Cs4PMo11VO40-Catalyzed Glycerol Ketalization to Produce Solketal: An Efficient Bioadditives Synthesis Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis and Characterization of Unsubstituted and Vanadium-Substituted Cesium Phosphomolybdate Salts
2.3. Catalytic Runs
3. Results and Discussion
3.1. Catalytic Characterization
3.2. Catalytic Runs
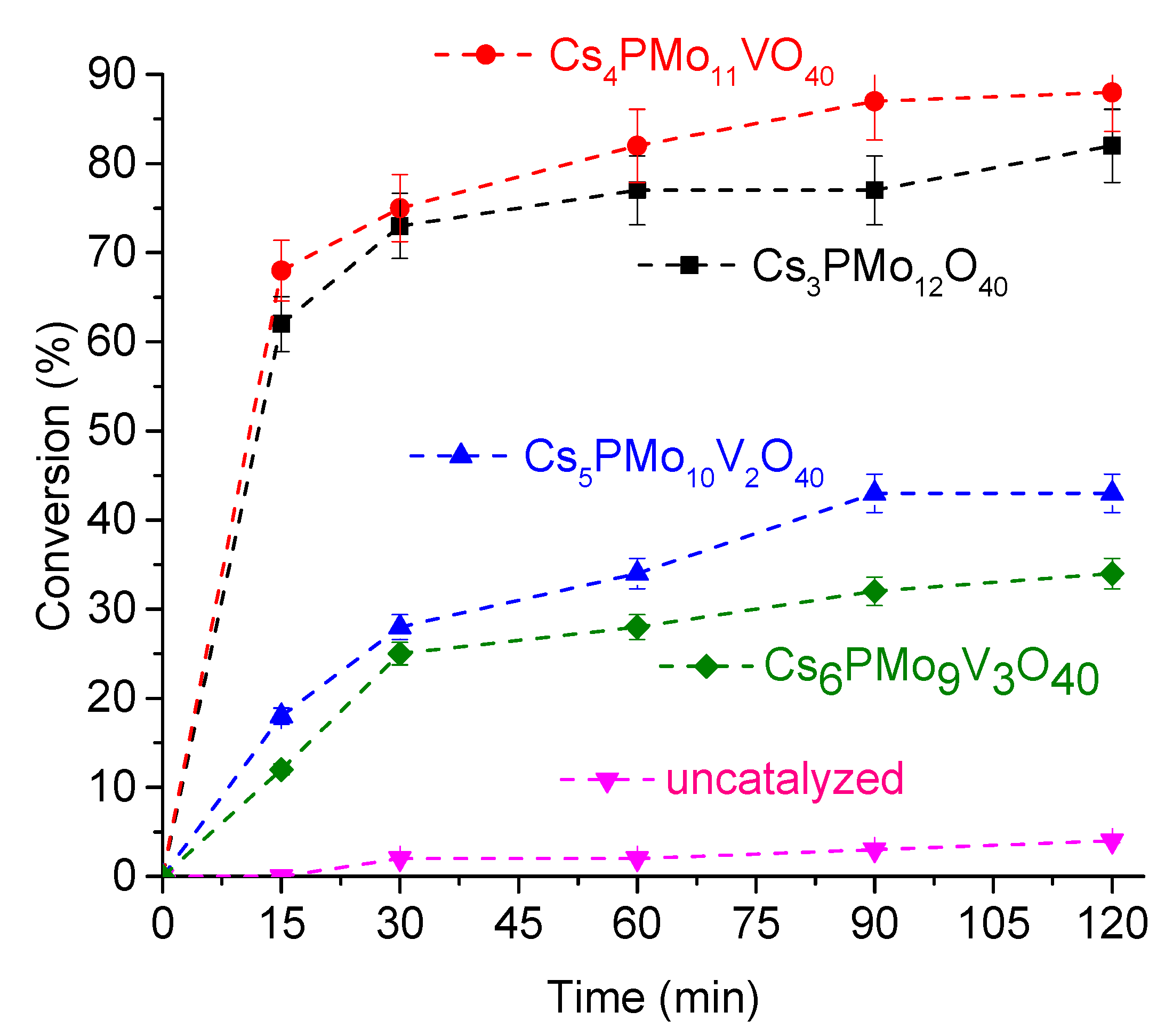
3.2.1. The Effect of Catalyst Nature
3.2.2. Mechanistic Insights
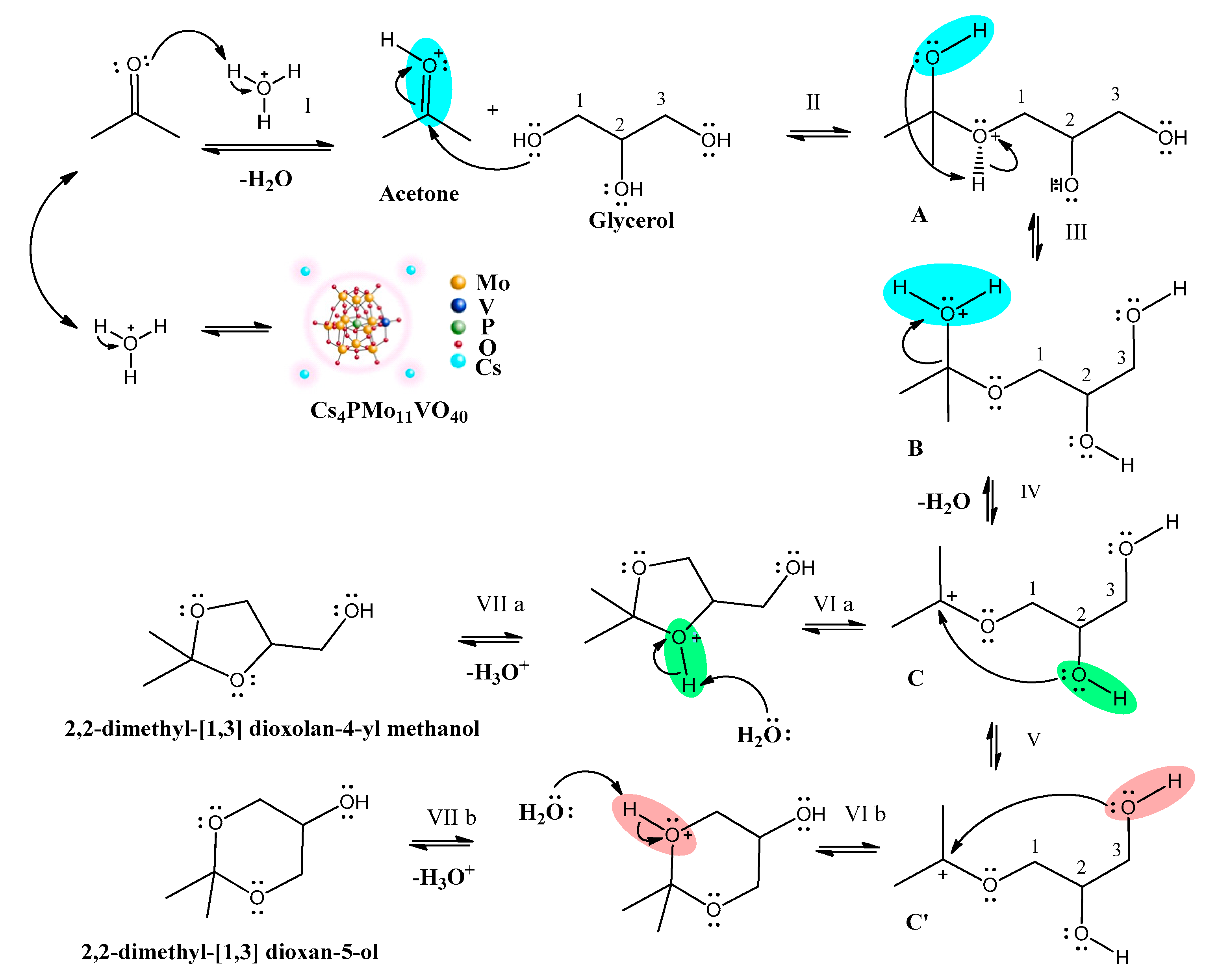
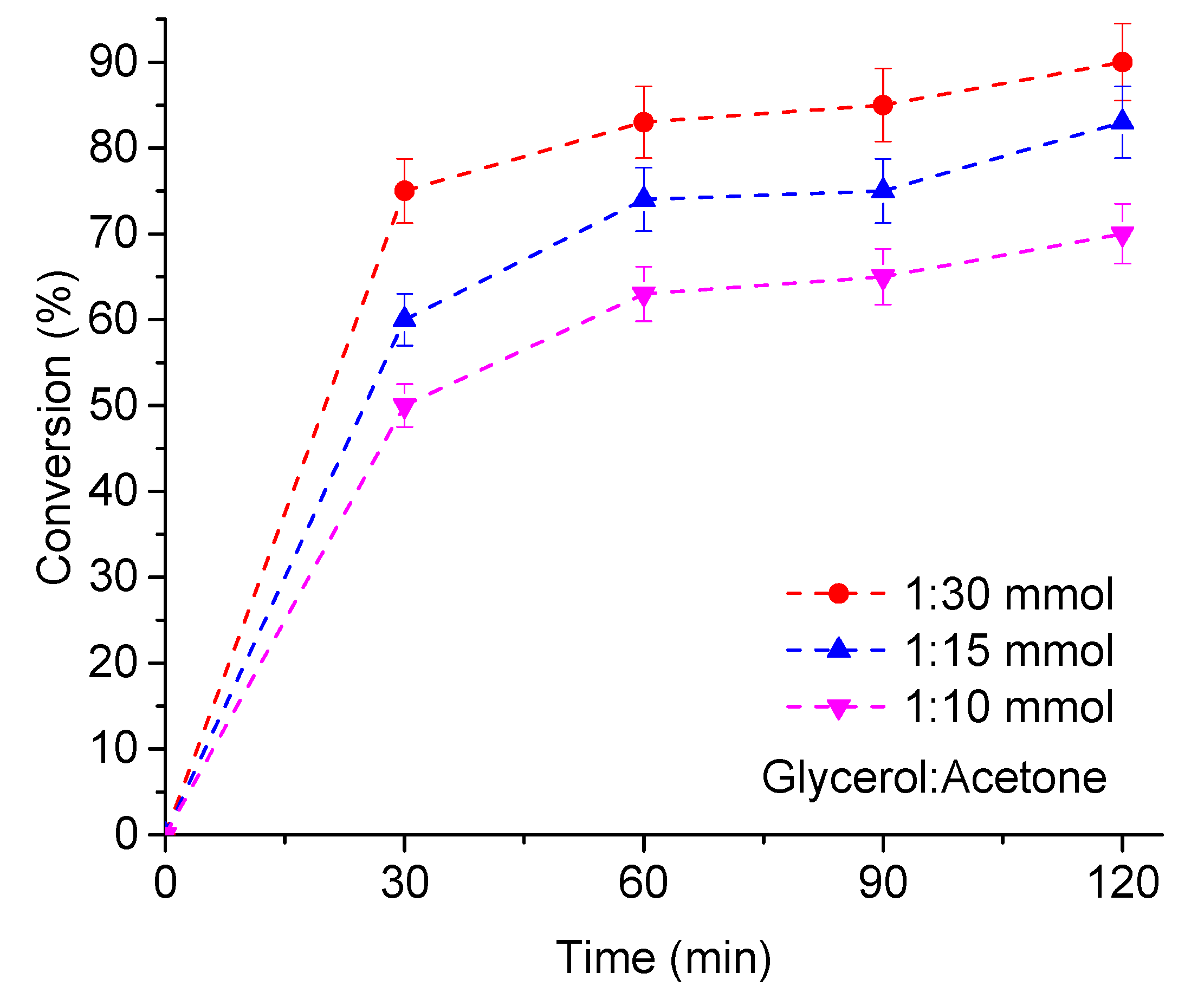
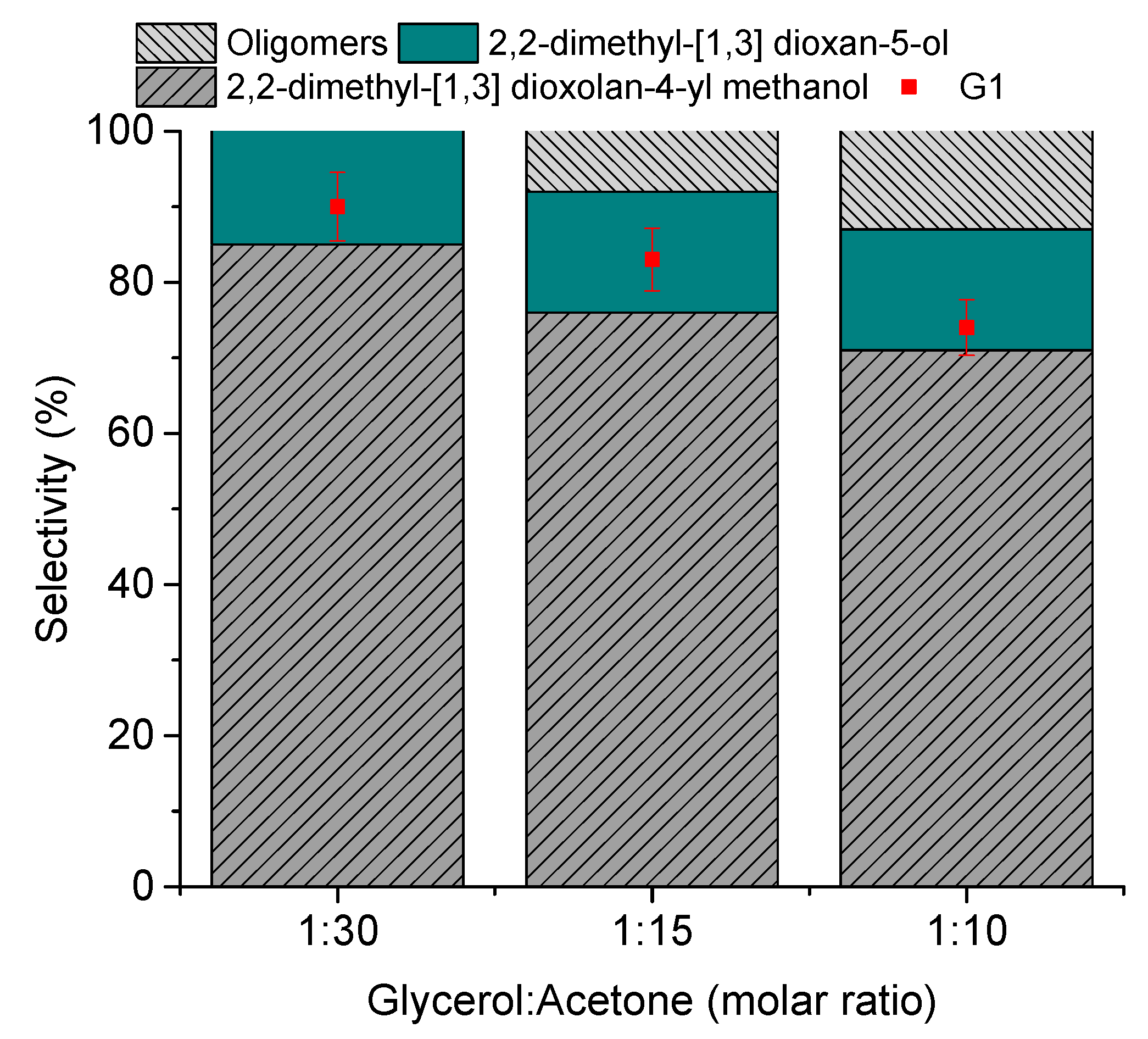
3.2.3. The Effect of the Stoichiometry of Reactants
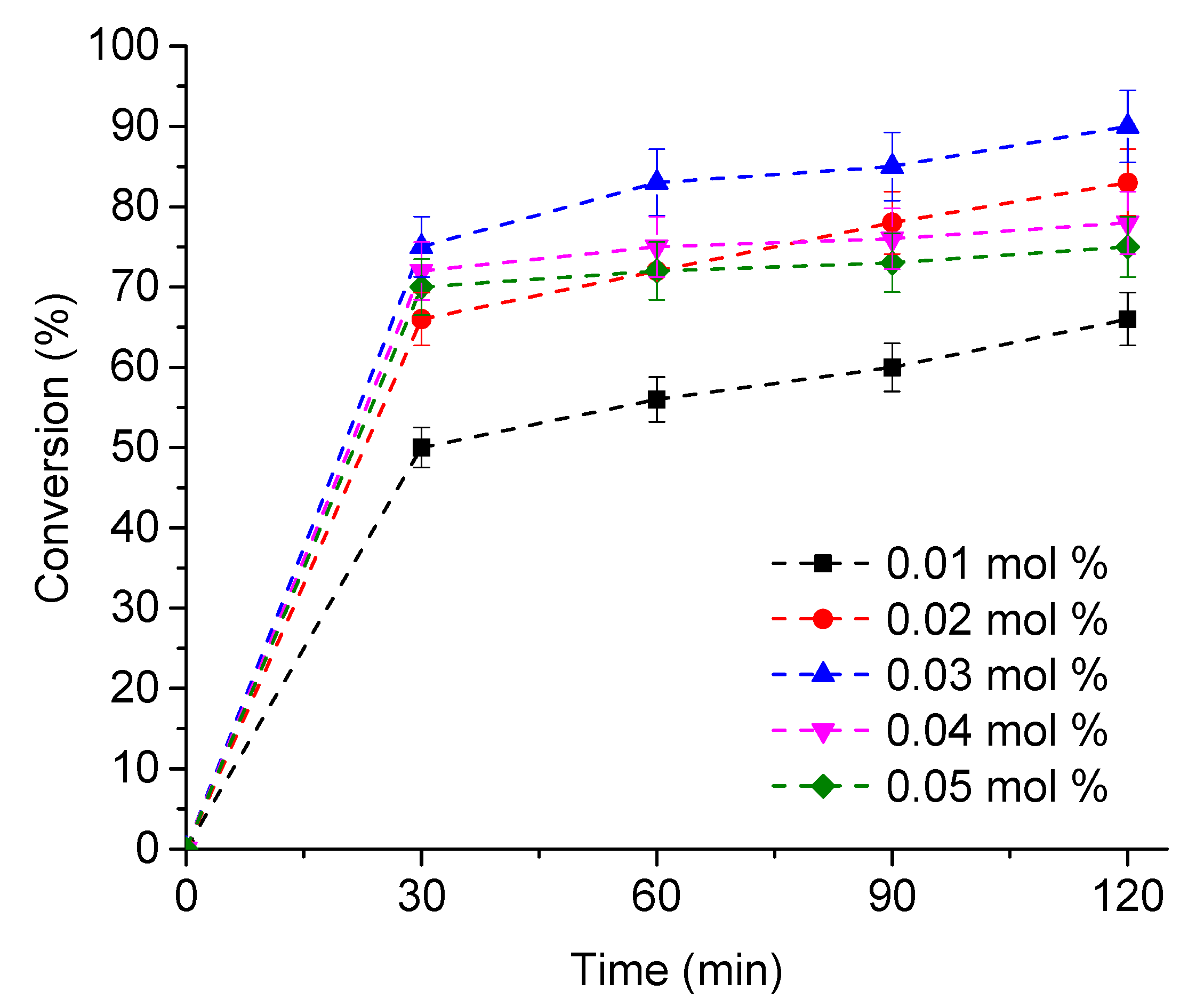
3.2.4. The Effect of Catalyst Load
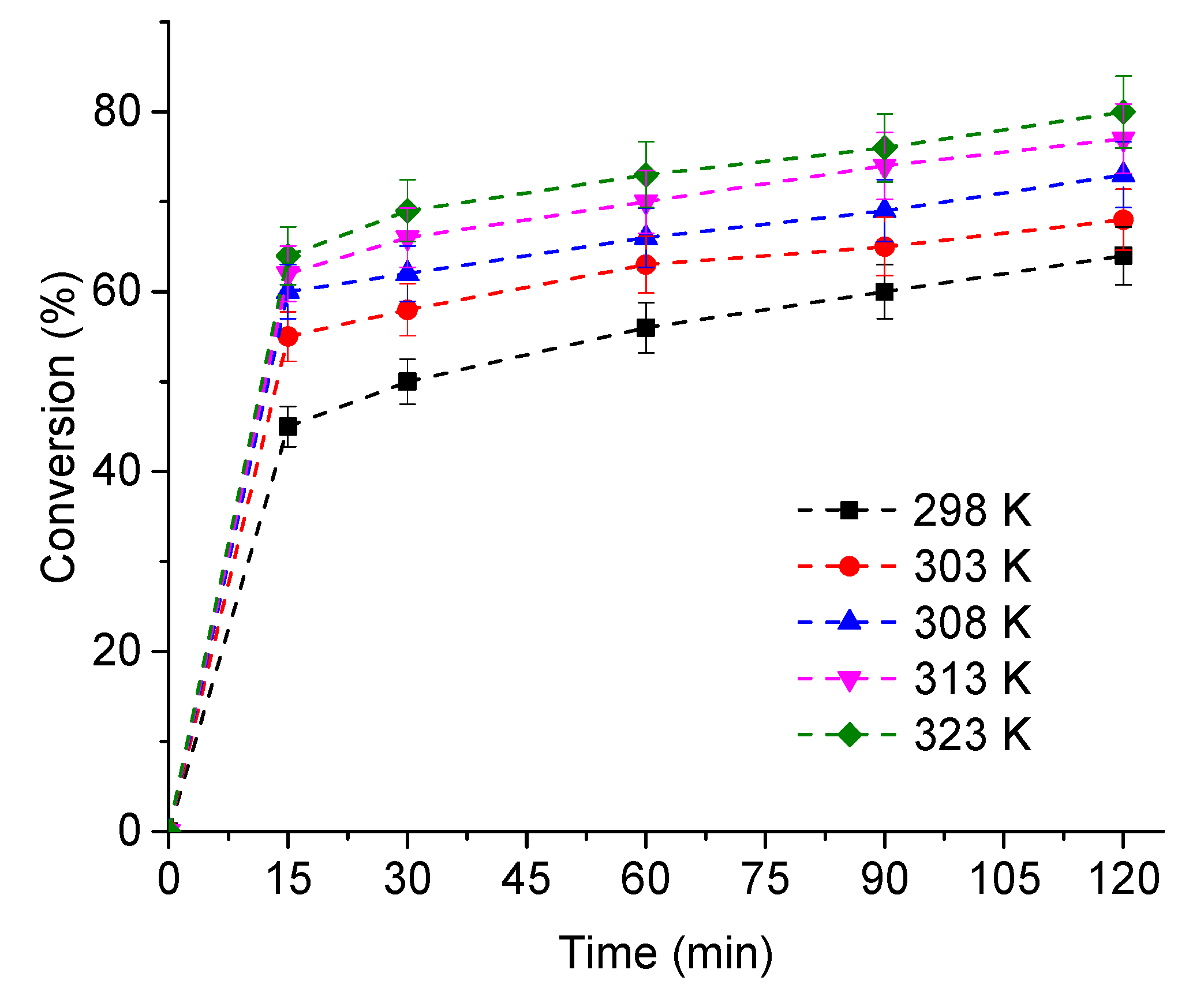
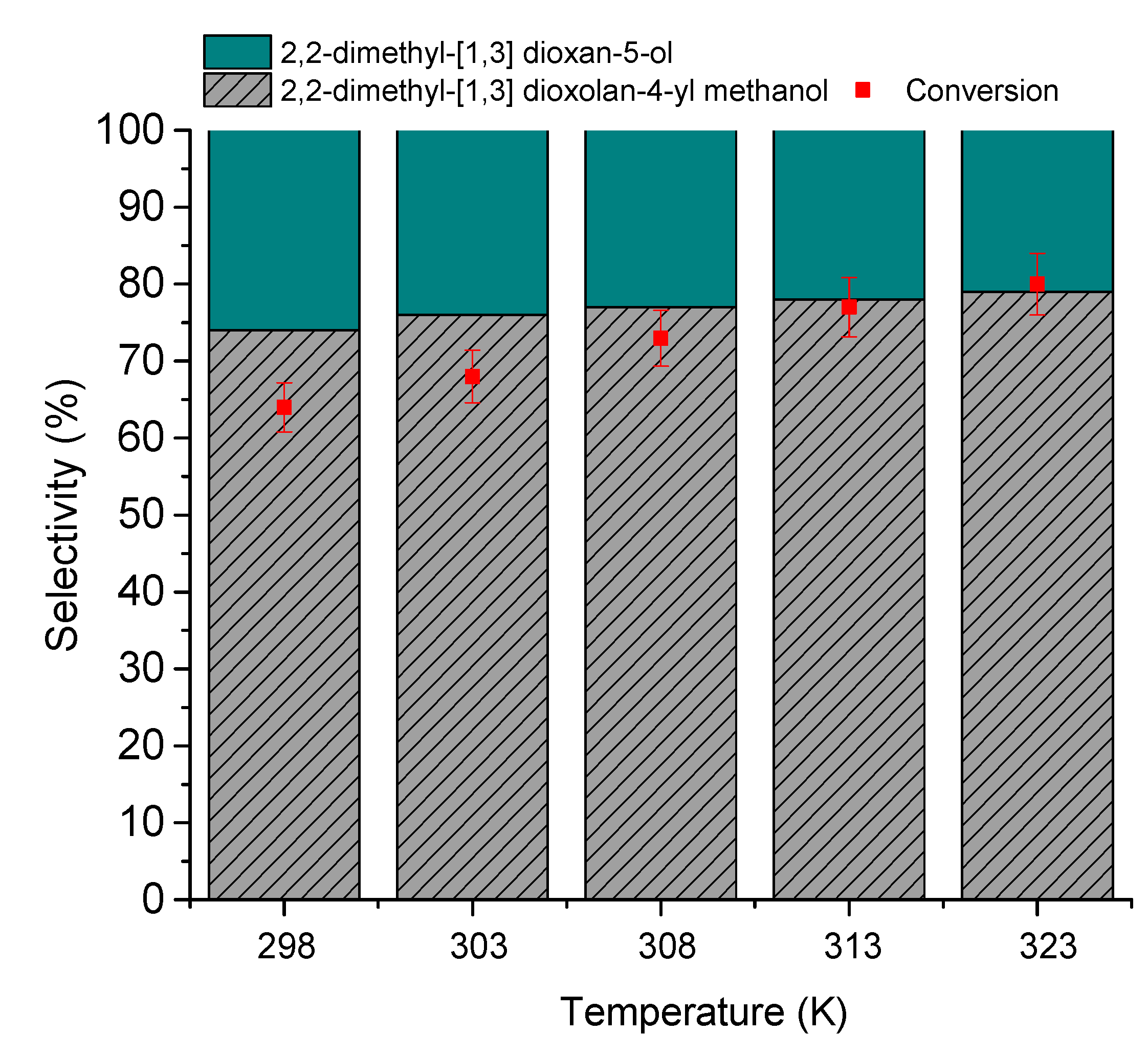
3.2.5. The Effects of Temperature
3.2.6. Recovery and Reuse of the Cs4PMo11VO40 Catalyst
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Monteiro, M.R.; Kugelmeier, C.L.; Pinheiro, R.S.; Batalha, M.O.; da Silva, C.A. Glycerol from biodiesel production: Techno logical paths for sustainability. Renew. Sustain. Energy Rev. 2018, 88, 109–122. [Google Scholar] [CrossRef]
- Olson, A.L.; Tunér, M.; Verhelst, S. A concise review of glycerol derivatives for use as fuel additives. Heliyon 2023, 9, e13041–e13054. [Google Scholar] [CrossRef] [PubMed]
- Kong, P.S.; Aroua, M.K.; Daud, W.M.A.W. Conversion of crude and pure glycerol into derivatives: A feasibility evaluation. Renew. Sustain. Energy Rev. 2016, 63, 533–555. [Google Scholar] [CrossRef]
- Checa, M.; Nogales-Delgado, S.; Montes, V.; Encinar, J.M. Recent advances in glycerol catalytic valorization: A review. Catalysts 2020, 10, 1279–1320. [Google Scholar] [CrossRef]
