Changes in Soil Microbial Parameters after Herbicide Application in Soils under Conventional Tillage and Non-Tillage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Herbicides
2.2. Field Experiment
2.3. Experimental Procedure
2.4. Data Analysis
3. Results and Discussion
3.1. Herbicide Residues in Soils under Tillage and Non-Tillage Management
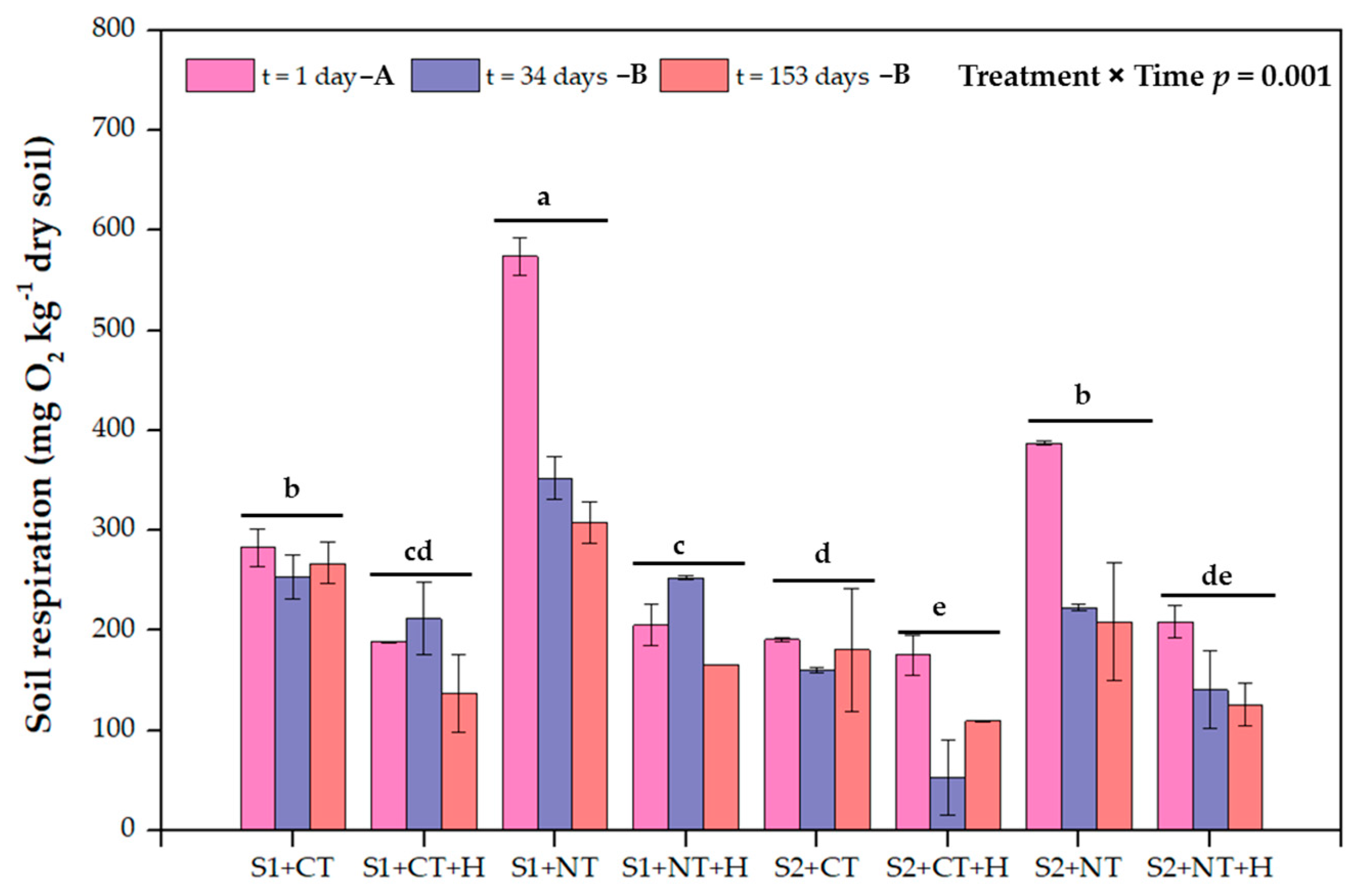
3.2. Soil Microbial Respiration
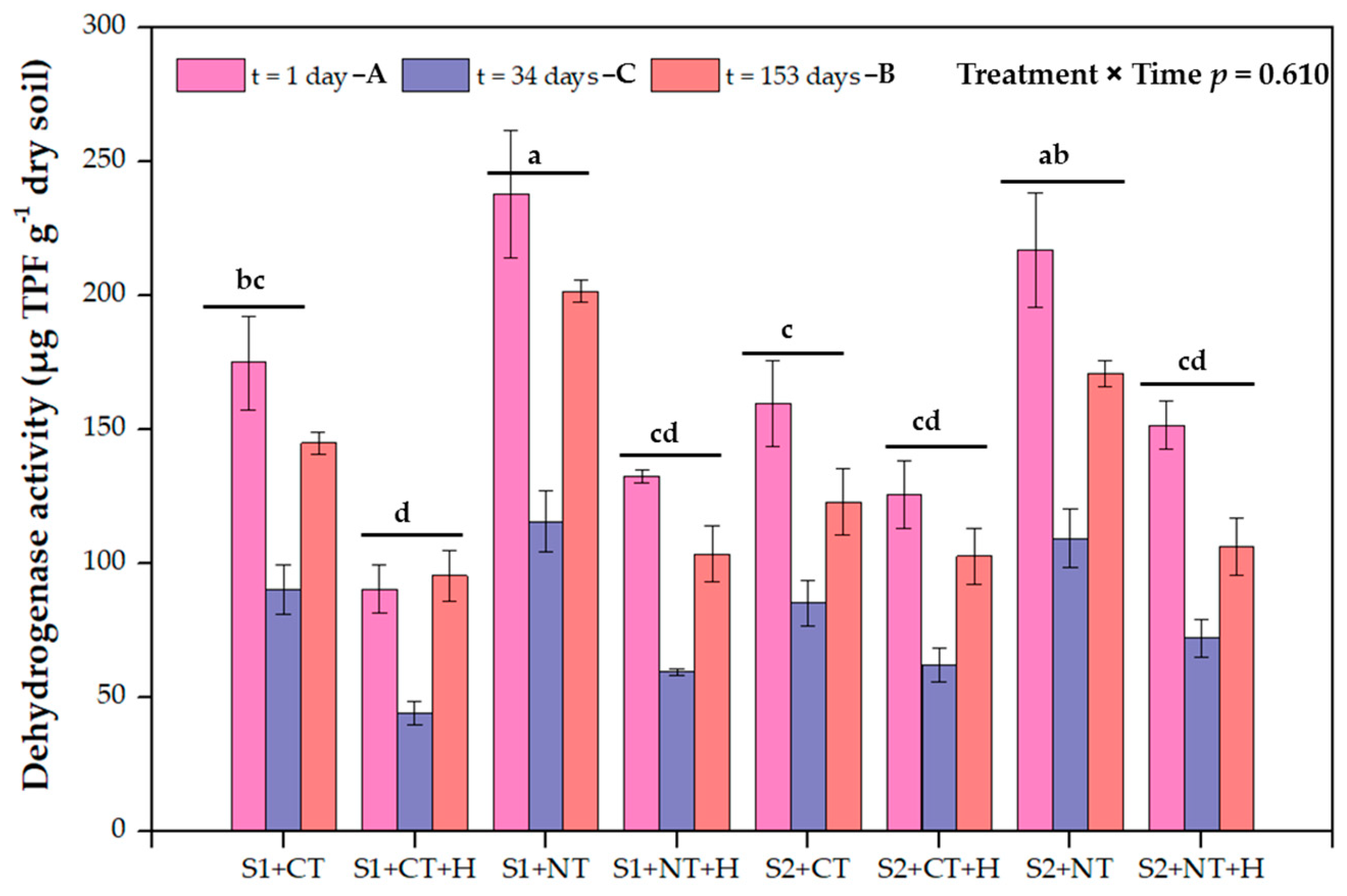
3.3. Soil Dehydrogenase Activity
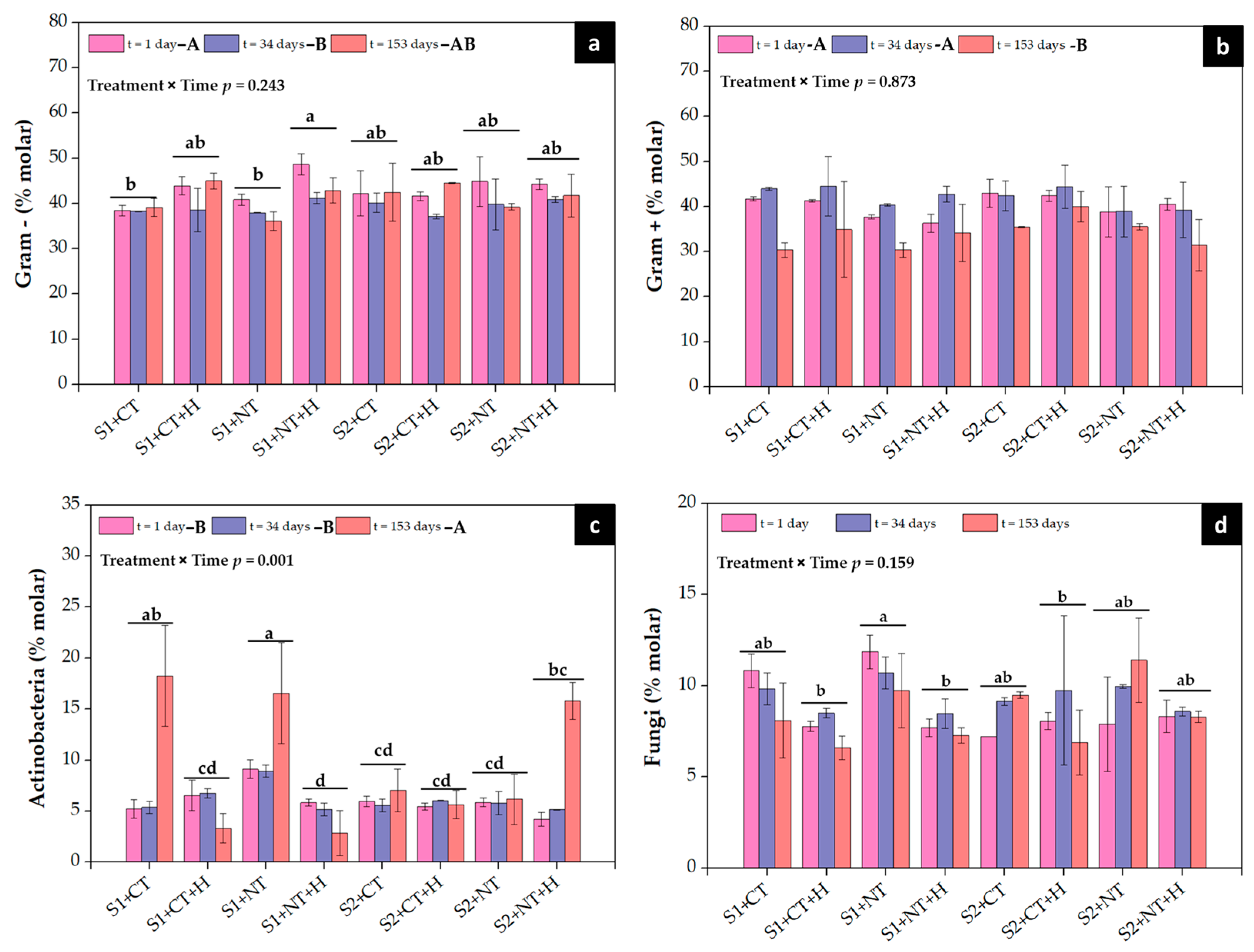
3.4. Soil Microbial Biomass and Structure
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boselli, R.; Fiorini, A.; Santelli, S.; Ardenti, F.; Capra, F.; Maris, S.C.; Tabaglio, V. Cover crops during transition to no-till maintain yield and enhance soil fertility in intensive agro-ecosystems. Field Crops Res. 2020, 255, 107871. [Google Scholar] [CrossRef]
- Kader, M.A.; Senge, M.; Mojid, M.A.; Ito, K. Recent advances in mulching materials and methods for modifying soil environment. Soil Tillage Res. 2017, 168, 155–166. [Google Scholar] [CrossRef]
- Jacobs, A.A.; Evans, R.S.; Allison, J.K.; Garner, E.R.; Kingery, W.L.; McCulley, R.L. Cover crops and no-tillage reduce crop production costs and soil loss, compensating for lack of short-term soil quality improvement in a maize and soybean production system. Soil Tillage Res. 2022, 218, 105310. [Google Scholar] [CrossRef]
- Wang, G.; Jia, H.; Zhuang, J.; Glatzel, S.; Bennett, J.M.; Zhu, Y. Growing-season soil microbial respiration response to long-term no tillage and spring ridge tillage. Int. J. Agric. Biol. Eng. 2020, 13, 143–150. [Google Scholar] [CrossRef]
- Zheng, F.; Wu, X.; Zhang, M.; Liu, X.; Song, X.; Lu, J.; Wang, B.; van Groenigen, K.J.; Li, S. Linking soil microbial community traits and organic carbon accumulation rate under long-term conservation tillage practices. Soil Tillage Res. 2022, 220, 105360. [Google Scholar] [CrossRef]
