Enhancing Alkaline Protease Stability through Enzyme-Catalyzed Crosslinking and Its Application in Detergents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.1.1. Preparation of Stained Fabric
2.1.2. Preparation of Laundry Detergents
2.2. Alkaline Protease Activity Determination
2.3. Modification of Alkaline Protease
2.4. Optimal Temperature and Thermal Stability of Modified Alkaline Protease
2.5. Optimal pH of Modified Alkaline Protease
2.6. Stability of Modified Alkaline Protease in Washing Aids and Commercial Laundry Detergents
2.7. Evaluation of Washing Performance of Modified Alkaline Protease
2.7.1. Stain Removal Efficiency of Modified Alkaline Protease
2.7.2. Stain Removal Efficacy Characterization
2.7.3. Residual Element Analysis
2.8. Statistical Analysis
3. Results and Discussion
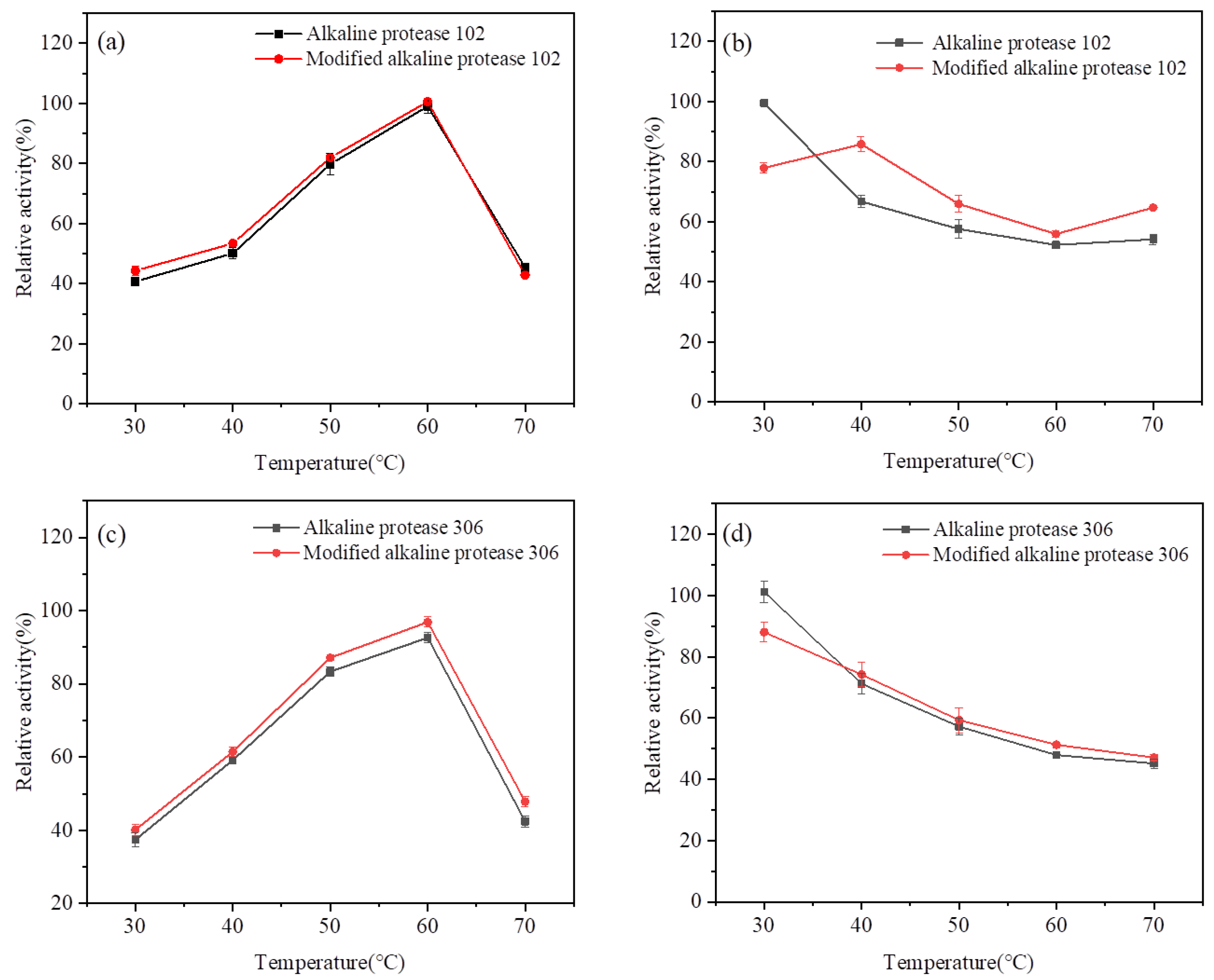
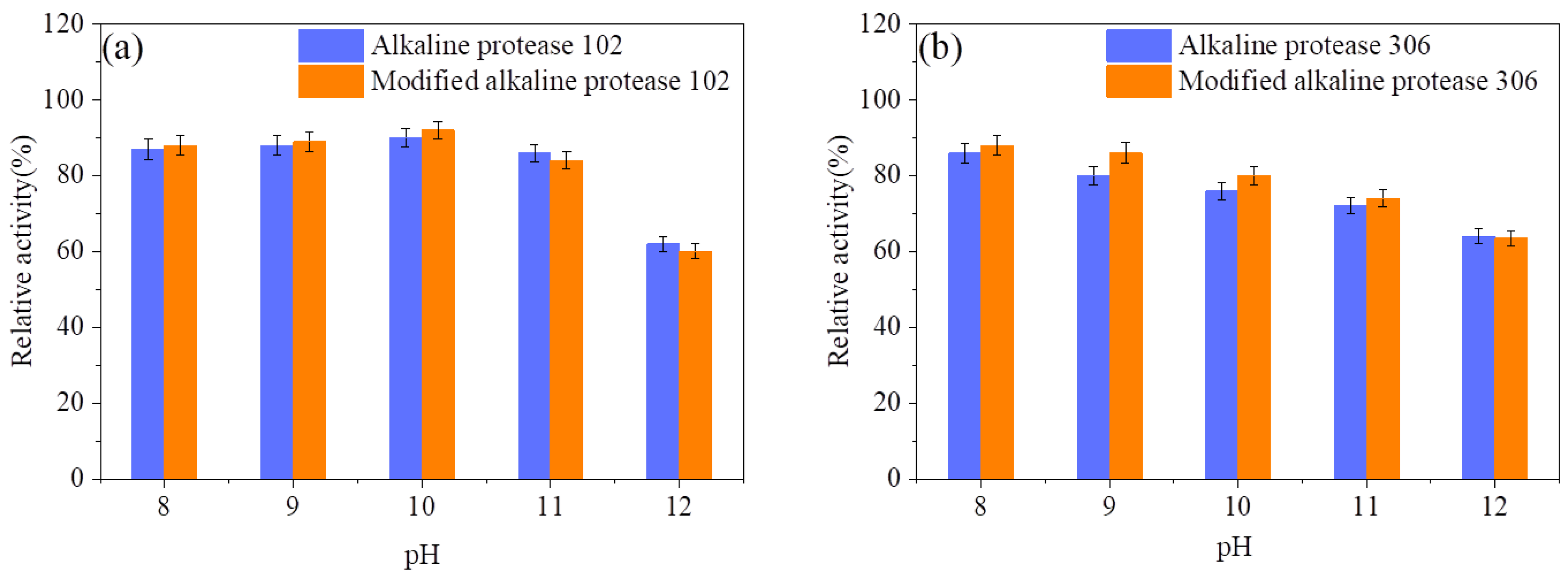