- Rodrigues, A.; Bordado, J.C.; Santos, R.G. Upgrading the glycerol from biodiesel production as a source of energy carriers and chemicals-A technological review for three chemical pathways. Energies 2017, 10, 1817–1853. [Google Scholar] [CrossRef]
- Fatimah, I.; Sahroni, I.; Fadillah, G.; Musawwa, M.M.; Mahlia, T.M.I.; Muraza, O. Glycerol to solketal for fuel additive: Recent progress in heterogeneous catalysts. Energies 2019, 12, 2872–2886. [Google Scholar] [CrossRef]
- Tan, H.W.; Aziz, A.A.R.; Aroua, M.K. Glycerol production and its applications as a raw material: A review. Renew. Sustain. Energy Rev. 2013, 27, 118–127. [Google Scholar] [CrossRef]
- Trifoi, A.R.; Agachi, P.Ş.; Pap, T. Glycerol acetals and ketals as possible diesel additives. A review of their synthesis proto cols. Renew. Sustain. Energy Rev. 2016, 62, 804–814. [Google Scholar] [CrossRef]
- Mota, C.J.A.; da Silva, C.X.A.; Rosenbach, N.; Costa, J.; da Silva, F. Glycerin derivatives as fuel additives: The addition of glycerol/acetone ketal (solketal) in gasolines. Energy Fuels 2010, 24, 2733–2736. [Google Scholar] [CrossRef]
- Nanda, M.R.; Zhang, Y.; Yuan, Z.; Qin, W.; Ghaziaskar, H.S.; Xu, C. Catalytic conversion of glycerol for sustainable pro duction of solketal as a fuel additive: A review. Renew. Sust. Energy Rev. 2016, 56, 1022–1031. [Google Scholar] [CrossRef]
- Yadav, N.; Yadav, G.; Ahmaruzzaman, M. Camellia sinensis leaf-assisted green synthesis of SO3H-functionalized ZnS/biochar nanocatalyst for highly selective solketal production and improved reusability in methylene blue dye adsorption. Renew. Energy 2024, 224, 120179. [Google Scholar] [CrossRef]