- Panettieri, M.; de Sosa, L.L.; Domínguez, M.T.; Madejón, E. Long-term impacts of conservation tillage on Mediterranean agricultural soils: Shifts in microbial communities despite limited effects on chemical properties. Agric. Ecosyst. Environ. 2020, 304, 107144. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Q.; Cai, Y.; Yang, Q.; Chang, S.X. Minimum tillage and residue retention increase soil microbial population size and diversity: Implications for conservation tillage. Sci. Total Environ. 2020, 716, 137164. [Google Scholar] [CrossRef]
- Baghel, J.K.; Das, T.K.; Raj, R.; Paul, S.; Mukherjee, I.; Bisht, M. Effect of conservation agriculture and weed management on weeds, soil microbial activity and wheat (Triticum aestivum) productivity under a rice (Oryza sativa)-wheat cropping system. Indian J. Agric. Sci. 2018, 88, 1709–1716. [Google Scholar] [CrossRef]
- Belmonte, S.A.; Celi, L.; Stahel, R.J.; Bonifacio, E.; Novello, V.; Zanini, E.; Steenwerth, K.L. Effect of Long-Term Soil Management on the Mutual Interaction Among Soil Organic Matter, Microbial Activity and Aggregate Stability in a Vineyard. Pedosphere 2018, 28, 288–298. [Google Scholar] [CrossRef]
- Arantes, A.C.C.; Cotta, S.R.; da Conceição, P.M.; Meneghin, S.P.; Martinelli, R.; Próspero, A.G.; Boaretto, R.M.; Andreote, F.D.; Mattos, D., Jr.; de Azevedo, F.A. Implication of Urochloa spp. Intercropping and Conservation Agriculture on Soil Microbiological Quality and Yield of Tahiti Acid Lime in Long Term Orchard Experiment. Agriculture 2020, 10, 491. [Google Scholar] [CrossRef]
- Jayaraman, S.; Dang, Y.P.; Naorem, A.; Page, K.L.; Dalal, R.C. Conservation Agriculture as a System to Enhance Ecosystem Services. Agriculture 2021, 11, 718. [Google Scholar] [CrossRef]
- Legrand, F.; Picot, A.; Cobo-Díaz, J.F.; Carof, M.; Chen, W.; Le Floch, G. Effect of tillage and static abiotic soil properties on microbial diversity. Appl. Soil Ecol. 2018, 132, 135–145. [Google Scholar] [CrossRef]
- Alletto, L.; Coquet, Y.; Benoit, P.; Heddadj, D.; Barriuso, E. Tillage management effects on pesticide fate in soils. A review. Agron. Sustain. Dev. 2010, 30, 367–400. [Google Scholar] [CrossRef]