3.1. Optimal Temperature, Heat Stability, and Optimal pH of Modified Alkaline Protease
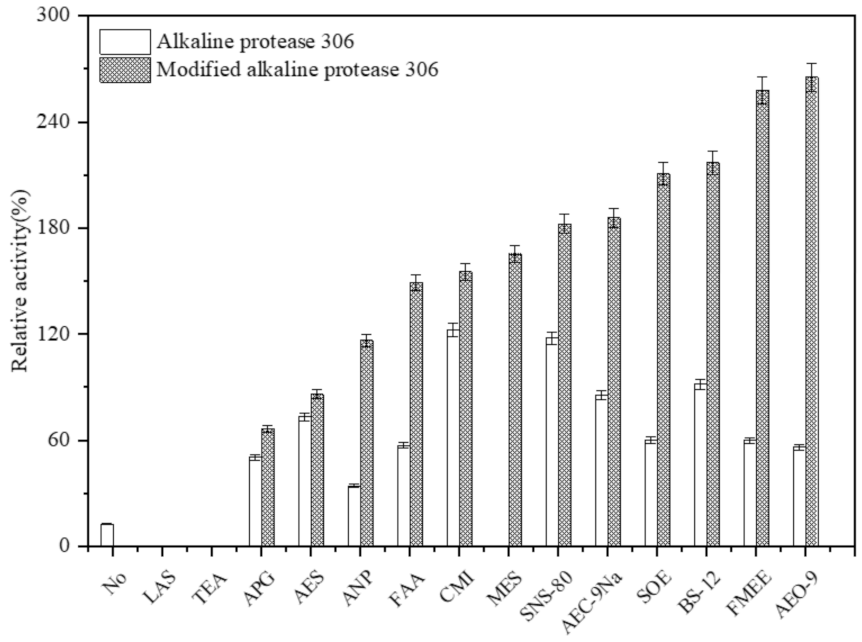
3.2. Stability of Modified Alkaline Protease in Laundry Detergent Additives
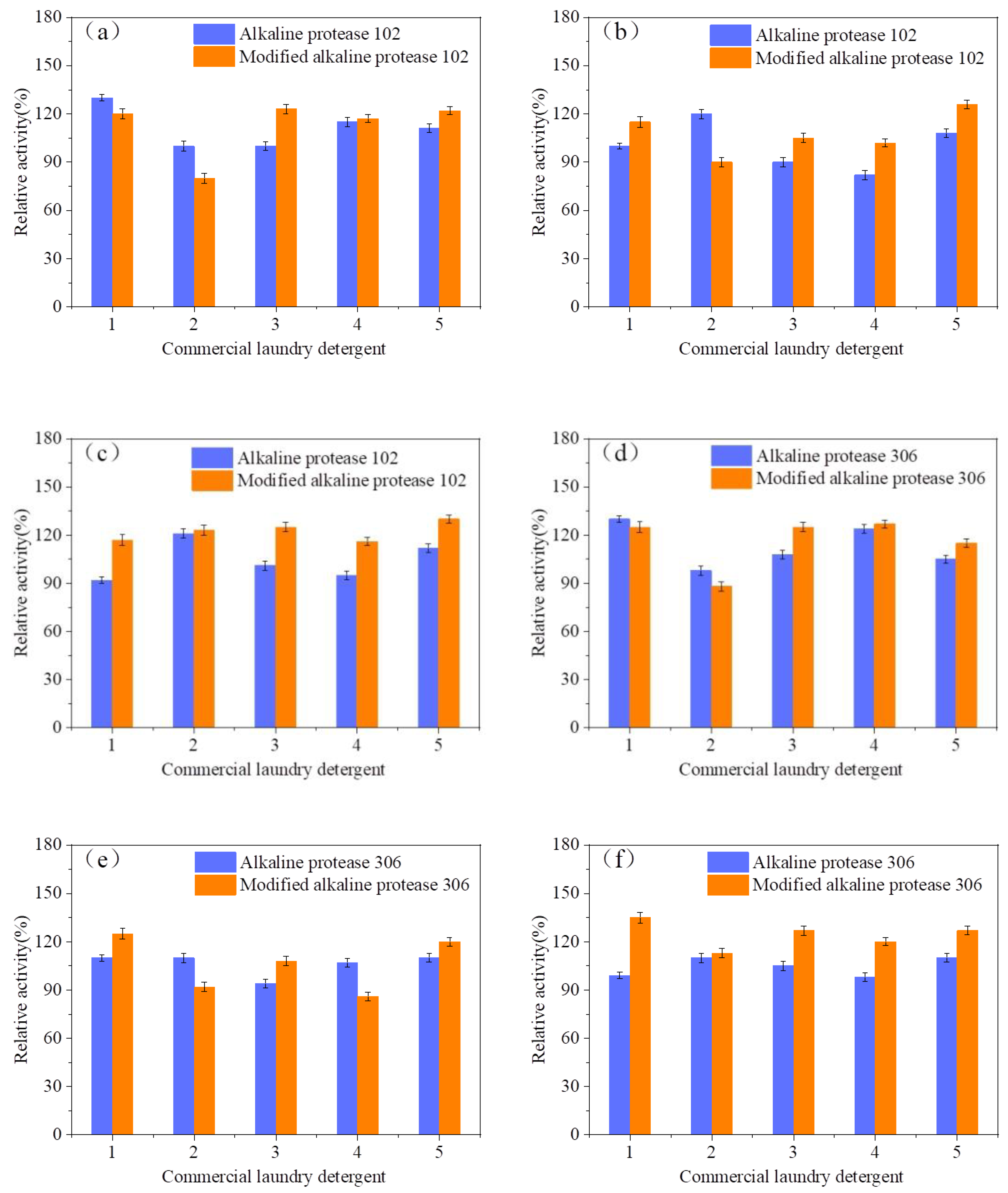
3.3. Stability of Modified Alkaline Protease in Commercial Laundry Detergents
3.4. Washing Performance Test of Modified Alkaline Protease
3.4.1. Stain Removal Performance of Modified Alkaline Protease
3.4.2. Characterization of Stain Removal Effect
3.4.3. Residual Element Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A


References
- Lu, G.Q.; Zhang, L.P. Study on application of protease in liquid detergent product. Mod. Chemother. Eng. 2010, 30 (Suppl. S2), 124–127. [Google Scholar]
- Han, M.G. The application of enzyme preparations provides a green and efficient solution for the cleaning of industrial textiles. Chin. Clean. Ind. 2015, 7, 28–31. [Google Scholar]