- Zahid, I.; Ayoub, M.; Nazir, M.H.; Sher, F.; Shamsuddin, R.; Abdullah, B.; Ameen, M. Kinetic & thermodynamic studies of green fuel additive solketal from crude glycerol over metakaolin clay catalyst. Biomass Bioenergy 2024, 181, 107036. [Google Scholar]
- Kumawat, S.; Singh, S.; Bhatt, T.; Maurya, A.; Vaidyanathan, S.; Natte, K.; Jagadeesh, R.V. Valorization of bio-renewable glycerol by catalytic amination reactions. Green Chem. 2024, 26, 3021–3038. [Google Scholar] [CrossRef]
- Li, L.; Korányi, T.I.; Sels, B.F.; Pescarmona, P.P. Highly-efficient conversion of glycerol to solketal over heterogeneous Lewis acid catalysts. Green Chem. 2012, 14, 1611–1619. [Google Scholar] [CrossRef]
- Yang, J.; Li, N.; Ma, W.J.; Zhou, J.H.; Sun, H.Z. Synthesis of solketal with catalyst sulfonic acid resin. Adv. Mater. Res. 2013, 830, 176–179. [Google Scholar] [CrossRef]
- Nakajima, K.; Hara, M. Amorphous carbon with SO3H groups as a solid Brønsted acid catalyst. ACS Catal. 2012, 2, 1296–1304. [Google Scholar] [CrossRef]
- Rodrigues, R.; Mandelli, D.; Gonçalves, N.S.; Pescarmona, P.P.; Carvalho, W.A. Acetalization of acetone with glycerol catalyzed by niobium-aluminum mixed oxides synthesized by a sol-gel process. J. Mol. Catal. A Chem. 2016, 422, 122–130. [Google Scholar] [CrossRef]
- Gonçalves, M.; Rodrigues, R.; Galhardo, T.S.; Carvalho, W.A. Highly selective acetalization of glycerol with acetone to solketal over acidic carbon-based catalysts from biodiesel waste. Fuel 2016, 181, 46–54. [Google Scholar] [CrossRef]
- Kowalska-Kus, J.; Held, A.; Frankowski, M.; Nowinska, K. Solketal formation from glycerol and acetone over hierarchical zeolites of different structure as catalysts. J. Mol. Catal. A Chem. 2017, 426, 205–212. [Google Scholar] [CrossRef]
