Electrolytic Regeneration of Spent Caustic Soda from CO2 Capture Systems
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
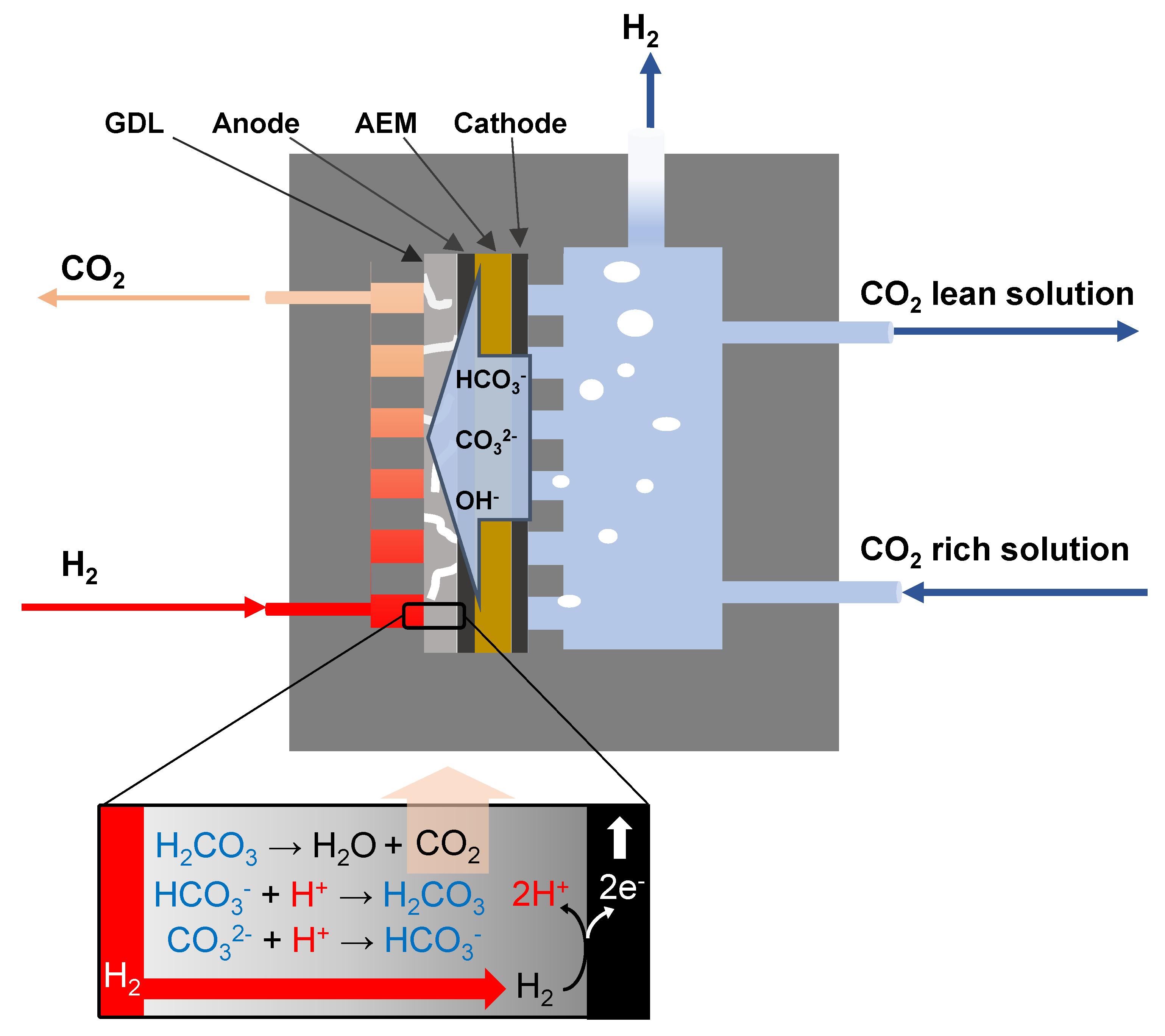
2.2. Hybrid Cell Fabrication and Testing
2.3. Calculation
3. Results and Discussion
3.1. Polarisation Performance
3.2. Effect of H2 Loading Rate on the Polarisation Performance of the Hybrid Cell
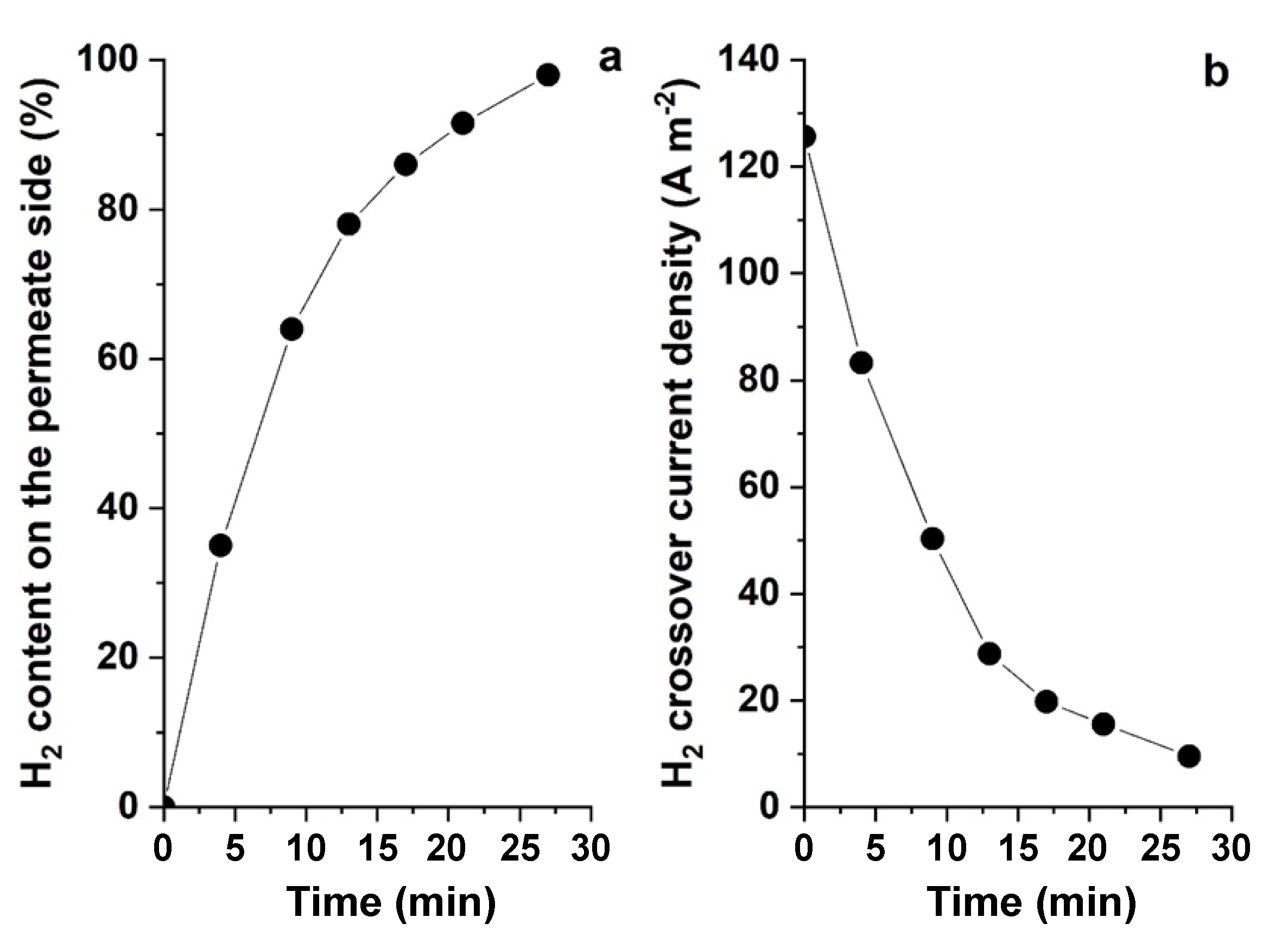
3.3. H2 Back Diffusion across the AEM
3.4. Faradaic Efficiency
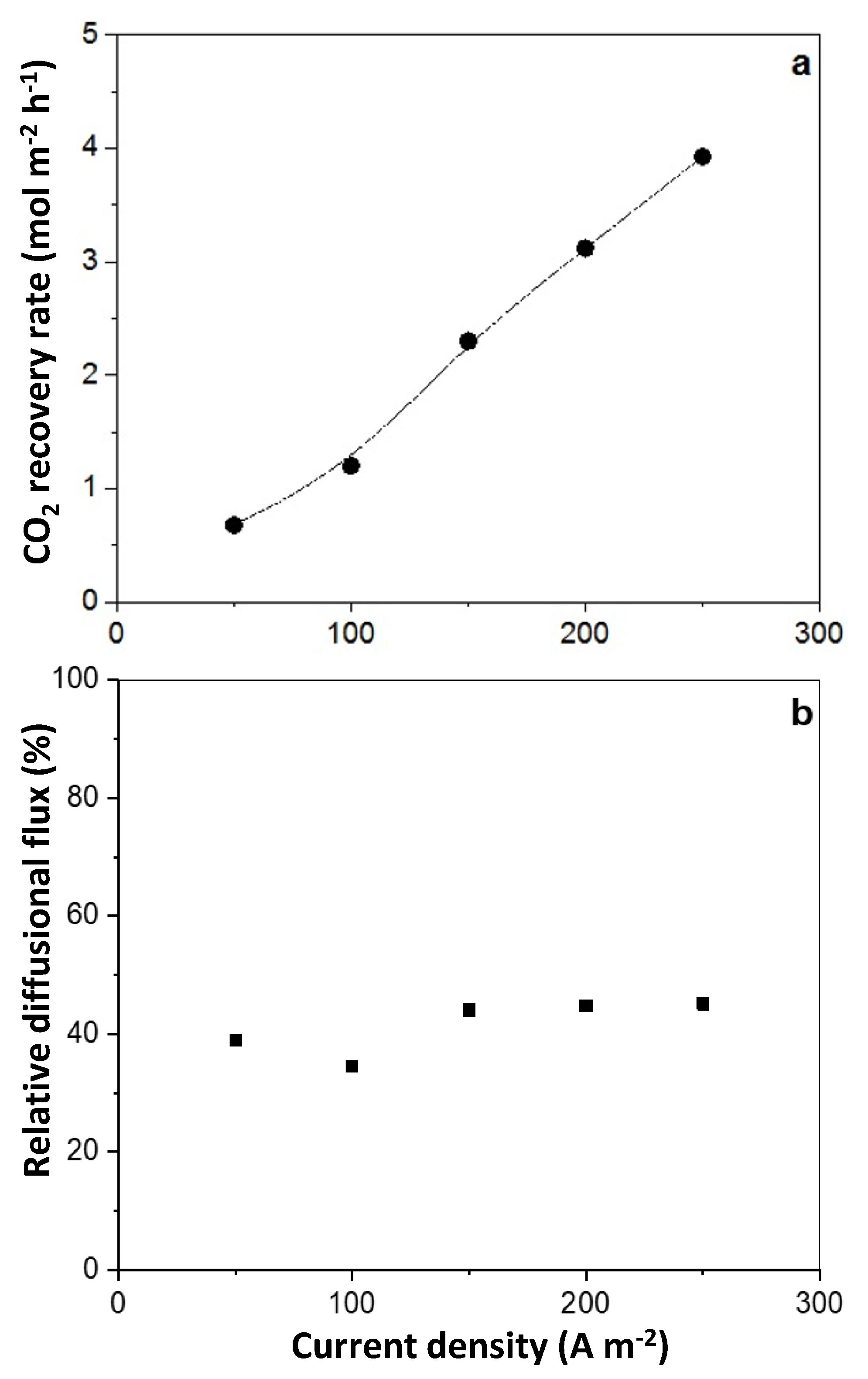
3.5. CO2 Recovery Investigation
3.6. Energy Requirement Analysis
| Cell Configuration | Mechanism | CO2 Sources | Faradaic Efficiency (%) | CO2 Recovery Rate (mol/m2/h) | Energy Requirement (kWh/kg CO2) | Reference |
|---|---|---|---|---|---|---|
| Non-redox cycling mode | Water electrolysis | Biogas (55% CO2, 45% CH4) | 40 | 0.7–11 | 0.31–3.8 | This study |
| Water Electrolysis | Biogas (CH4 60%, 40% CO2) | 20–80 | 1.8–6 | 1.01–5.8 | [25] | |
| Water Electrolysis | Aqueous carbonate/bicarbonate | 10–100 | 0.37–7.4 | 0.63–5.6 | [56] | |
| Redox cycling mode | H2 cycling | Biogas (55% CO2, 45% CH4) | 40 | 0.7–11 | 0.19–2.8 | This study |
| H2 cycling | CO2 gas mixtures (50% CO2, 50% N2) | 80 | 1.8–3.7 | 0.18 | [34] | |
| H2 cycling | Aqueous carbonate/bicarbonate | N.A | 1.8–5.5 | 2.3–3.2 | [57] | |
| O2 cycling | Flue gas | <25 | 0.37–1.8 | 0.48–0.73 | [24] | |
| O2 cycling | Flue gas | 45–65 | 0.07–0.7 | 0.8–1.1 | [47] | |
| Copper ion cycling | Flue gas | 45–60 | 0.4–0.7 | 0.22–0.31 | [51] | |
| Quinone cycling | Flue gas | 100 | 8.7 | 0.66 | [53] |
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AEM | Anion exchange membrane. |