- Xie, S.O.; Luo, Y.; Zhang, L.P. The application of enzyme in pretreated petergent. Chin. Clean. Ind. 2018, 8, 43–47. [Google Scholar]
- Klibanov, A.M. Improving enzymes by using them in organic solvents. Nature 2001, 409, 241–246. [Google Scholar] [CrossRef]
- Li, L. Optimization of the detergent formulation by using alkaline protease and lipase. J. Daily Chem. Sci. 2015, 38, 45–48+56. [Google Scholar]
- Luo, L.B.; Li, J.L. Study on the improvement of enzyme stability in liquid detergent. J. Daily Chem. Sci. 2010, 33, 27–29. [Google Scholar]
- Wang, P.H.; Wang, W.X.; Yang, T.; Zeng, H.; Rui, Z.B.; Li, D.H.; Huang, P. Influencing factors on washing performance of alkaline protease in liquid detergent. China Surfactant Deterg. Cosmet. 2021, 51, 1109–1117. [Google Scholar]
- Russell, G.L.; Britton, L.N. Use of certain alcohol ethoxylates to maintain protease stability in the presence of anionic surfactants. J. Surfact. Deterg. 2002, 5, 5–10. [Google Scholar] [CrossRef]
- Zhang, H.; Li, H.; Liu, H.; Lang, D.A.; Xu, H.; Zhu, H. The application of a halotolerant metalloprotease from marine bacterium Vibrio sp. LA-05 in liquid detergent formulations. Int. Biodeterior. Biodegrad. 2019, 142, 18–25. [Google Scholar] [CrossRef]
- Severson, R.G., Jr. Liquid Detergents Containing Boric Acid and Formate to Stabilize Enzymes. U.S. Patent 4,537,707, 27 August 1985. [Google Scholar]
- Maurer, K.H. Detergent proteases. Curr. Opin. Biotechnol. 2004, 15, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Canalle, L.A.; Löwik, D.W.P.M.; van Hest, J.C.M.V. Polypeptide–polymer bioconjugates. Chem. Soc. Rev. 2010, 39, 329–353. [Google Scholar] [CrossRef]
- WO2008116915A1; Stable Enzyme Solutions and Method of Manufacturing. World Intellectual Property Organization: Geneva, Switzerland, 2008.
