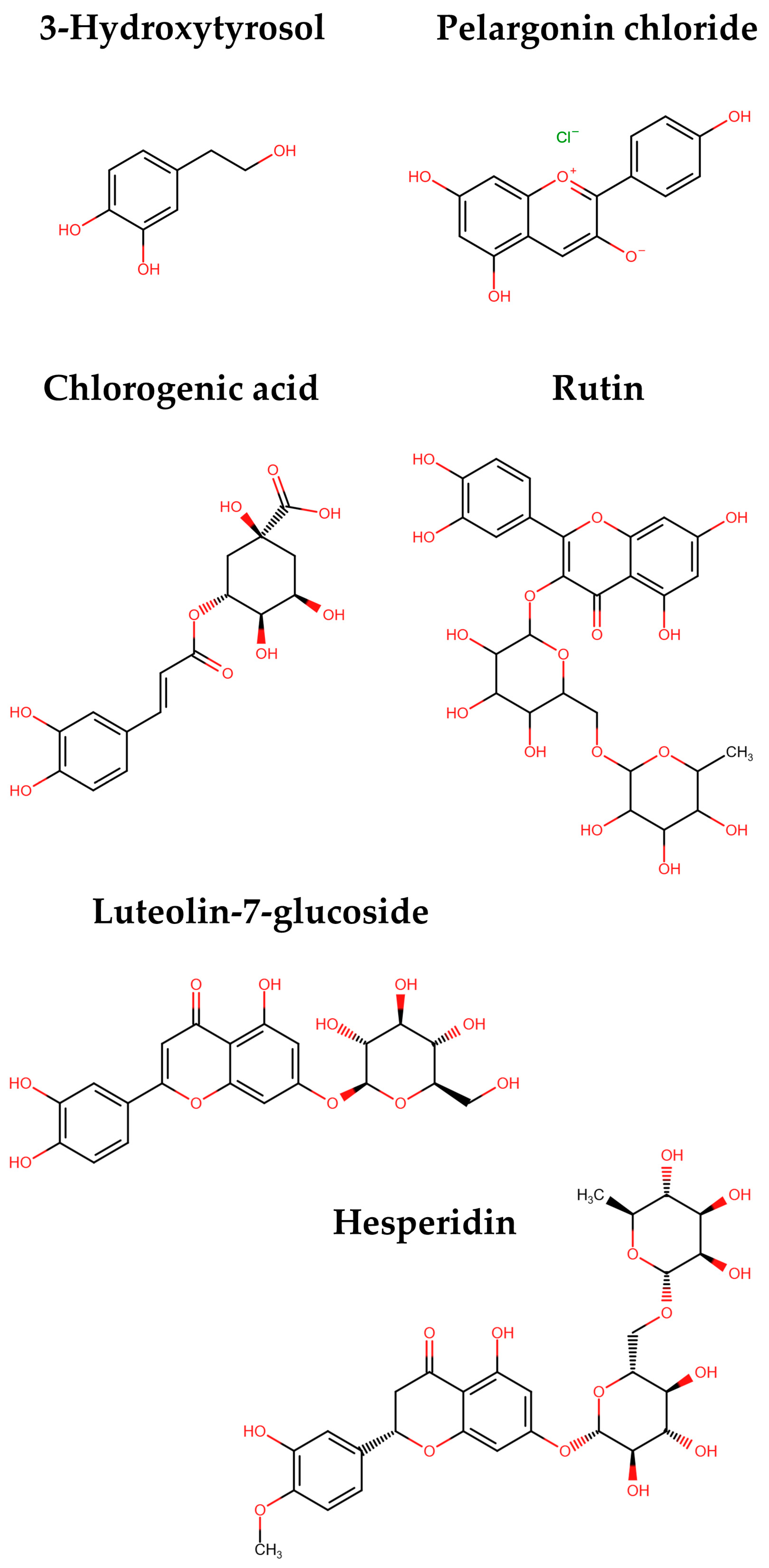
3.2.2. Polyphenolic Compounds of the Optimum Extract
3.2.3. Antioxidant Properties of the Extracts
3.2.4. TCC and AAC of the Extracts
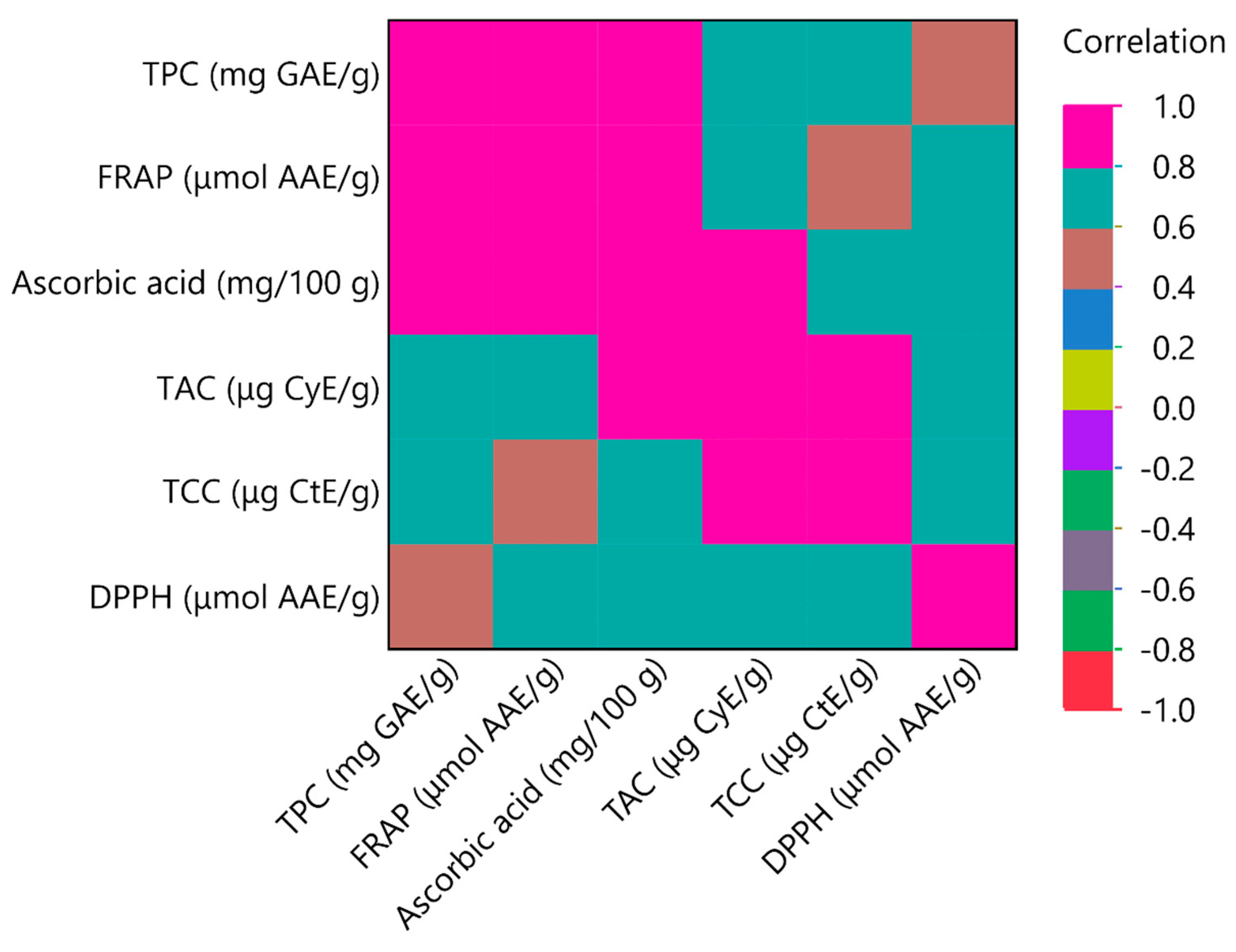
3.3. Principal Component Analysis (PCA) and Multivariate Correlation Analysis (MCA)
3.4. Partial Least Squares (PLS) Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bedi, P.S.; Bekele, M.; Gure, G. Phyto-Chemistry and Pharmacological Activities of Hibiscus sabdariffa Linn.—A Review. Int. Res. J. Pure Appl. Chem. 2020, 21, 41–54. [Google Scholar] [CrossRef]
- Solangi, A.H.; Junejo, S.; Siddiqui, A.A.; Arain, M.Y.; Ansari, M.A.; Talpur, U.A.; AhadKolach, A. Roselle (Hibiscus sabdariffa L.) a Multipurpose Medicinal Plant and Its Uses: A Review. Int. J. Biol. Res. 2017, 5, 21–24. [Google Scholar]
- Izquierdo-Vega, J.A.; Arteaga-Badillo, D.A.; Sánchez-Gutiérrez, M.; Morales-González, J.A.; Vargas-Mendoza, N.; Gómez-Aldapa, C.A.; Castro-Rosas, J.; Delgado-Olivares, L.; Madrigal-Bujaidar, E.; Madrigal-Santillán, E. Organic Acids from Roselle (Hibiscus sabdariffa L.)—A Brief Review of Its Pharmacological Effects. Biomedicines 2020, 8, 100. [Google Scholar] [CrossRef] [PubMed]
- Sarbini, D.W.I.; Huriyati, E.M.Y.; Sadewa, H.; Wahyuningsih, M.S.H. The Effect of Rosella (Hibiscus sabdariffa Linn) on Insulin Resistance in Patients with Type 2 Diabetes Mellitus: A Randomized Clinical Trial. Int. J. Pharm. Res. 2019, 11, 547–557. [Google Scholar]
- Abubakar, S.M.; Ukeyima, M.T.; Spencer, J.P.E.; Lovegrove, J.A. Acute Effects of Hibiscus sabdariffa Calyces on Postprandial Blood Pressure, Vascular Function, Blood Lipids, Biomarkers of Insulin Resistance and Inflammation in Humans. Nutrients 2019, 11, 341. [Google Scholar] [CrossRef]
- Nwachukwu, D.C.; Aneke, E.I.; Nwachukwu, N.Z.; Azubike, N.; Obika, L.F. Does Consumption of an Aqueous Extract of Hibiscus sabdariffa Affect Renal Function in Subjects with Mild to Moderate Hypertension? J. Physiol. Sci. 2017, 67, 227–234. [Google Scholar] [CrossRef]
- Sakhaei, R.; Nadjarzadeh, A.; Esmaeili, A.; Mohammadi, M.; Hemayati, R.; Reza, J.Z.; Mozaffari-Khosravi, H.; Ramezani-Jolfaie, N. Cardiovascular and Renal Effects of Hibiscus sabdariffa Linnaeus. in Patients with Diabetic Nephropathy: A Randomized, Double-Blind, Controlled Trial. J. Nutr. Food Secur. 2021, 6, 116–126. [Google Scholar] [CrossRef]
- Peter, E.L.; Rumisha, S.F.; Mashoto, K.O.; Minzi, O.M.; Mfinanga, S. Efficacy of Standardized Extract of Hibiscus sabdariffa L. (Malvaceae) in Improving Iron Status of Adults in Malaria Endemic Area: A Randomized Controlled Trial. J. Ethnopharmacol. 2017, 209, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Nwachukwu, D.C.; Aneke, E.I.; Nwachukwu, N.Z.; Azubike, N.; Obika, L.F.O. Thirst Perception in Mild to Moderate Hypertensive Nigerians Treated with Aqueous Extract of Hibiscus sabdariffa L. Afr. J. Pharm. Pharmacol. 2016, 10, 403–410. [Google Scholar] [CrossRef]