- Malone, M.; Foster, E. A mixed-methods approach to determine how conservation management programs and techniques have affected herbicide use and distribution in the environment over time. Sci. Total Environ. 2019, 660, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Mahía, J.; Martín, A.; Carballas, T.; Díaz-Raviña, M. Atrazine degradation and enzyme activities in an agricultural soil under two tillage systems. Sci. Total Environ. 2007, 378, 187–194. [Google Scholar] [CrossRef]
- Okada, E.; Costa, J.L.; Bedmar, F. Glyphosate Dissipation in Different Soils Under No-Till and Conventional Tillage. Pedosphere 2019, 29, 773–783. [Google Scholar] [CrossRef]
- Hussain, S.; Arshad, M.; Springael, D.; Sørensen, S.R.; Bending, G.D.; Devers-Lamrani, M.; Maqbool, Z.; Martin-Laurent, F. Abiotic and Biotic Processes Governing the Fate of Phenylurea Herbicides in Soils: A Review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1947–1998. [Google Scholar] [CrossRef]
- Álvarez-Martín, A.; Hilton, S.L.; Bending, G.D.; Rodríguez-Cruz, M.S.; Sánchez-Martín, M.J. Changes in activity and structure of the soil microbial community after application of azoxystrobin or pirimicarb and an organic amendment to an agricultural soil. Appl. Soil Ecol. 2016, 106, 47–57. [Google Scholar] [CrossRef]
- Marín-Benito, J.M.; Herrero-Hernández, E.; Andrades, M.S.; Sánchez-Martín, M.J.; Rodríguez-Cruz, M.S. Effect of different organic amendments on the dissipation of linuron, diazinon and myclobutanil in an agricultural soil incubated for different time periods. Sci. Total Environ. 2014, 476–477, 611–621. [Google Scholar] [CrossRef]
- Hussain, S.; Siddique, T.; Arshad, M.; Saleem, M. Bioremediation and Phytoremediation of Pesticides: Recent Advances. Crit. Rev. Environ. Sci. Technol. 2009, 39, 843–907. [Google Scholar] [CrossRef]
- Pose-Juan, E.; Igual, J.M.; Curto, N.; Sánchez-Martín, M.J.; Rodríguez-Cruz, M.S. Mesotrione dissipation and response of soil microbial communities in a soil amended with organic residues. Span. J. Soil Sci. 2015, 5, 12–25. [Google Scholar] [CrossRef]