- Lund, H.; Kaasgaard, S.G.; Skagerlind, P.; Jorgensen, L.; Jorgensen, C.I.; van de Weert, M.V.D. Correlation Between Enzyme Activity and Stability of a Protease, an Alpha-Amylase and a Lipase in a Simplified Liquid Laundry Detergent System, Determined by Differential Scanning Calorimetry. J. Surfact. Deterg. 2012, 15, 9–21. [Google Scholar] [CrossRef]
- Zhang, J. Study on the Interaction of Alkaline Protease with Surfactants and Protease’ the Application in Liquid Detergent. Master’s Thesis, Shanxi University, Taiyuan, China, 2016. [Google Scholar]
- Wu, M.N. The Stability and Application of Enzymes in Laundry Liquids. Master’s Thesis, Jiangnan University, Wuxi, China, 2020. [Google Scholar]
- Klibanov, A.M. What is remembered and why? Nature 1995, 374, 596. [Google Scholar] [CrossRef]
- Fishman, A.; Cogan, U. Bio-imprinting of lipases with fatty acids. J. Mol. Catal. B Enzymatic. 2003, 22, 193–202. [Google Scholar] [CrossRef]
- Saylan, Y.; Kılıç, S.; Denizli, A. Biosensing applications of molecularly imprinted-polymer-based nanomaterials. Processes 2024, 12, 177. [Google Scholar] [CrossRef]
- Zhao, Q.Y. Physicochemical Properties of Different fractions of Cowpea Proteins and the Mechanism of Their TGase-Induced Gel Formation. Master’s Thesis, Shenyang Agricultural University, Shenyang, China, 2023. [Google Scholar]
- Yan, W.H. Heterologous Expression of Glutamine Transaminase and Its Application in Preparation of Antimiciobial Emulsifier. Master’s Thesis, Qilu University of Technology, Jinan, China, 2023. [Google Scholar]
- Duarte, L.; Matte, C.R.; Bizarro, C.V.; Ayub, M.A.Z. Transglutaminases: Part I—Origins, sources, and biotechnological characteristics. World J. Microbiol. Biotechnol. 2020, 36, 3–21. [Google Scholar] [CrossRef] [PubMed]
- GB/T 23527-2009; Protease Preparations. National Standards of the People’s Republic of China: Beijing, China, 2009.