| OER | Oxygen evolution reaction. |
| HOR | Hydrogen oxidation reaction. |
| GDL | Gas diffusion layer. |
| EMAR | Electrochemically mediated amine regeneration. |
| F | Faradaic constant (96,485 A s/mol). |
| E | Net energy requirement (kWh/kg CO2). |
References
- Mondal, M.K.; Balsora, H.K.; Varshney, P. Progress and trend in CO2 capture/separation technologies: A review. Energy 2012, 46, 431. [Google Scholar] [CrossRef]
- Al-Ghussain, L. Global warming: Review on driving forces and mitigation. Environ. Prog. Sustain. Energy 2019, 38, 13. [Google Scholar] [CrossRef]
- Madejski, P.; Chmiel, K.; Subramanian, N.; Kuś, T. Methods and techniques for CO2 capture: Review of potential solutions and applications in modern technologies. Energies 2022, 15, 887. [Google Scholar] [CrossRef]
- Sharifian, R.; Wagterveld, R.; Digdaya, I.; Xiang, C.; Vermaas, D. Electrochemical carbon dioxide capture to close the carbon cycle. Energy Environ. Sci. 2021, 14, 781. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, L.; Otto, A.; Robinius, M.; Stolten, D. A review of post-cobustion CO2 capture technologies from coal-fired power plants. Energy Procedia 2017, 114, 650. [Google Scholar] [CrossRef]
- Koytsoumpa, E.I.; Bergins, C.; Kakaras, E. The CO2 economy: Review of CO2 capture and reuse technologies. J. Supercrit. Fluids 2018, 132, 3. [Google Scholar] [CrossRef]
- Borhani, T.N.; Wang, M. Role of solvents in CO2 capture processes: The review of selection and design methods. Renew. Sustain. Energy Rev. 2019, 114, 109299. [Google Scholar] [CrossRef]