- Imfeld, G.; Vuilleumier, S. Measuring the effects of pesticides on bacterial communities in soil: A critical review. Eur. J. Soil Biol. 2012, 49, 22–30. [Google Scholar] [CrossRef]
- Jose Carpio, M.; Garcia-Delgado, C.; Maria Marin-Benito, J.; Jesus Sanchez-Martin, M.; Sonia Rodriguez-Cruz, M. Soil Microbial Community Changes in a Field Treatment with Chlorotoluron, Flufenacet and Diflufenican and Two Organic Amendments. Agronomy 2020, 10, 1166. [Google Scholar] [CrossRef]
- García-Delgado, C.; Barba, V.; Marín-Benito, J.M.; Igual, J.M.; Sánchez-Martín, M.J.; Rodríguez-Cruz, M.S. Simultaneous application of two herbicides and green compost in a field experiment: Implications on soil microbial community. Appl. Soil Ecol. 2018, 127, 30–40. [Google Scholar] [CrossRef]
- Wendt, M.J.; Kenter, C.; Ladewig, E.; Wegener, M.; Märländer, B. Duration of Soil Activity of Foramsulfuron Plus Thiencarbazone-methyl Applied to Weed Species Typical of Sugar Beet Cultivation. Weed Technol. 2017, 31, 291–300. [Google Scholar] [CrossRef]
- Lewis, K.A.; Tzilivakis, J.; Warner, D.J.; Green, A. An international database for pesticide risk assessments and management. Hum. Ecol. Risk Assess. Int. J. 2016, 22, 1050–1064. [Google Scholar] [CrossRef]
- Douibi, M.; Krishtammagari, A.; Sánchez-Martín, M.J.; Rodríguez-Cruz, M.S.; Marín-Benito, J.M. Mulching vs. organic soil amendment: Effects on adsorption-desorption of herbicides. Sci. Total Environ. 2023, 892, 164749. [Google Scholar] [CrossRef] [PubMed]
- Douibi, M.; Sánchez-Martín, M.J.; Rodríguez-Cruz, M.S.; Marín-Benito, J.M. Impacto de la Temperatura y de Prácticas Agrícolas Sostenibles en la Degradación de Herbicidas. Rev. Ciências Agrárias 2022, 45, 583–586. [Google Scholar]
- Cassigneul, A.; Benoit, P.; Nobile, C.; Bergheaud, V.; Dumeny, V.; Etiévant, V.; Maylin, A.; Justes, E.; Alletto, L. Behaviour of S-metolachlor and its oxanilic and ethanesulfonic acids metabolites under fresh vs. partially decomposed cover crop mulches: A laboratory study. Sci. Total Environ. 2018, 631–632, 1515–1524. [Google Scholar]