- Rahaman, M.S.; Phung, T.K.; Hossain, M.A.; Chowdhury, E.; Tulaphol, S.; Lalvani, S.B.; O’Toole, M.; Willing, G.A.; Jasinski, J.B.; Crocker, M.; et al. Hydrophobic functionalization of HY zeolites for efficient conversion of glycerol to solketal. Appl. Catal. A 2020, 592, 117369–117378. [Google Scholar] [CrossRef]
- Poly, S.S.; Jamil, M.A.R.; Touchy, A.S.; Yasumura, S.; Siddiki, S.M.A.H.; Toyao, T.; Maenoa, Z.; Shimizu, K.-I. Acetalization of glycerol with ketones and aldehydes catalyzed by high silica Hβ zeolite. Mol. Catal. 2019, 479, 110608–110614. [Google Scholar] [CrossRef]
- Stawicka, K.; Díaz-Álvarez, A.E.; Calvino-Casilda, V.; Trejda, M.; Bañares, M.A.; Ziolek, M. The role of Brønsted and Lewis acid sites in acetalization of glycerol over modified mesoporous cellular foams. J. Phys. Chem. C 2016, 120, 16699–16711. [Google Scholar] [CrossRef]
- Chen, L.; Nohair, B.; Zhao, D.; Kaliaguine, S. Glycerol acetalization with formaldehyde using heteropolyacid salts sup ported on mesostructured silica. Appl. Catal. A 2018, 549, 207–215. [Google Scholar] [CrossRef]
- Ferreira, P.; Fonseca, I.M.; Ramos, A.M.; Vital, J.; Castanheiro, J.E. Valorisation of glycerol by condensation with acetone over silica-included heteropolyacids. Appl. Catal. B Environ. 2010, 98, 94–99. [Google Scholar] [CrossRef]
- Abreu, T.H.; Meyer, C.I.; Padró, C.; Martins, L. Acidic V-MCM-41 catalysts for the liquid-phase ketalization of glycerol with acetone. Microporous Mesoporous Mater. 2019, 273, 219–225. [Google Scholar] [CrossRef]
- Li, X.; Jiang, Y.; Zhou, R.; Hou, Z. Acetalization of glycerol with acetone over appropriately-hydrophobic zirconium or organophosphonates. Appl. Clay Sci. 2020, 189, 105555–105563. [Google Scholar] [CrossRef]