- Mulder, G.; Six, D.; Claessens, B.; Broes, T.; Omar, N.; Van Mierlo, J. The dimensioning of PV battery systems depending on the incentive and selling price conditions. Appl. Energy 2013, 111, 1126. [Google Scholar] [CrossRef]
- Spigarelli, B.P.; Kawatra, S.K. Opportunities and challenges in carbon dioxide capture. J. CO2 Util. 2013, 1, 69. [Google Scholar] [CrossRef]
- Wilberforce, T.; Olabi, A.; Sayed, E.T.; Elsaid, K.; Abdelkareem, M.A. Progress in carbon capture technologies. Sci. Total Environ. 2021, 761, 143203. [Google Scholar] [CrossRef]
- Yadav, S.; Mondal, S. A review on the progress and prospects of oxy-fuel carbon capture and sequestration (CCS) technologies. Fuel 2022, 308, 122057. [Google Scholar]
- Siagian, U.W.; Raksajati, A.; Himma, N.F.; Khoiruddin, K.; Wenten, I. Membrane-based carbon capture technologies: Membrane gas separation vs. membrane contractor. J. Nat. Gas Sci. Eng. 2019, 67, 172. [Google Scholar] [CrossRef]
- Aaron, D.; Tsouris, C. Separation of CO2 from flue gas—A review. Sep. Sci. Technol. 2005, 40, 321. [Google Scholar] [CrossRef]
- Mahmoudkhani, M.; Heidel, K.; Ferreira, J.; Keith, D.; Cherry, R.S. Low energy packed tower and caustic recovery for direct capture of CO2 from air. Energy Procedia 2009, 1, 1535. [Google Scholar] [CrossRef]
- Baciocchi, R.; Costa, G.; Gavasci, R.; Lombardi, L.; Zingaretti, D. Invitigation of an innovative process for biogas upgrading- pilot plant preliminary results. Chem. Eng. J. 2012, 179, 63. [Google Scholar] [CrossRef]
- Vega, F.; Cano, M.; Gallego, L.M.; Camino, S.; Camino, J.A.; Navarrete, B. Evaluation of MEA 5M performance at different CO2 concentrations of flue gas tested at a CO2 capture lab-scale plant. Energy Procedia 2017, 114, 6222. [Google Scholar] [CrossRef]