- Guo, C.H.; Chen, Z.H.; Lu, Z.H.; Yu, X. Studies on extraction, purification and partial property of protease from tilapia intestine. J. Chin. Inst. Food Sci. Technol. 2010, 10, 100–105. [Google Scholar]
- Shen, W.Y.; Zhu, Y.R.; Qian, K.L. The effects of temperature and pH on activities of digestive enzymes in jade perch (Scortum barcoo) in Australia. J. Dalian Ocean Univ. 2006, 02, 189–192. [Google Scholar]
- Gulmez, C.; Altinkaynak, C.; Özdemir, N.; Atakisi, O. Proteinase K hybrid nanoflowers (P-hNFs) as a novel nanobiocatalytic detergent additive. Int. J. Biol. Macromol. 2018, 119, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Márquez, O.; Fernández-Serrano, M.; Pilamala, M.; Jácome, M.B.; Luzón, G. Stability studies of an amylase and a protease for cleaning processes in the food industry. Food Bioprod. Process. 2019, 117, 64–73. [Google Scholar] [CrossRef]
- Chen, K. Construction of the Engineering Bacteria with High Yield of Alkaline Protease 2709 and Its Application Performance. Master’s Thesis, Tianjin University of Science and Technology, Tianjin, China, 2018. [Google Scholar]
- Wang, P.H.; Wang, W.X.; Zeng, H.; Rui, Z.B. Research progress in improving the washing performance of alkaline protease. China Surfactant Deterg. Cosmet. 2022, 52, 180–189. [Google Scholar]
