Biomimetic Approach for Enhanced Mechanical Properties and Stability of Self-Mineralized Calcium Phosphate Dibasic–Sodium Alginate–Gelatine Hydrogel as Bone Replacement and Structural Building Material
Abstract
:1. Introduction
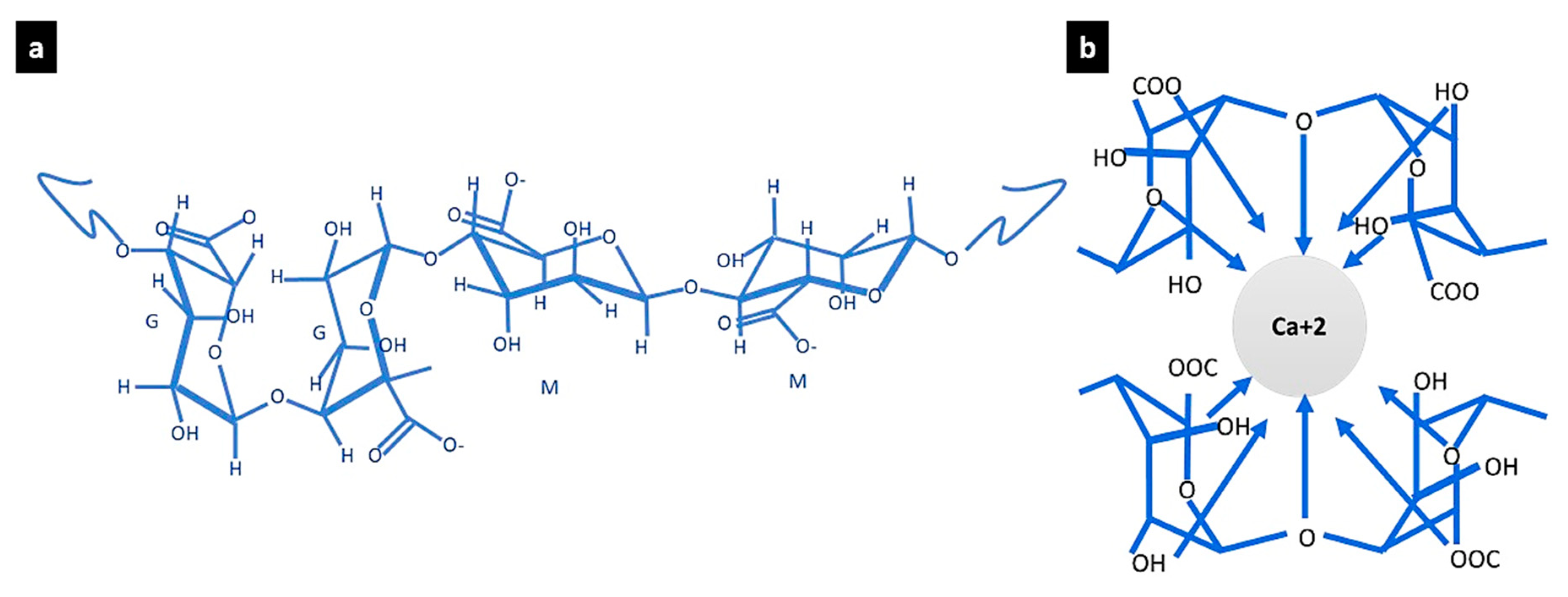
Alginate–Gelatine–Calcium Phosphate Trio-Hydrogels for Bone Tissue Regeneration
2. Materials and Methods
2.1. Rheological Properties of Alginate–Gelatine Hydrogel Enhanced with Different Concentrations of Calcium Phosphate
2.2. Rheological Properties of Alginate–Gelatine–Calcium Phosphate Hydrogel Enhanced with Different Concentrations of Epigallocatechin Gallate