- Shim, J.-G.; Lee, D.W.; Lee, J.H.; Kwak, N.-S. Experimental study on capture of carbon dioxide and production of sodium carbonate from sodium hydroxide. Environ. Eng. Res. 2016, 21, 297. [Google Scholar] [CrossRef]
- Finney, K.N.; Akram, M.; Diego, M.E.; Yang, X.; Pourkashanian, M. Bioenergy with Carbon Capture and Storage; Elsevier: Amsterdam, The Netherlands, 2019; p. 15. [Google Scholar]
- Zhang, F.; Zhang, W.; Guo, J.; Lei, Y.; Dar, M.A.; Almutairi, Z.; Alshareef, H.N. All-carbon hybrid mobile ion capacitors enabled by 3D laser-scribed graphene. Energy Technol. 2020, 8, 2000193. [Google Scholar] [CrossRef]
- Mahmoudkhani, M.; Keith, D.W. Low-energy sodium hydroxide recovery for CO2 capture from atmosphere air-thermodynamic analysis. Int. J. Greenh. Gas Control. 2009, 3, 376. [Google Scholar] [CrossRef]
- Lide, D.R. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Nohlgren, I. Non-conventional causticization technology—A review. Nord. Pulp Pap. Res. J. 2004, 19, 470. [Google Scholar] [CrossRef]
- Walke, L.; Atkinson, K.; Clark, D.; Scardaville, D.; Winnick, J. Recovery of CO2 from flue gas using an electrochemical membrane. Gas Sep. Purif. 1988, 2, 72. [Google Scholar] [CrossRef]
- Pennline, H.W.; Granite, E.J.; Luebke, D.R.; Kitchin, J.R.; Landon, J.; Weiland, L.M. Separation of CO2 from flue gas using electrochemical cells. Fuel 2010, 89, 1307. [Google Scholar] [CrossRef]