- Talal Al-Malki, A.; Abbas Ahmed Sindi, H.; Hussain Al-Qahtani, M. Regulation of CD36 Gene Expression by Hibiscus sabdariffa Tea Extracts to Affect the Atherosclerosis Biomarkers in Saudi Women. J. Food Nutr. Res. 2021, 9, 45–49. [Google Scholar] [CrossRef]
- Islam, M. Food and Medicinal Values of Roselle (Hibiscus sabdariffa L. Linne Malvaceae) Plant Parts: A Review. Open J. Nutr. Food Sci. 2019, 1, 14–20. [Google Scholar]
- Montalvo-González, E.; Villagrán, Z.; González-Torres, S.; Iñiguez-Muñoz, L.E.; Isiordia-Espinoza, M.A.; Ruvalcaba-Gómez, J.M.; Arteaga-Garibay, R.I.; Acosta, J.L.; González-Silva, N.; Anaya-Esparza, L.M. Physiological Effects and Human Health Benefits of Hibiscus sabdariffa: A Review of Clinical Trials. Pharmaceuticals 2022, 15, 464. [Google Scholar] [CrossRef]
- Sindi, H.A.; Marshall, L.J.; Morgan, M.R.A. Comparative Chemical and Biochemical Analysis of Extracts of Hibiscus sabdariffa. Food Chem. 2014, 164, 23–29. [Google Scholar] [CrossRef]
- Cisse, M.; Vaillant, F.; Acosta, O.; Claudie, D.M.; Dornier, M. Thermal Degradation Kinetics of Anthocyanins from Blood Orange, Blackberry, and Roselle Using the Arrhenius, Eyring, and Ball Models. J. Agric. Food Chem. 2009, 57, 6285–6291. [Google Scholar] [CrossRef]
- Wong, S.P.; Leong, L.P.; William Koh, J.H. Antioxidant Activities of Aqueous Extracts of Selected Plants. Food Chem. 2006, 99, 775–783. [Google Scholar] [CrossRef]
- Oboh, G.; Rocha, J.B.T. Antioxidant and Neuroprotective Properties of Sour Tea (Hibiscus sabdariffa, Calyx) and Green Tea (Camellia sinensis) on Some pro-Oxidant-Induced Lipid Peroxidation in Brain In Vitro. Food Biophys. 2008, 3, 382–389. [Google Scholar] [CrossRef]
- Farombi, E.O.; Fakoya, A. Free Radical Scavenging and Antigenotoxic Activities of Natural Phenolic Compounds in Dried Flowers of Hibiscus sabdariffa L. Mol. Nutr. Food Res. 2005, 49, 1120–1128. [Google Scholar] [CrossRef] [PubMed]
- Mohd-Esa, N.; Hern, F.S.; Ismail, A.; Yee, C.L. Antioxidant Activity in Different Parts of Roselle (Hibiscus sabdariffa L.) Extracts and Potential Exploitation of the Seeds. Food Chem. 2010, 122, 1055–1060. [Google Scholar] [CrossRef]
- Amin, A.; Hamza, A.A.; Kambal, A.; Daoud, S. Herbal Extracts Counteract Cisplatin-Mediated Cell Death in Rat Testis. Asian J. Androl. 2008, 10, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Raj, B.; John, S.; Chandrakala, V.; Kumari, G.H. Green Extraction Techniques for Phytoconstituents from Natural Products. In Medicinal Plants; Kumar, S., Ed.; IntechOpen: Rijeka, Croatia, 2022. [Google Scholar]
- Picot-Allain, C.; Mahomoodally, M.F.; Ak, G.; Zengin, G. Conventional versus Green Extraction Techniques—A Comparative Perspective. Curr. Opin. Food Sci. 2021, 40, 144–156. [Google Scholar] [CrossRef]
- Romano, R.; De Luca, L.; Aiello, A.; Rossi, D.; Pizzolongo, F.; Masi, P. Bioactive Compounds Extracted by Liquid and Supercritical Carbon Dioxide from Citrus Peels. Int. J. Food Sci. Technol. 2022, 57, 3826–3837. [Google Scholar] [CrossRef]