- Hadj-Ali, N.E.; Agrebi, R.; Ghorbel-Frikha, B.; Sellami-Kamoun, A.; Kanoun, S.; Nasri, M. Biochemical and molecular characterization of a detergent stable alkaline serine-protease from a newly isolated Bacillus licheniformis NH1. Enzym. Microb. Technol. 2007, 40, 515–523. [Google Scholar] [CrossRef]
- Wu, M.N.; Li, L.; Xia, Y.H. Improvement of proteases stability in liquid laundry detergent. China Surfact. Deterg. Cosmet. 2019, 49, 103–107. [Google Scholar]
- Tanksale, A.; Chandra, P.M. Immobilization of alkaline protease from Conidiobolus macrosporus for reuse and improved thermal stability. Biotechnol. Lett. 2001, 23, 51–54. [Google Scholar] [CrossRef]
- Shen, B.B. Application of Composite Enzymes in Liquid Laundry Detergent. Master’s Thesis, South China University of Technology, Guangzhou, China, 2012. [Google Scholar]
- Fuciños, C.; Estévez, N.; Míguez, M.; Fajardo, P.; Chapela, M.J.; Gondar, D.; Rúa, M.L. Effectiveness of proteolytic enzymes to remove gluten residues and feasibility of incorporating them into cleaning products for industrial purposes. Food Res. Int. 2019, 120, 167–177. [Google Scholar] [CrossRef] [PubMed]

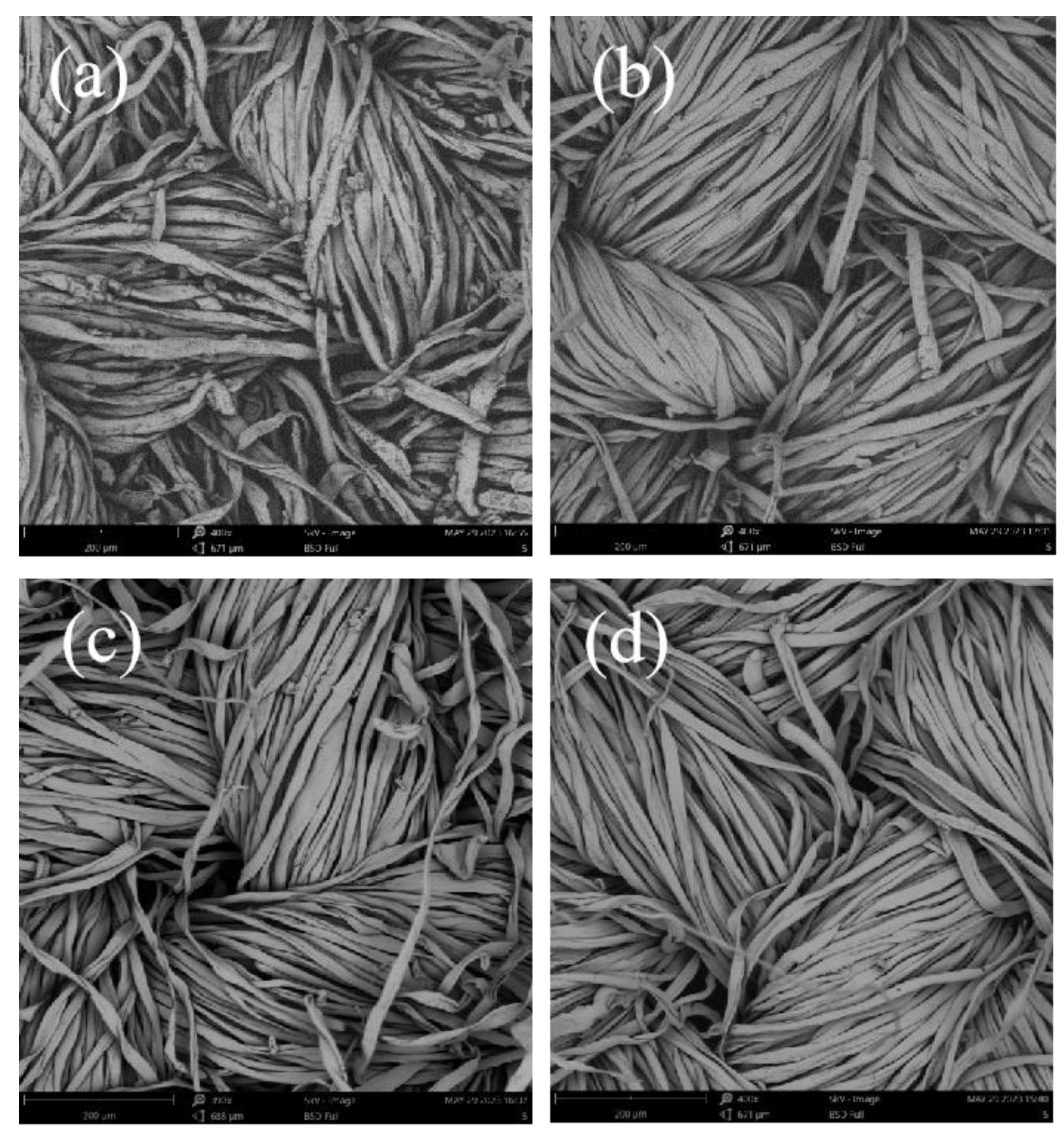
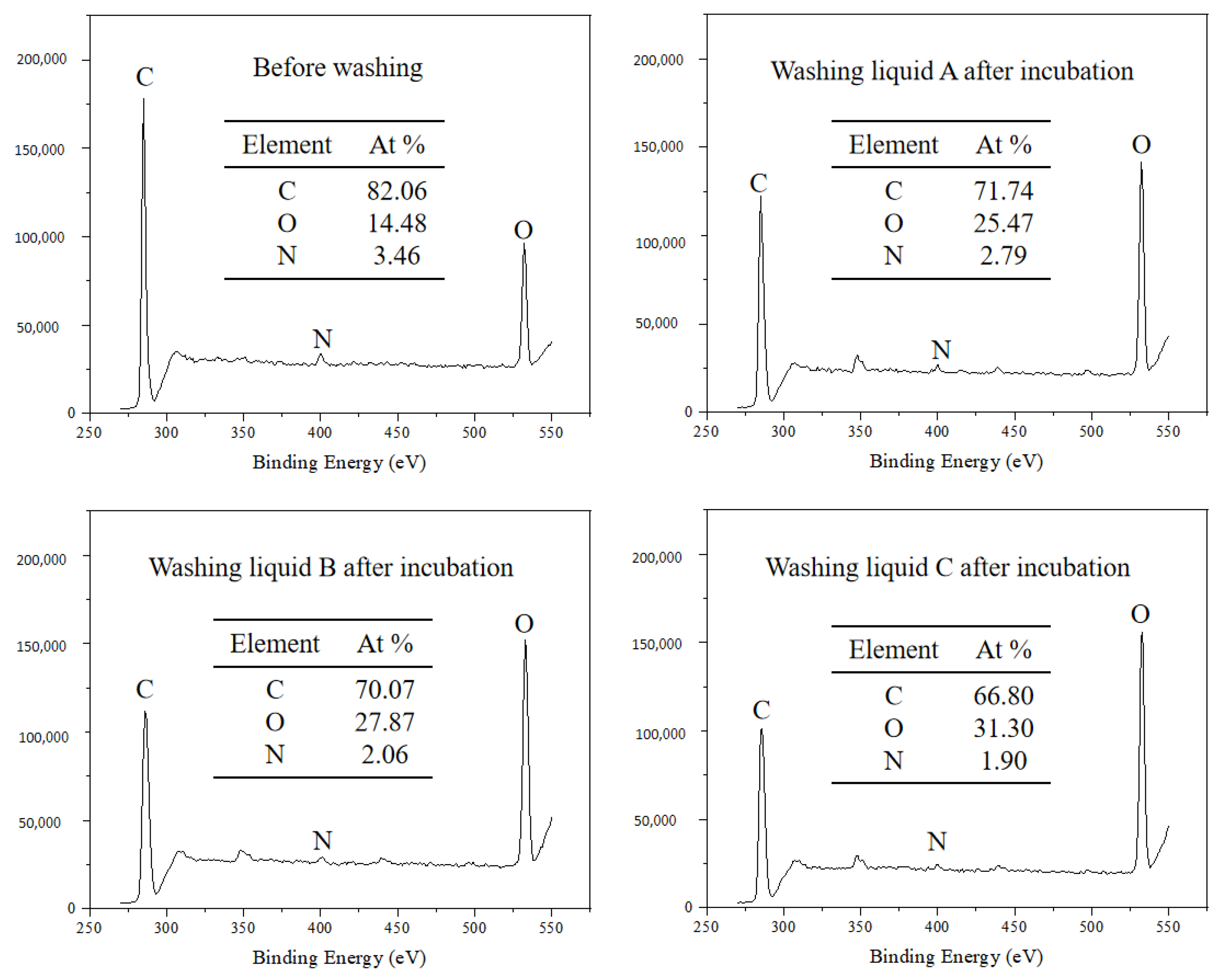