- Verbeeck, K.; De Vrieze, J.; Biesemans, M.; Rabaey, K. Membrane electrolysis-assisted CO2 and H2S extraction as innovative pretreatment method for biological biogas upgrading. Chem. Eng. J. 2019, 361, 1479. [Google Scholar] [CrossRef]
- Mohammadpour, H.; Pivrikas, A.; Cheng, K.Y.; Ho, G. A three-chamber electrochemical cell facilitated biogas upgrading and high-purity oxygen producion. J. Appl. Electrochem. 2022, 52, 919–927. [Google Scholar] [CrossRef]
- Baysinger, G.; Berger, L.I.; Goldberg, R.; Kehiaian, H.; Kuchitsu, K.; Rosenblatt, G.; Roth, D.; Zwillinger, D. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Xiang, C.; Papadantonakis, K.M.; Lewis, N.S. Principles and implementations of electrolysis systems for water splitting. Mater. Horiz. 2016, 3, 169. [Google Scholar] [CrossRef]
- Zahran, Z.N.; Mohamed, E.A.; Tsubonouchi, Y.; Ishizaki, M.; Togashi, T.; Kurihara, M.; Saito, K.; Yui, T.; Yagi, M. Electrocatalytic water splitting with unprecedentedly low overpotentials by nickel and sulfide nanowires stuffed into carbon nitride scabbards. Energy Environ. Sci. 2021, 14, 5358. [Google Scholar] [CrossRef]
- Carmo, M.; Fritz, D.L.; Mergel, J.; Stolten, D. A comprehensive review on PEM water electrolysis. Int. J. Hydrog. Energy 2013, 38, 4901. [Google Scholar] [CrossRef]
- Kaninski, M.P.M.; Miulovic, S.M.; Tasic, G.S.; Maksic, A.D.; Nikolic, V.M. A study on the Co-W activated Ni electrodes for the hydrogen production from alkaline water electrolysis-energy saving. Int. J. Hydrog. Energy 2011, 36, 5227. [Google Scholar] [CrossRef]
- Melaina, M.W.; Antonia, O.; Penev, M. Blending Hydrogen into Natural Gas Pipeline Networks: A Review of Key Issues; Technical Report; NREL: Golden, CO, USA, 2013.