- Putra, N.R.; Yustisia, Y.; Heryanto, R.B.; Asmaliyah, A.; Miswarti, M.; Rizkiyah, D.N.; Yunus, M.A.C.; Irianto, I.; Qomariyah, L.; Rohman, G.A.N. Advancements and Challenges in Green Extraction Techniques for Indonesian Natural Products: A Review. S. Afr. J. Chem. Eng. 2023, 46, 88–98. [Google Scholar] [CrossRef]
- Redondo, D.; Venturini, M.E.; Luengo, E.; Raso, J.; Arias, E. Pulsed Electric Fields as a Green Technology for the Extraction of Bioactive Compounds from Thinned Peach By-Products. Innov. Food Sci. Emerg. Technol. 2018, 45, 335–343. [Google Scholar] [CrossRef]
- Somwongin, S.; Sirilun, S.; Chantawannakul, P.; Anuchapreeda, S.; Yawootti, A.; Chaiyana, W. Ultrasound-Assisted Green Extraction Methods: An Approach for Cosmeceutical Compounds Isolation from Macadamia integrifolia Pericarp. Ultrason. Sonochem. 2023, 92, 106266. [Google Scholar] [CrossRef]
- Otero, P.; Quintana, S.E.; Reglero, G.; Fornari, T.; García-Risco, M.R. Pressurized Liquid Extraction (PLE) as an Innovative Green Technology for the Effective Enrichment of Galician Algae Extracts with High Quality Fatty Acids and Antimicrobial and Antioxidant Properties. Mar. Drugs 2018, 16, 156. [Google Scholar] [CrossRef]
- Llompart, M.; Garcia-Jares, C.; Celeiro, M.; Dagnac, T. Microwave-Assisted Extraction. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Worsfold, P., Poole, C., Townshend, A., Miró, M., Eds.; Elsevier: Oxford, UK, 2018; pp. 67–77. ISBN 978-0-08-101984-9. [Google Scholar]
- Singh, S.; Verma, D.K.; Thakur, M.; Tripathy, S.; Patel, A.R.; Shah, N.; Utama, G.L.; Srivastav, P.P.; Benavente-Valdés, J.R.; Chávez-González, M.L.; et al. Supercritical Fluid Extraction (SCFE) as Green Extraction Technology for High-Value Metabolites of Algae, Its Potential Trends in Food and Human Health. Food Res. Int. 2021, 150, 110746. [Google Scholar] [CrossRef]
- Koina, I.M.; Sarigiannis, Y.; Hapeshi, E. Green Extraction Techniques for the Determination of Active Ingredients in Tea: Current State, Challenges, and Future Perspectives. Separations 2023, 10, 121. [Google Scholar] [CrossRef]
- Kunhermanti, D.; Mahfud, M. Optimization of Peppermint (Mentha Piperita L.) Extraction Using Solvent-Free Microwave Green Technology. Adv. Food Sci. Sustain. Agric. Agroindustrial Eng. 2023, 33–40. [Google Scholar]
- Hasni, S.; Riguene, H.; Rigane, G.; Ghazghazi, H.; Salem, R.B.; Cifuentes, A.; Mendiola, J.A.; Ibáñez, E. Extraction of Neuroprotective Compounds from Eucalyptus Leaves Using Green Technologies. Ternary Mixture Design. In Proceedings of the 19th European Meeting on Supercritical Fluids, Budapest, Hungary, 21–24 May 2023. [Google Scholar]
- Santos, L.G.; Martins, V.G. Optimization of the Green Extraction of Polyphenols from the Edible Flower Clitoria Ternatea by High-Power Ultrasound: A Comparative Study with Conventional Extraction Techniques. J. Appl. Res. Med. Aromat. Plants 2023, 34, 100458. [Google Scholar] [CrossRef]