- Kozhevnikov, I.V. Catalysis by heteropoly acids and multicomponent polyoxometalates in 424 liquid-phase reactions. Chem. Rev. 1998, 98, 171–198. [Google Scholar] [CrossRef] [PubMed]
- Pope, M.T.; Müller, A. Polyoxometalate chemistry: An old field with new dimensions in several disciplines. Angew. Chem. Int. Ed. Engl. 1991, 30, 34–48. [Google Scholar] [CrossRef]
- Li, D.; Ma, P.; Niu, J.; Wang, J. Recent advances in transition-metal-containing Keggin-type polyoxometalate-based coor dination polymers. Coord. Chem. Rev. 2019, 392, 49–80. [Google Scholar] [CrossRef]
- da Silva, M.J.; Liberto, N.A. Soluble and solid supported Keggin heteropolyacids as catalysts in reactions for biodiesel production: Challenges and recent advances. Curr. Org. Chem. 2016, 20, 1263–1283. [Google Scholar] [CrossRef]
- Devasan, R.; Gouda, S.P.; Halder, H.; Rokhum, S.L. Microwave-assisted valorization of biodiesel byproduct glycerol to solketal over Musa acuminata peel waste derived solid acid catalyst: Process optimization, kinetics, and thermodynamics. Energy Adv. 2024, 3, 316–329. [Google Scholar] [CrossRef]
- da Silva, M.J.; de Oliveira, C.M. Catalysis by Keggin Heteropolyacid Salts. Curr. Catal. 2018, 7, 26–34. [Google Scholar] [CrossRef]
- Gromov, N.V.; Medvedeva, T.B.; Lukoyanov, I.A.; Panchenko, V.N.; Prikhod’ko, S.A.; Parmon, V.N.; Timofeeva, M.N. Hydrolysis-oxidation of cellulose to formic acid in the presence of micellar vanadium-containing molybdophosphoric het eropoly acids. Results Eng. 2023, 17, 100913. [Google Scholar] [CrossRef]
- da Silva, M.J.; Teixeira, M.G.; Chaves, D.M.; Siqueira, L. An efficient process to synthesize solketal from glycerol over tin(II) silicotungstate catalyst. Fuel 2020, 281, 118724. [Google Scholar] [CrossRef]