- Huang, X.; He, J.; Yan, X.; Hong, Q.; Chen, K.; He, Q.; Zhang, L.; Liu, X.; Chuang, S.; Li, S.; et al. Microbial catabolism of chemical herbicides: Microbial resources, metabolic pathways and catabolic genes. Pestic. Biochem. Physiol. 2017, 143, 272–297. [Google Scholar] [CrossRef]
- Ahmad, K.S.; Hafeez, N.; Gul, M.M.; Ali, D.; Shaheen, A.; Aslam, B. Xenobiotic thiencarbazone-methyl biotransformation investigation by bacteria Streptococcus pneumoniae, Escherichia coli and Streptococcus pyogenes. Int. J. Env. Sci. Technol. 2021, 18, 1753–1760. [Google Scholar] [CrossRef]
- Wołejko, E.; Kaczyński, P.; Łozowicka, B.; Wydro, U.; Borusiewicz, A.; Hrynko, I.; Konecki, R.; Snarska, K.; Dec, D.; Malinowski, P. Dissipation of S-metolachlor in plant and soil and effect on enzymatic activities. Env. Monit Assess 2017, 189, 355. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Qu, Q.; Zhang, Z.; Yuan, W.; Cui, H.; Shen, Y.; Lin, W.; Lu, T.; Qian, H. Effects of residual S-metolachlor in soil on the phyllosphere microbial communities of wheat (Triticum aestivum L.). Sci. Total Environ. 2020, 748, 141342. [Google Scholar] [CrossRef] [PubMed]
- Borowik, A.; Wyszkowska, J.; Kucharski, J.; Baćmaga, M.; Tomkiel, M. Response of microorganisms and enzymes to soil contamination with a mixture of terbuthylazine, mesotrione, and S-metolachlor. Environ. Sci. Pollut. Res. 2017, 24, 1910–1925. [Google Scholar] [CrossRef] [PubMed]
- Rabelo, J.S.; Dos Santos, E.A.; de Melo, E.I.; Vaz, M.G.M.V.; de Oliveira Mendes, G. Tolerance of microorganisms to residual herbicides found in eucalyptus plantations. Chemosphere 2023, 329, 138630. [Google Scholar] [CrossRef] [PubMed]
- Koçak, B.; Cenkseven, S. Effects of Three Commonly Used Herbicides in Maize on Short-Term Soil Organic Carbon Mineralization. Water Air Soil Pollut. 2021, 232, 386. [Google Scholar] [CrossRef]
- World Reference Base; Schád, P.; van Huyssteen, C.; Micheli, E. World Reference Base for Soil Resources 2014, Update 2015. Available online: https://www.fao.org/3/i3794en/I3794en.pdf (accessed on 30 March 2023).