- Haida, Z.; Ab Ghani, S.; Juju Nakasha, J.; Hakiman, M. Determination of Experimental Domain Factors of Polyphenols, Phenolic Acids and Flavonoids of Lemon (Citrus Limon) Peel Using Two-Level Factorial Design. Saudi J. Biol. Sci. 2022, 29, 574–582. [Google Scholar] [CrossRef]
- Chatzimitakos, T.; Athanasiadis, V.; Kotsou, K.; Bozinou, E.; Lalas, S.I. Response Surface Optimization for the Enhancement of the Extraction of Bioactive Compounds from Citrus limon Peel. Antioxidants 2023, 12, 1605. [Google Scholar] [CrossRef]
- Bozinou, E.; Lakka, A.; Poulianiti, K.; Lalas, S.; Makris, D.P. Cyclodextrins as High-Performance Green Co-Solvents in the Aqueous Extraction of Polyphenols and Anthocyanin Pigments from Solid Onion Waste. Eur. Food Res. Technol. 2021, 247, 2831–2845. [Google Scholar] [CrossRef]
- Shehata, E.; Grigorakis, S.; Loupassaki, S.; Makris, D.P. Extraction Optimisation Using Water/Glycerol for the Efficient Recovery of Polyphenolic Antioxidants from Two Artemisia Species. Sep. Purif. Technol. 2015, 149, 462–469. [Google Scholar] [CrossRef]
- Ayour, J.; Alahyane, A.; Harrak, H.; Neffa, M.; Taourirte, M.; Benichou, M. Assessment of Nutritional, Technological, and Commercial Apricot Quality Criteria of the Moroccan Cultivar “Maoui” Compared to Introduced Spanish Cultivars “Canino” and “Delpatriarca” towards Suitable Valorization. J. Food Qual. 2021, 2021, 6679128. [Google Scholar] [CrossRef]
- Dani, H.M.; Jagota, S.K. A New Calorimetric Technique for the Estimation of Vitamin C Using Folin Phenol Reagent. Anal. Biochem. 1982, 127, 178–182. [Google Scholar]
- Ghosh, P.; Priyadarshini, S.; Munshi, M.; Beula, P.; Atthoti, L.; Kruthi, G.S.; Sravan, B.S.; Irshaan, S.; Rana, S.S.; Abdullah, S. Physicochemical Characterization and Mass Modelling of Roselle (Hibiscus sabdariffa L.) Calyx and Seeds. Food Chem. Adv. 2023, 3, 100400. [Google Scholar] [CrossRef]
- Uddin, R.; Saha, M.R.; Subhan, N.; Hossain, H.; Jahan, I.A.; Akter, R.; Alam, A. HPLC-Analysis of Polyphenolic Compounds in Gardenia Jasminoides and Determination of Antioxidant Activity by Using Free Radical Scavenging Assays. Adv. Pharm. Bull. 2014, 4, 273–281. [Google Scholar] [CrossRef]
- Kuntić, V.; Pejić, N.; Mićić, S. Direct Spectrophotometric Determination of Hesperidin in Pharmaceutical Preparations. Acta Chim. Slov. 2012, 59, 436–441. [Google Scholar]
- Miralles, P.; Chisvert, A.; Salvador, A. Determination of Hydroxytyrosol and Tyrosol by Liquid Chromatography for the Quality Control of Cosmetic Products Based on Olive Extracts. J. Pharm. Biomed. Anal. 2015, 102, 157–161. [Google Scholar] [CrossRef]
- Lee, J.; Rennaker, C.; Wrolstad, R.E. Correlation of Two Anthocyanin Quantification Methods: HPLC and Spectrophotometric Methods. Food Chem. 2008, 110, 782–786. [Google Scholar] [CrossRef]
- Pauliuc, D.; Dranca, F.; Oroian, M. Antioxidant Activity, Total Phenolic Content, Individual Phenolics and Physicochemical Parameters Suitability for Romanian Honey Authentication. Foods 2020, 9, 306. [Google Scholar] [CrossRef]