- Budych, M.J.W.; Staszak, K.K.; Bajek, A.; Pniewski, F.; Jastrzab, R.; Staszak, M.; Tylkowski, B.; Wieszczycka, K. The future of polyoxymetalates for biological and chemical apllications. Coord. Chem. Rev. 2023, 493, 215306. [Google Scholar] [CrossRef]
- Castro, G.A.D.; Lopes, N.P.G.; Fernandes, A.S.; da Silva, M.J. Copper phosphotungstate-catalyzed microwave-assisted synthesis of 5-hydroxymethylfurfural in a biphasic system. Cellulose 2022, 29, 5529–5545. [Google Scholar] [CrossRef]
- Hombach, L.; Simitsis, N.; Vossen, J.T.; Vorhoit, A.J.; Beine, A.K. Solidified and Immobilized Heteropolyacids for the Valorization of Lignocellulose. ChemCatChem 2022, 14, e202101838. [Google Scholar] [CrossRef]
- Narasimharao, K.; Brown, D.; Lee, A.F.; Newman, A.; Siril, P.; Tavener, S.; Wilson, K. Structure–activity rela tions in Cs-doped heteropolyacid catalysts for biodiesel production. J. Catal. 2007, 248, 226–234. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, Y.; Liu, X.; Shi, X.; Wang, C.; Zhong, H.; Jin, F. Sustainable production of formic acid and acetic acid from biomass. Mol. Catal. 2023, 545, 113199. [Google Scholar] [CrossRef]
- Pope, M.T.; Jeannin, Y.; Fournier, M. Heteropoly and Isopoly Oxometalates; Springer: Berlin/Heidelberg, Germany, 1983; Volume 8, p. 420. [Google Scholar]
- Mouanni, S.; Amitouche, D.; Mazari, T.; Rabia, C. Transition metal-substituted Keggin-type polyoxometalates as catalysts for adipic acid production. Appl. Petrochem. Res. 2019, 9, 67–75. [Google Scholar] [CrossRef]
- da Silva, M.J.; Andrade, P.H.S.; Ferreira, S.O.; Vilanculo, C.B.; Oliveira, C.M. Monolacunary K8SiW11O39-Catalyzed terpenic alcohols oxidation with hydrogen peroxide. Catal. Lett. 2018, 148, 2516–2527. [Google Scholar] [CrossRef]