- Shi, Q.; Zhu, C.; Du, D.; Lin, Y. Robust noble metal based electrocatalysts for oxygen evolution reaction. Chem. Soc. Rev. 2019, 48, 3181. [Google Scholar] [CrossRef]
- Muroyama, A.P.; Beard, A.; Pribyl-Kranewitter, B.; Gubler, L. Separation of CO2 from dilute gas streams using a membrane electrochemical cell. ACS EST Eng. 2021, 1, 905. [Google Scholar] [CrossRef]
- Mohammadpour, H.; Cord-Ruwisch, R.; Pivrikas, A.; Ho, G. Simple energy-efficient electrochemically -driven CO2 scrubbing for biogas upgrading. Renew. Energy 2022, 195, 274. [Google Scholar] [CrossRef]
- Cong, Y.; Yi, B.; Song, Y. Hydrogen oxidation reaction in alkaline media: From mechanism to recent electrolysis. Nano Energy 2018, 44, 288. [Google Scholar] [CrossRef]
- Hu, J.; Kuttiyiel, K.A.; Sasaki, K.; Zhang, C.; Adzic, R.R. Determination of hydrogen oxidation reaction mechanism based on Pt-had energetics in alkaline electrolyte. J. Electrochem. Soc. 2018, 165, J3355. [Google Scholar] [CrossRef]
- Ströbel, R.; Oszcipok, M.; Fasil, M.; Rohland, B.; Jörissen, L.; Garche, J. The compression of hydrogen in an electrochemical cell based on PE fuel cell design. J. Power Sources 2002, 105, 208. [Google Scholar] [CrossRef]
- Li, D.; Chung, H.T.; Maurya, S.; Matanovic, I.; Kim, Y.S. Impact of ionomer adsoprtion on alkaline hydrogen oxidation acticity and fuel cell performance. Curr. Opin. Electrochem. 2018, 12, 189. [Google Scholar] [CrossRef]
- Cui, D.; Ji, Y.; Chang, C.; Wang, Z.; Xiao, X. Influence of fuel flow rate on the performance of micro tubular solid oxide fuel cell. Int. J. Hydrog. Energy 2020, 45, 13459. [Google Scholar] [CrossRef]
- Nordio, M.; Rizzi, F.; Manzolini, G.; Mulder, M.; Raymakers, L.; Annaland, M.V.S.; Gallucci, F. Experimental and modelling study of an electrochemical hydrogen compressor. Chem. Eng. J. 2019, 369, 432. [Google Scholar] [CrossRef]
- Baik, K.D.; Hong, B.K.; Kim, M.S. Effect of operating parameters on hydrogen crossover rate through Nafion membranes in polymer electrolyte membrane fuel cells. Renew. Energy 2013, 57, 234. [Google Scholar] [CrossRef]
- Pushkareva, I.; Pushkarev, A.; Grigoriev, S.; Modisha, P.; Bessarabov, D. Comparative study of anion exchange membranes for low cost water electrolysis. Int. J. Hydrog. Energy 2020, 45, 26070. [Google Scholar] [CrossRef]
- Rocourt, X.; Mélani, L.; Sochet, I.; Jallais, S. In Proceedings of the 31st Meeting on Combustion-Italian Section of the Combustion Institute. Torino, Italy, 17–20 June 2008; p. 17. [Google Scholar]
- Hemme, C.; Van Berk, W. Hydrogeochemical modeling to identify potential reisks of underground hydrogen storage in depleted gas fields. Appl. Sci. 2018, 8, 2282. [Google Scholar] [CrossRef]
- Huang, T.; Qiu, X.; Zhang, J.; Li, X.; Pei, Y.; Jiang, H.; Yue, R.; Yin, Y.; Jiang, Z.; Zhang, X. Hydrogen crossover through microporous anion exchange membranes for fuel cells. J. Power Sources 2022, 527, 231143. [Google Scholar] [CrossRef]
- Rigdon, W.A.; Omasta, T.J.; Lewis, C.; Hickner, M.A.; Varcoe, J.R.; Renner, J.N.; Ayers, K.E.; Mustain, W.E. Carbonate dynamics and opportunities with low temperature, anion exchange membrane-based electrochemical carbon dioxide separators. J. Electrochem. Energy Convers. Storage 2017, 14, 020701. [Google Scholar] [CrossRef]
- Krol, J.J.; Wessling, M.; Strathmann, H. Concentration polarisation with monopolar ion exchange membranes: Current-voltage curves and water dissocation. J. Membr. Sci. 1999, 162, 145. [Google Scholar] [CrossRef]
- McCarty, R.D.; Hord, J.; Roder, H.M. Selected Properties of Hydrogen (Engineering Design Data); US Department of Commerce, National Bureau of Standards: Gaithersburg, MD, USA, 1981.