- Marín-Benito, J.M.; Barba, V.; Ordax, J.M.; Sánchez-Martín, M.J.; Rodríguez-Cruz, M.S. Recycling organic residues in soils as amendments: Effect on the mobility of two herbicides under different management practices. J. Environ. Manag. 2018, 224, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Sparks, D.L. Methods of Soil Analysis. Part 3: Chemical Methods; SSSA Series; Wiley: Hoboken, NJ, USA, 1996. [Google Scholar]
- Tabatabai, M.A. Soil Enzymes. In Methods of Soil Analysis, Part 2-Microbiological and Biochemical Properties; Weaver, R.W., Angl, J.S., Bottomley, P.S., Eds.; Soil Science Society of America (SSSA): Madison, WI, USA, 1994; pp. 903–947. [Google Scholar]
- Frostegård, Å.; Bååth, E.; Tunlio, A. Shifts in the structure of soil microbial communities in limed forests as revealed by phospholipid fatty acid analysis. Soil Biol. Biochem. 1993, 25, 723–730. [Google Scholar] [CrossRef]
- Alletto, L.; Benoit, P.; Bolognsi, B.; Couffignal, M.; Bergheaud, V.; Dumny, V.; Longueval, C.; Barriuso, E. Sorption and mineralisation of S-metolachlor in soils from fields cultivated with different conservation tillage systems. Soil Tillage Res. 2013, 128, 97–103. [Google Scholar] [CrossRef]
- Schmitz, G.L.; Witt, W.W.; Mueller, T.C. The Effect of Wheat (Triticum aestivum) Straw Levels on Chlorimuron, Imazaquin, and Imazethapyr Dissipation and Interception. Weed Technol. 2001, 15, 129–136. [Google Scholar] [CrossRef]
- Sperry, B.P.; Ferguson, J.C.; Bond, J.A.; Kruger, G.R.; Johnson, A.B.; Reynolds, D.B. Effect of differential levels of simulated overhead irrigation on residual herbicides applied to wheat straw–covered soil for barnyardgrass control. Weed Technol. 2022, 36, 648–654. [Google Scholar] [CrossRef]
- Sapkota, T.B.; Mazzoncini, M.; Bàrberi, P.; Antichi, D.; Silvestri, N. Fifteen years of no till increase soil organic matter, microbial biomass and arthropod diversity in cover crop-based arable cropping systems. Agron. Sustain. Dev. 2012, 32, 853–863. [Google Scholar] [CrossRef]
- Mirzavand, J.; Asadi-Rahmani, H.; Moradi-Talebbeigi, R. Biological indicators of soil quality under conventional, reduced, and no-tillage systems. Arch. Agron. Soil Sci. 2022, 68, 311–324. [Google Scholar] [CrossRef]
- Khan, M.H.; Liu, H.; Zhu, A.; Khan, M.H.; Hussain, S.; Cao, H. Conservation tillage practices affect soil microbial diversity and composition in experimental fields. Front. Microbiol. 2023, 14, 1227–1297. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, W.; Ye, H. [Effects of pesticides metolachlor and S-metolachlor on soil microorganisms in aquisols. II. Soil Respiration]. Ying Yong Sheng Tai Xue Bao 2006, 17, 1305–1309. [Google Scholar] [PubMed]
- García-Orenes, F.; Guerrero, C.; Roldán, A.; Mataix-Solera, J.; Cerdà, A.; Campoy, M.; Zornoza, R.; Bárcenas, G.; Caravaca, F. Soil microbial biomass and activity under different agricultural management systems in a semiarid Mediterranean agroecosystem. Soil Tillage Res. 2010, 109, 110–115. [Google Scholar] [CrossRef]
- Zuber, S.M.; Villamil, M.B. Meta-analysis approach to assess effect of tillage on microbial biomass and enzyme activities. Soil Biol. Biochem. 2016, 97, 176–187. [Google Scholar] [CrossRef]
- Dang, Y.P.; Page, K.L.; Dalal, R.C.; Menzies, N.W. No-till Farming Systems for Sustainable Agriculture: An Overview. In No-till Farming Systems for Sustainable Agriculture; Dang, Y.P., Dalal, R.C., Menzies, N.W., Eds.; Springer International Publushing: Cham, Switzerland, 2020; pp. 3–20. Available online: http://link.springer.com/10.1007/978-3-030-46409-7_1. (accessed on 30 January 2023).