| Laundry Detergent | Stain Removal Value | Stain Removal Ratio |
|---|---|---|
| Laundry detergent A before incubation | 17.66 ± 0.69 d | 1.00 d |
| Laundry detergent A after incubation | 17.75 ± 0.80 d | 1.01 ± 0.05 d |
| Laundry detergent B before incubation | 25.40 ± 1.08 a | 1.44 ± 0.07 a |
| Laundry detergent B after incubation | 19. 27 ± 0.64 c | 1.09 ± 0.04 c |
| Laundry detergent C before incubation | 25.38 ± 0.71 a | 1.44 ± 0.07 a |
| Laundry detergent C after incubation | 23.10 ± 0.88 b | 1.31 ± 0.05 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Ren, X.; Zhao, Y.; Xu, T.; Xiao, J.; Chen, H. Enhancing Alkaline Protease Stability through Enzyme-Catalyzed Crosslinking and Its Application in Detergents. Processes 2024, 12, 624. https://doi.org/10.3390/pr12030624
Yang H, Ren X, Zhao Y, Xu T, Xiao J, Chen H. Enhancing Alkaline Protease Stability through Enzyme-Catalyzed Crosslinking and Its Application in Detergents. Processes. 2024; 12(3):624. https://doi.org/10.3390/pr12030624
Chicago/Turabian StyleYang, Haichuan, Xiankun Ren, Yating Zhao, Tengjiao Xu, Jing Xiao, and Hao Chen. 2024. "Enhancing Alkaline Protease Stability through Enzyme-Catalyzed Crosslinking and Its Application in Detergents" Processes 12, no. 3: 624. https://doi.org/10.3390/pr12030624