- Kotsou, K.; Stoikou, M.; Athanasiadis, V.; Chatzimitakos, T.; Mantiniotou, M.; Sfougaris, A.I.; Lalas, S.I. Enhancing Antioxidant Properties of Prunus Spinosa Fruit Extracts via Extraction Optimization. Horticulturae 2023, 9, 942. [Google Scholar] [CrossRef]
- Pappas, V.M.; Lakka, A.; Palaiogiannis, D.; Athanasiadis, V.; Bozinou, E.; Ntourtoglou, G.; Makris, D.P.; Dourtoglou, V.G.; Lalas, S.I. Optimization of Pulsed Electric Field as Standalone “Green” Extraction Procedure for the Recovery of High Value-Added Compounds from Fresh Olive Leaves. Antioxidants 2021, 10, 1554. [Google Scholar] [CrossRef] [PubMed]
- Athanasiadis, V.; Chatzimitakos, T.; Bozinou, E.; Kotsou, K.; Palaiogiannis, D.; Lalas, S.I. Maximizing the Extraction of Bioactive Compounds from Diospyros kaki Peel through the Use of a Pulsed Electric Field and Ultrasound Extraction. Biomass 2023, 3, 422–440. [Google Scholar] [CrossRef]
- Wardy, W.; Saalia, F.K.; Steiner-Asiedu, M.; Budu, A.S.; Sefa-Dedeh, S. A Comparison of Some Physical, Chemical and Sensory Attributes of Three Pineapple (Ananas comosus) Varieties Grown in Ghana. Afr. J. Food Sci. 2009, 3, 22–25. [Google Scholar]
- Amaya-Cruz, D.; Peréz-Ramírez, I.F.; Pérez-Jiménez, J.; Nava, G.M.; Reynoso-Camacho, R. Comparison of the Bioactive Potential of Roselle (Hibiscus sabdariffa L.) Calyx and Its by-Product: Phenolic Characterization by UPLC-QTOF MSE and Their Anti-Obesity Effect in Vivo. Food Res. Int. 2019, 126, 108589. [Google Scholar] [CrossRef]
- Singh, M.; Thrimawithana, T.; Shukla, R.; Adhikari, B. Extraction and Characterization of Polyphenolic Compounds and Potassium Hydroxycitrate from Hibiscus sabdariffa. Future Foods 2021, 4, 100087. [Google Scholar] [CrossRef]
- dos Santos Martins, D.R.; Lescano, C.H.; Justo, A.F.O.; Vicente, J.M.; Santos, S.H.S.; Aguilar, C.M.; Borges, A.; Pires de Oliveira, I.; Sanjinez-Argandoña, E.J. Effect of Different Extraction Methods on Anthocyanin Content in Hibiscus sabdariffa L. and Their Antiplatelet and Vasorelaxant Properties. Plant Foods Hum. Nutr. 2023, 78, 342–350. [Google Scholar] [CrossRef]
- Amer, S.A.; Al-Khalaifah, H.S.; Gouda, A.; Osman, A.; Goda, N.I.A.; Mohammed, H.A.; Darwish, M.I.M.; Hassan, A.M.; Mohamed, S.K.A. Potential Effects of Anthocyanin-Rich Roselle (Hibiscus sabdariffa L.) Extract on the Growth, Intestinal Histomorphology, Blood Biochemical Parameters, and the Immune Status of Broiler Chickens. Antioxidants 2022, 11, 544. [Google Scholar] [CrossRef]
- Li, S.; Li, W.; Wang, C.; Wu, R.; Yin, R.; Kuo, H.-C.; Wang, L.; Kong, A.-N. Pelargonidin Reduces the TPA Induced Transformation of Mouse Epidermal Cells -Potential Involvement of Nrf2 Promoter Demethylation. Chem. Biol. Interact. 2019, 309, 108701. [Google Scholar] [CrossRef]
- Karasawa, K.; Otani, H. Anti-Allergic Properties of a Matured Fruit Extract of the Date Palm Tree (Phoenix dactylifera L.) in Mite-Sensitized Mice. J. Nutr. Sci. Vitaminol. 2012, 58, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Cao, G.; Prior, R.L. Oxygen Radical Absorbing Capacity of Anthocyanins. J. Agric. Food Chem. 1997, 45, 304–309. [Google Scholar] [CrossRef]