- Ravichandran, M.; Samiappan, S.C.; Pandiyan, R.; Velu, R.K. Improvement of crop and soil management practices through mulching for enhancement of soil fertility and environmental sustainability: A review. J. Exp. Biol. Agric. Sci. 2022, 10, 697–712. [Google Scholar] [CrossRef]
- Mathew, R.P.; Feng, Y.; Githinji, L.; Ankumah, R.; Balkcom, K.S. Impact of No-Tillage and Conventional Tillage Systems on Soil Microbial Communities. Appl. Environ. Soil Sci. 2012, 2012, e548620. [Google Scholar] [CrossRef]
- Six, J.; Frey, S.D.; Thiet, R.K.; Batten, K.M. Bacterial and Fungal Contributions to Carbon Sequestration in Agroecosystems. Soil Sci. Soc. Amer. J 2006, 70, 555–569. [Google Scholar] [CrossRef]
- Lipșa, F.D.; Ulea, E.; Chiriac, I.P.; Coroi, I.G. Effect of Herbicide S-Metolachlor on Soil Microorganisms; Seria Agronomie; Lucrări Științifice, Universitatea de Stiinte Agricole si Medicină Veterinară ‘Ion Ionescu de la Brad’: Iași, Romania, 2010; Volume 53, pp. 110–113. [Google Scholar]
- Filimon, M.N.; Roman, D.L.; Caraba, I.V.; Isvoran, A. Assessment of the Effect of Application of the Herbicide S-Metolachlor on the Activity of Some Enzymes Found in Soil. Agriculture 2021, 11, 469. [Google Scholar] [CrossRef]
- Kaschuk, G.; Alberton, O.; Hungria, M. Three decades of soil microbial biomass studies in Brazilian ecosystems: Lessons learned about soil quality and indications for improving sustainability. Soil Biol. Biochem. 2010, 42, 1–13. [Google Scholar] [CrossRef]
- Kaur, P.; Jain, D.; Singh Bhullar, M. Effect of Conventional and Conservation Agriculture Practices on Dissipation of Metribuzin and Clodinafop-Propargyl, Soil Enzymatic and Microbial Activities in Wheat in a Rice-Wheat System. Soil Sediment Contam. Int. J. 2023, 1–21. [Google Scholar] [CrossRef]
- Zhang, B.; Li, Y.; Ren, T.; Tian, Z.; Wang, G.; He, X.; Tian, C. Short-term effect of tillage and crop rotation on microbial community structure and enzyme activities of a clay loam soil. Biol. Fertil Soils 2014, 50, 1077–1085. [Google Scholar] [CrossRef]
- Kumar, S.; Rana, S.S.; Kumar, R.; Sharma, N. Effects of Conservation Tillage and Weed Management On Soil Microbial Community and Enzymatic Activity. Bangladesh J. Bot. 2022, 51, 425–431. [Google Scholar] [CrossRef]
- Schmidt, R.; Gravuer, K.; Bossange, A.V.; Mitchell, J.; Scow, K. Long-term use of cover crops and no-till shift soil microbial community life strategies in agricultural soil. PLoS ONE 2018, 13, e0192953. [Google Scholar] [CrossRef]
- Helgason, B.L.; Walley, F.L.; Germida, J.J. Long-term no-till management affects microbial biomass but not community composition in Canadian prairie agroecosytems. Soil Biol. Biochem. 2010, 42, 2192–2202. [Google Scholar] [CrossRef]
- Lupwayi, N.Z.; May, W.E.; Kanashiro, D.A.; Petri, R.M. Soil bacterial community responses to black medic cover crop and fertilizer N under no-till. Appl. Soil Ecol. 2018, 124, 95–103. [Google Scholar] [CrossRef]
- Allison, S.D.; Martiny, J.B.H. Resistance, resilience, and redundancy in microbial communities. Proc. Natl. Acad. Sci. USA 2008, 105 (Suppl. 1), 11512–11519. [Google Scholar] [CrossRef]