- Gao, X.; Liu, X.; Zang, W.; Dong, H.; Pang, Y.; Kou, Z.; Wang, P.; Pan, Z.; Wei, S.; Mu, S. Synergizing in-grown Ni3N/Ni heterostructured core and ultrathin Ni3N surface shell enables self-adaptive surface reconfiguration and efficient oxygen evolution reaction. Nano Energy 2020, 78, 105355. [Google Scholar] [CrossRef]
- Rahimi, M.; Diederichsen, K.M.; Ozbek, N.; Wang, M.; Choi, W.; Hatton, T.A. An electrochemically mediated amine regenration process with a mixed absorbent for postcombuiustion CO2 capture. Environ. Sci. Technol. 2020, 54, 8999. [Google Scholar] [CrossRef] [PubMed]
- Gouedard, C.; Picq, D.; Launay, F.; Carrette, P.-L. Amine degradation in CO2 capture: I. A review. Int. J. Greenh. Gas Control. 2012, 10, 244. [Google Scholar] [CrossRef]
- Huang, C.; Liu, C.; Wu, K.; Yue, H.; Tang, S.; Lu, H.; Liang, B. CO2 capture from flue gas using an electrochemically reversible hydroqinone/quinone solution. Energy Fuels 2019, 33, 3380. [Google Scholar] [CrossRef]
- Zeman, F. Energy and material balance of CO2 capture from ambient air. Environ. Sci. Technol. 2007, 41, 7558. [Google Scholar] [CrossRef] [PubMed]
- Havlík, T.H.; Havlík, T. (Eds.) Hydrometallurgy: Principles and Appliations. Woodhead Publishing: Sawston, UK, 2008; p. 255. [Google Scholar]
- Eisaman, M.D.; Alvarado, L.; Larner, D.; Wang, P.; Garg, B.; Littau, K.A. CO2 separation using bipolar membrane electrodialysis. Energy Environ. Sci. 2011, 4, 1319. [Google Scholar] [CrossRef]
- Shu, Q.; Legrand, L.; Kuntke, P.; Tedesco, M.; Hamelers, H.V. Electrochemical regenration of spent alkaline absorbent from direct air capture. Environ. Sci. Technol. 2020, 54, 8990. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammadpour, H.; Pivrikas, A.; Cheng, K.Y.; Ho, G. Electrolytic Regeneration of Spent Caustic Soda from CO2 Capture Systems. Processes 2024, 12, 723. https://doi.org/10.3390/pr12040723
Mohammadpour H, Pivrikas A, Cheng KY, Ho G. Electrolytic Regeneration of Spent Caustic Soda from CO2 Capture Systems. Processes. 2024; 12(4):723. https://doi.org/10.3390/pr12040723
Chicago/Turabian StyleMohammadpour, Hossein, Almantas Pivrikas, Ka Yu Cheng, and Goen Ho. 2024. "Electrolytic Regeneration of Spent Caustic Soda from CO2 Capture Systems" Processes 12, no. 4: 723. https://doi.org/10.3390/pr12040723