- Hapsari, B.W.; Manikharda; Setyaningsih, W. Methodologies in the Analysis of Phenolic Compounds in Roselle (Hibiscus sabdariffa L.): Composition, Biological Activity, and Beneficial Effects on Human Health. Horticulturae 2021, 7, 35. [Google Scholar] [CrossRef]
- Wang, J.; Cao, X.; Jiang, H.; Qi, Y.; Chin, K.L.; Yue, Y. Antioxidant Activity of Leaf Extracts from Different Hibiscus sabdariffa Accessions and Simultaneous Determination Five Major Antioxidant Compounds by LC-Q-TOF-MS. Molecules 2014, 19, 21226–21238. [Google Scholar] [CrossRef] [PubMed]
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Chlorogenic Acid: Recent Advances on Its Dual Role as a Food Additive and a Nutraceutical against Metabolic Syndrome. Molecules 2017, 22, 358. [Google Scholar] [CrossRef]
- Haider, K.; Haider, M.R.; Neha, K.; Yar, M.S. Free Radical Scavengers: An Overview on Heterocyclic Advances and Medicinal Prospects. Eur. J. Med. Chem. 2020, 204, 112607. [Google Scholar] [CrossRef]
- Li, X.; Huang, W.; Tan, R.; Xu, C.; Chen, X.; Li, S.; Liu, Y.; Qiu, H.; Cao, H.; Cheng, Q. The Benefits of Hesperidin in Central Nervous System Disorders, Based on the Neuroprotective Effect. Biomed. Pharmacother. 2023, 159, 114222. [Google Scholar] [CrossRef]
- Kumar, S.S.; Manoj, P.; Shetty, N.P.; Giridhar, P. Effect of Different Drying Methods on Chlorophyll, Ascorbic Acid and Antioxidant Compounds Retention of Leaves of Hibiscus sabdariffa L. J. Sci. Food Agric. 2015, 95, 1812–1820. [Google Scholar] [CrossRef]
- Cooperstone, J.L.; Schwartz, S.J. Recent Insights Into Health Benefits of Carotenoids. In Handbook on Natural Pigments in Food and Beverages; Carle, R., Schweiggert, R.M., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Cambridge, UK, 2016; pp. 473–497. ISBN 978-0-08-100371-8. [Google Scholar]
- Black, H.S.; Boehm, F.; Edge, R.; Truscott, T.G. The Benefits and Risks of Certain Dietary Carotenoids That Exhibit Both Anti- and Pro-Oxidative Mechanisms-A Comprehensive Review. Antioxidants 2020, 9, 264. [Google Scholar] [CrossRef]
- Doseděl, M.; Jirkovský, E.; Macáková, K.; Krčmová, L.K.; Javorská, L.; Pourová, J.; Mercolini, L.; Remião, F.; Nováková, L.; Mladěnka, P. Vitamin c—Sources, Physiological Role, Kinetics, Deficiency, Use, Toxicity, and Determination. Nutrients 2021, 13, 615. [Google Scholar] [CrossRef]
- Yin, X.; Chen, K.; Cheng, H.; Chen, X.; Feng, S.; Song, Y.; Liang, L. Chemical Stability of Ascorbic Acid Integrated into Commercial Products: A Review on Bioactivity and Delivery Technology. Antioxidants 2022, 11, 153. [Google Scholar] [CrossRef]
- Maganha, E.G.; da Costa Halmenschlager, R.; Rosa, R.M.; Henriques, J.A.P.; de Paula Ramos, A.L.L.; Saffi, J. Pharmacological Evidences for the Extracts and Secondary Metabolites from Plants of the Genus Hibiscus. Food Chem. 2010, 118, 1–10. [Google Scholar] [CrossRef]