- Khmelevtsova, L.E.; Sazykin, I.S.; Azhogina, T.N.; Sazykina, M.A. Influence of Agricultural Practices on Bacterial Community of Cultivated Soils. Agriculture 2022, 12, 371. [Google Scholar] [CrossRef]

| Herbicide | Chemical Structure | WS a (mg L−1) | Log Kow b | Kfoc/Koc c (mL g−1) | DT50 d Field/Lab (Days) | GUS Index e |
|---|---|---|---|---|---|---|
| S-metolachlor (SMOC) [2-chloro-N-(2-ethyl-6-methylphenyl)-N-[(1S)-2-meth-oxy-1-methylethyl] acetamide] | 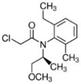 | 480 | 3.05 | 200.2 | 23.2/51.8 | 2.32 |
| Foramsulfuron (FORAM) [2-[[[[(4,6-dimethoxy-2-pyrimidinyl) amino] carbonyl] amino] sulfony]-4-(formylamino)-N,N-dimethylbenzamide] |  | 3293 | −0.78 | 78.4 | -/25.3 | 2.95 |
| Thiencarbazone-methyl (TCM) [methyl 4-[[[(4,5-dihydro-3-methoxy-4-methyl-5-oxo-1H-1,2,4-triazol-1-yl) carbonyl] amino] sulfonyl]-5-methyl-3-thiophenecarboxylic acid] |  | 436 | −1.98 | 100 | 17/51.5 | 2.46 |
| Treatment/Parameter | S1 + CT | S2 + CT | S1 + NT | S2 + NT |
|---|---|---|---|---|
| Sand (%) | 80.4 | 76.7 | 80.4 | 76.7 |
| Silt (%) | 4.7 | 6.8 | 4.7 | 6.8 |
| Clay (%) | 14.9 | 16.5 | 14.9 | 16.5 |
| pH | 6.81 | 7.67 | 6.8 | 7.67 |
| OC (%) | 0.69 | 1.01 | 0.68 | 1.01 |
| Herbicides/Soil | Residual Herbicide (μg Herbicide g−1 Dry Soil) ± SD a | ||
|---|---|---|---|
| 1 Day | 34 Days | 153 Days | |
| S-metolachlor | |||
| S1 + CT | 0.898 ± 0.09 aA | 0.421 ± 0.08 aB | 0.024 ± 0.00 -C |
| S1 + NT | 0.013 ± 0.02 cA | 0.007 ± 0.00 bB | 0.000 ± 0.00 -C |
| S2 + CT | 0.659 ± 0.04 bA | 0.423 ± 0.14 aB | 0.036 ± 0.05 -C |
| S2 + NT | 0.000 ± 0.00 c- | 0.050 ± 0.05 b- | 0.014 ± 0.02 -- |
| Foramsulfuron | |||
| S1 + CT | 0.555 ± 0.28 ab- | 0.005 ± 0.00 c- | 0.002 ± 0.00 -- |
| S1 + NT | 0.022 ± 0.02 b- | 0.011± 0.00 bc- | 0.041 ± 0.00 -B |
| S2 + CT | 0.675 ± 0.72 aA | 0.023 ± 0.00 aB | 0.041 ± 0.00 -B |
| S2 + NT | 0.009 ± 0.10 bB | 0.018 ± 0.00 abA | 0.002 ± 0.00 -C |
| Thiencarbazone-methyl | |||
| S1 + CT | 0.102 ± 0.02 aA | 0.031 ± 0.00 -B | 0.003 ± 0.00 -B |
| S1 + NT | 0.003 ± 0.01 bB | 0.012 ± 0.00 -A | 0.001 ± 0.00 -B |
| S2 + CT | 0.097 ± 0.00 aA | 0.041 ± 0.01 -B | 0.004 ± 0.00 -C |
| S2 + NT | 0.001 ± 0.00 b- | 0.021 ± 0.00 -- | 0.003 ± 0.00 -- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Douibi, M.; Carpio, M.J.; Rodríguez-Cruz, M.S.; Sánchez-Martín, M.J.; Marín-Benito, J.M. Changes in Soil Microbial Parameters after Herbicide Application in Soils under Conventional Tillage and Non-Tillage. Processes 2024, 12, 827. https://doi.org/10.3390/pr12040827
Douibi M, Carpio MJ, Rodríguez-Cruz MS, Sánchez-Martín MJ, Marín-Benito JM. Changes in Soil Microbial Parameters after Herbicide Application in Soils under Conventional Tillage and Non-Tillage. Processes. 2024; 12(4):827. https://doi.org/10.3390/pr12040827
Chicago/Turabian StyleDouibi, Marwa, María José Carpio, María Sonia Rodríguez-Cruz, María J. Sánchez-Martín, and Jesús M. Marín-Benito. 2024. "Changes in Soil Microbial Parameters after Herbicide Application in Soils under Conventional Tillage and Non-Tillage" Processes 12, no. 4: 827. https://doi.org/10.3390/pr12040827