- Wang, S.-S.; Yang, G.-Y. Recent advances in polyoxometalate-catalyzed reactions. Chem. Rev. 2015, 115, 4893–4992. [Google Scholar] [CrossRef] [PubMed]
- da Silva, M.J.; Ribeiro, C.J.; Vilanculo, C.B. How the content of protons and vanadium affects the activity of H3+nPMo12-nVnO40 (n = 0, 1, 2, or 3) catalysts on the oxidative esterification of benzaldehyde with hydrogen peroxide. Catal. Lett. 2023, 153, 2045–2056. [Google Scholar] [CrossRef]
- Tsigdinos, G.A.; Hallada, C.J. Molybdovanadophosphoric acids and their salts. I. Investigation of methods of preparation and characterization. Inorg. Chem. 1968, 7, 437–441. [Google Scholar] [CrossRef]
- da Silva, M.J.; Ribeiro, C.J.A.; de Araújo, E.N.; Torteloti, I.M. Acetalization of alkyl alcohols with benzaldehyde over cesium phosphor molybdovanadate salts. Processes 2023, 11, 2220. [Google Scholar] [CrossRef]
- Yua, B.-Y.; Tsenga, T.-Y.; Yanga, Z.-Y.; Shen, S.-J. Evaluation on the solketal production processes: Rigorous design, optimization, environmental analysis, and control. Process Saf. Environ. Prot. 2022, 157, 140–155. [Google Scholar] [CrossRef]
- Ray, P.C.; Roberts, S.M. Overcoming intrinsic diastereoselection using polyleucine as a chiral epoxidate ion catalyst. Tetrahedron Lett. 1999, 40, 1779–1782. [Google Scholar] [CrossRef]
- Deutsch, J.A.; Martin, A.; Lieske, H. Investigations on heterogeneously catalysed condensations of glycerol to cyclic acetals. J. Catal. 2007, 245, 428–435. [Google Scholar] [CrossRef]
- Roldán, L.L.; Mallada, R.; Fraile, J.M.; Mayoral, J.A.; Menéndez, M. Glycerol upgrading by ketalization in a zeolite membrane reactor. Asia-Pac. J. Chem. Eng. 2009, 4, 279–284. [Google Scholar] [CrossRef]




| Catalyst | Initial Electrode Potential (mV) | Acidity Strength a | Acid Site Numbers (mEq/g) |