- Enedina, R.; Luisa, M.; Iván, E.; Antonio, J.; Teresa, M. Free Radical Scavenging Properties and Their Relationship with Bioactive Compounds Content of Dehydrated Calyces of Roselle (Hibiscus sabdariffa L.). Afr. J. Agric. Res. 2015, 10, 1203–1210. [Google Scholar] [CrossRef]
- Wong, P.-K.; Yusof, S.; Ghazali, H.M.; Che Man, Y.B. Physico-chemical Characteristics of Roselle (Hibiscus sabdariffa L.). Nutr. Food Sci. 2002, 32, 68–73. [Google Scholar] [CrossRef]




| Independent Variables | Code Units | Coded Variable Level | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Technique | X1 | ST | PEF + ST | US + ST | PEF + US + ST | – |
| CEtOH (%, v/v) | X2 | 0 | 25 | 50 | 75 | 100 |
| t (min) | X3 | 30 | 60 | 90 | 120 | 150 |
| T (°C) | X4 | 20 | 35 | 50 | 65 | 80 |
| Design Point | Independent Variables | Responses | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | TPC 1 | TAC 2 | FRAP 3 | DPPH 4 | TCC 5 | AAC 6 | |
| 1 | 3 | 1 | 3 | 4 | 25.30 | 341.08 | 295.60 | 164.82 | 710.02 | 381.32 |
| 2 | 3 | 2 | 1 | 3 | 29.62 | 604.39 | 477.68 | 243.42 | 763.65 | 480.75 |
| 3 | 2 | 3 | 4 | 3 | 21.29 | 543.96 | 226.20 | 119.58 | 921.84 | 441.42 |
| 4 | 2 | 4 | 5 | 4 | 12.27 | 347.47 | 149.64 | 106.69 | 708.51 | 327.26 |
| 5 | 3 | 5 | 4 | 2 | 3.90 | 135.53 | 82.72 | 66.59 | 256.92 | 267.87 |
| 6 | 4 | 1 | 4 | 5 | 32.33 | 285.56 | 354.95 | 105.94 | 578.57 | 448.03 |
| 7 | 4 | 2 | 3 | 1 | 34.43 | 465.42 | 358.60 | 108.00 | 690.92 | 401.23 |
| 8 | 1 | 3 | 3 | 2 | 29.42 | 578.07 | 274.41 | 81.21 | 847.53 | 484.04 |
| 9 | 1 | 4 | 4 | 1 | 23.38 | 398.22 | 301.91 | 82.18 | 689.48 | 386.66 |
| 10 | 1 | 5 | 1 | 4 | 12.75 | 162.97 | 216.52 | 32.14 | 231.75 | 303.81 |
| 11 | 1 | 1 | 2 | 3 | 32.26 | 468.95 | 386.91 | 119.12 | 545.52 | 461.45 |
| 12 | 1 | 2 | 5 | 5 | 35.97 | 430.21 | 487.51 | 123.12 | 594.45 | 487.20 |
| 13 | 4 | 3 | 2 | 4 | 37.82 | 539.70 | 467.69 | 135.80 | 768.40 | 507.19 |
| 14 | 3 | 4 | 2 | 5 | 24.51 | 479.91 | 472.39 | 109.09 | 450.20 | 507.44 |
| 15 | 2 | 5 | 3 | 5 | 6.60 | 80.51 | 100.24 | 25.01 | 215.26 | 239.33 |
| 16 | 2 | 1 | 1 | 1 | 14.78 | 444.93 | 155.15 | 102.84 | 428.31 | 351.04 |
| 17 | 2 | 2 | 2 | 2 | 23.50 | 610.42 | 269.15 | 137.85 | 769.89 | 480.28 |
| 18 | 3 | 3 | 5 | 1 | 22.39 | 599.57 | 474.49 | 125.81 | 750.33 | 481.11 |
| 19 | 4 | 4 | 1 | 2 | 25.11 | 361.03 | 265.08 | 76.82 | 427.64 | 393.68 |
| 20 | 4 | 5 | 5 | 3 | 8.66 | 145.46 | 111.72 | 1.44 | 176.06 | 298.24 |
| Responses | Second-Order Polynomial Equations (Models) | R2 Predicted | R2 Adjusted | p-Value | Equation |
|---|---|---|---|---|---|
| TPC 1 | Y = 17.33 − 18.64X1 + 15.67X2 + 1.46X3 + 6.2X4 + 4.43X12 − 3.31X22 − 0.53X32 + 1.0X42 + 0.09X1X2 − 0.06X1X3 − 1.12X1X4 + 1.01X2X3 − 1.22X2X4 − 1.1X3X4 | 0.9738 | 0.9004 | 0.0050 | (2) |
| TAC 2 | Y = 294.78 − 66.1X1 + 466.69X2 − 128.22X3 + 14.9X4 − 16.9X12 − 93.75X22 + 2.13X32 + 8.85X42 + 15.6X1X2 + 28.93X1X3 − 0.13X1X4 + 11.21X2X3 − 12.1X2X4 − 8.57X3X4 | 0.9795 | 0.9221 | 0.0027 | (3) |
| FRAP 3 | Y = 71.14 − 120.69X1 + 316.19X2 − 151.96X3 + 120.44X4 + 26.76X12 − 60.63X22 + 13.76X32 + 44.87X42 + 8.92X1X2 + 15.58X1X3 − 31.57X1X4 + 32.22X2X3 − 40.81X2X4 − 41.59X3X4 | 0.9652 | 0.8676 | 0.0097 | (4) |