|---|---|---|---|
| H3PMo12O40 | 695 | Very strong | 1.3 |
| Cs3PMo12O40 | 450 | Very strong | 0.2 |
| Cs4PMo11VO40 | 460 | Very strong | 0.6 |
| Cs5PMo10V2O40 | 330 | Very strong | 0.2 |
| Cs6PMo9V3O40 | 90 | strong | 0.1 |
| Catalyst | Surface Area (m2·g−1) | Pore Volume (m3·g−1) × 10−3 | Diameter Pore (Å) |
|---|---|---|---|
| H3PMo12O40 | 2.8 | 1.7 | 3.8 |
| Cs3PMo12O40 | 104.1 | 37.3 | 2.0 |
| Cs4PMo11VO40 | 55.6 | 17.4 | 3.5 |
| Cs5PMo10V2O40 | 4.1 | 1.0 | 3.1 |
| Cs6PMo9V3O40 | 3.0 | 0.5 | 3.1 |
| Catalyst | Load (wt. %) | Temperature (K) | Conv. | TON | Ref. |
|---|---|---|---|---|---|
| Amberlyst-36 | 5 | 313 | 88 | 33 | [48] |
| Zeolite beta | 13 | 343 | 60 | 22 | [49] |
| Zeolite USY | 19 | 343 | 90 | 33 | [49] |
| H3PW12O40/SiO2 | 5 | 343 | 100 | 121 | [50] |
| Cs4PMo11VO40 | 0.36 | 298 | 90 | 180 | This work |
| Run | Conversion | Selectivity % Dioxane/Dioxolane | Recovery % |
|---|---|---|---|
| 1 | 89 | 12/88 | 97 |
| 2 | 88 | 10/90 | 98 |
| 3 | 85 | 11/89 | 97 |
| 4 | 86 | 10/90 | 97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, M.J.; Andrade Ribeiro, C.J. Cs4PMo11VO40-Catalyzed Glycerol Ketalization to Produce Solketal: An Efficient Bioadditives Synthesis Method. Processes 2024, 12, 854. https://doi.org/10.3390/pr12050854
da Silva MJ, Andrade Ribeiro CJ. Cs4PMo11VO40-Catalyzed Glycerol Ketalization to Produce Solketal: An Efficient Bioadditives Synthesis Method. Processes. 2024; 12(5):854. https://doi.org/10.3390/pr12050854
Chicago/Turabian Styleda Silva, Márcio José, and Cláudio Júnior Andrade Ribeiro. 2024. "Cs4PMo11VO40-Catalyzed Glycerol Ketalization to Produce Solketal: An Efficient Bioadditives Synthesis Method" Processes 12, no. 5: 854. https://doi.org/10.3390/pr12050854