| DPPH 4 | Y = −192.21 + 141.65X1 + 107.58X2 − 79.76X3 + 107.46X4 − 14.19X12 − 18.06X22 + 3.26X32 + 3.36X42 − 10.9X1X2 − 0.22X1X3 − 13.91X1X4 + 20.1X2X3 − 17.83X2X4 − 7.57X3X4 | 0.9626 | 0.8580 | 0.0114 | (5) |
| TCC 5 | Y = −458.87 + 164.06X1 + 344.69X2 + 251.8X3 + 215.7X4 − 10.31X12 − 67.16X22 − 6.52X32 − 55.52X42 − 35.63X1X2 − 31.84X1X3 + 26.19X1X4 − 10.7X2X3 + 24.33X2X4 − 13.78X3X4 | 0.9636 | 0.8618 | 0.0107 | (6) |
| AAC 6 | Y = 376.88 − 137.83X1 + 313.01X2 − 95.12X3 + 4.76X4 + 0.03X12 − 54.98X22 − 1.87X32 + 18.3X42 + 17.99X1X2 + 28.4X1X3 − 2.75X1X4 + 5.54X2X3 − 22.93X2X4 − 4.29X3X4 | 0.9717 | 0.8925 | 0.0059 | (7) |
| Responses | Optimal Conditions | ||||
|---|---|---|---|---|---|
| Maximum Predicted Response | Technique (X1) | C (%, v/v) (X2) | t (min) (X3) | T (°C) (X4) | |
| TPC (mg GAE/g dw) | 41.17 ± 7.73 | PEF + US + ST (4) | 25 (2) | 60 (2) | 65 (4) |
| TAC (μg CyE/g dw) | 655.67 ± 113.56 | US + ST (3) | 50 (3) | 30 (1) | 65 (4) |
| FRAP (μmol AAE/g dw) | 603.11 ± 121.21 | US + ST (3) | 50 (3) | 30 (1) | 65 (4) |
| DPPH (μmol AAE/g dw) | 243.42 ± 46.76 | US + ST (3) | 25 (2) | 30 (1) | 50 (3) |
| TCC (μg CtE/g dw) | 944.36 ± 194.3 | ST (1) | 50 (3) | 120 (4) | 50 (3) |
| AAC (mg/100 g dw) | 561.11 ± 67.68 | US + ST (3) | 50 (3) | 30 (1) | 65 (4) |
| Variables | PLS Model Values | Experimental Values |
|---|---|---|
| TPC (mg GAE/g dw) | 36.56 | 40.49 ± 2.67 |
| TAC (μg CyE/g dw) | 681.96 | 642.18 ± 34.68 |
| FRAP (μmol AAE/g dw) | 690.18 | 697.1 ± 23.7 |
| DPPH (μmol AAE/g dw) | 227.12 | 216.14 ± 14.91 |
| TCC (μg CtE/g dw) | 781.14 | 676.86 ± 41.29 |
| AAC (mg/100 g dw) | 595.96 | 536.05 ± 15.55 |
| Polyphenolic Compound | Optimal Extract (mg/g dw) |
|---|---|
| 3-Hydroxytyrosol | 1.33 ± 0.05 |
| Pelargonin chloride | 34.63 ± 1.94 |
| Chlorogenic acid | 1.27 ± 0.09 |
| Rutin | 0.68 ± 0.03 |
| Luteolin-7-glucoside | 0.54 ± 0.03 |
| Hesperidin | 0.98 ± 0.06 |
| Total identified | 39.44 ± 2.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kourelatou, A.; Chatzimitakos, T.; Athanasiadis, V.; Kotsou, K.; Makrygiannis, I.; Bozinou, E.; Lalas, S.I. Seeking Optimal Extraction Method for Augmenting Hibiscus sabdariffa Bioactive Compounds and Antioxidant Activity. Processes 2024, 12, 581. https://doi.org/10.3390/pr12030581
Kourelatou A, Chatzimitakos T, Athanasiadis V, Kotsou K, Makrygiannis I, Bozinou E, Lalas SI. Seeking Optimal Extraction Method for Augmenting Hibiscus sabdariffa Bioactive Compounds and Antioxidant Activity. Processes. 2024; 12(3):581. https://doi.org/10.3390/pr12030581
Chicago/Turabian StyleKourelatou, Athanasia, Theodoros Chatzimitakos, Vassilis Athanasiadis, Konstantina Kotsou, Ioannis Makrygiannis, Eleni Bozinou, and Stavros I. Lalas. 2024. "Seeking Optimal Extraction Method for Augmenting Hibiscus sabdariffa Bioactive Compounds and Antioxidant Activity" Processes 12, no. 3: 581. https://doi.org/10.3390/pr12030581