2.3. Mineralization Test of the CPDB-Modified SA–Gelatine without Cells, Incubated in Fetal Bovine Serum and Air, Respectively, for 7 and 14 Days, Respectively
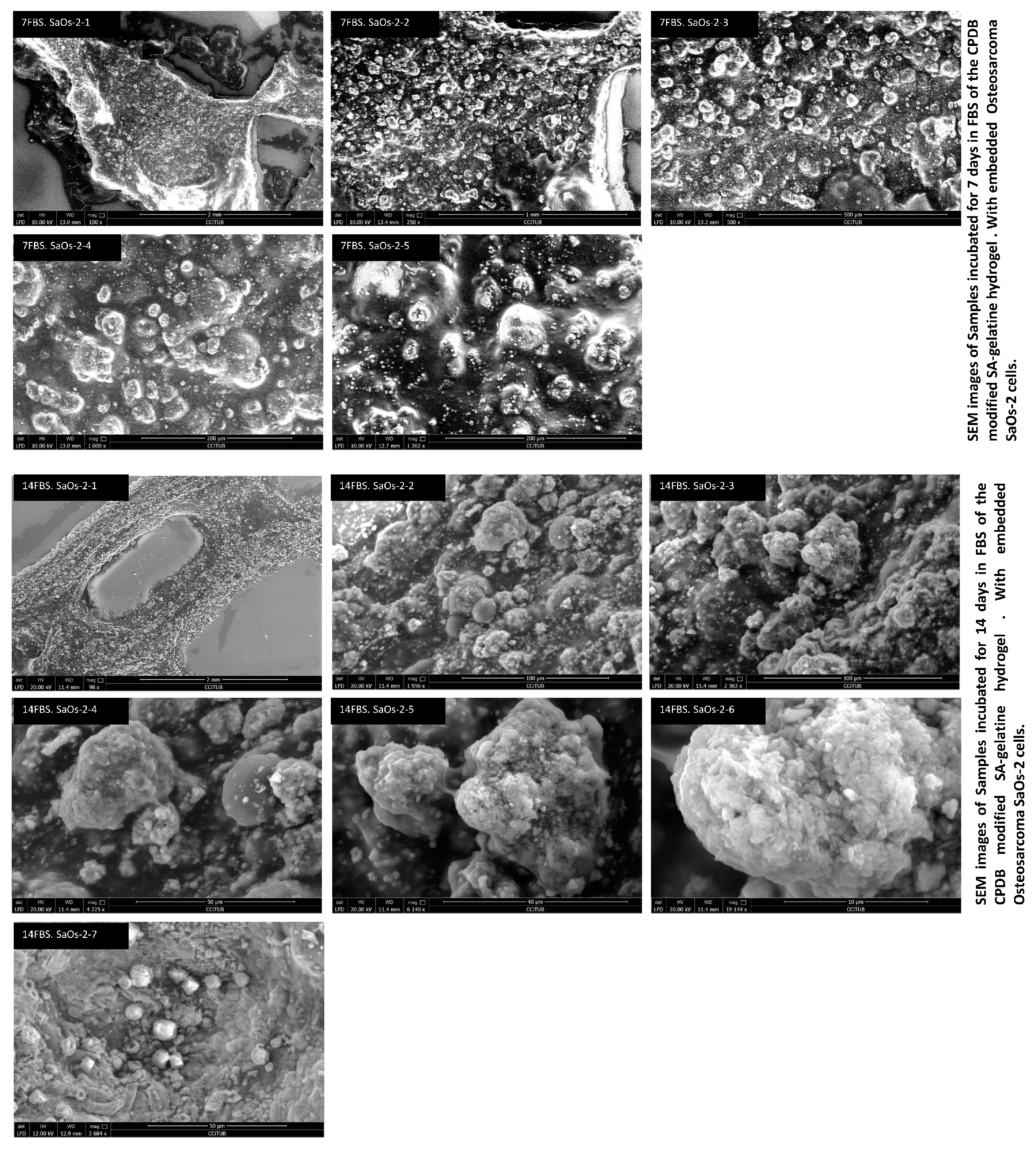
2.4. Mineralization Test of CPDB-Modified SA–Gelatine with Osteosarcoma Cells Incubated in Fatal Bovine Serum and Air for 7 and 14 Days, Respectively
3. Results and Discussion
3.1. Rheological Properties of SA–Gelatine Hydrogel Enhanced with Different Concentrations of Calcium Phosphate Dibasic
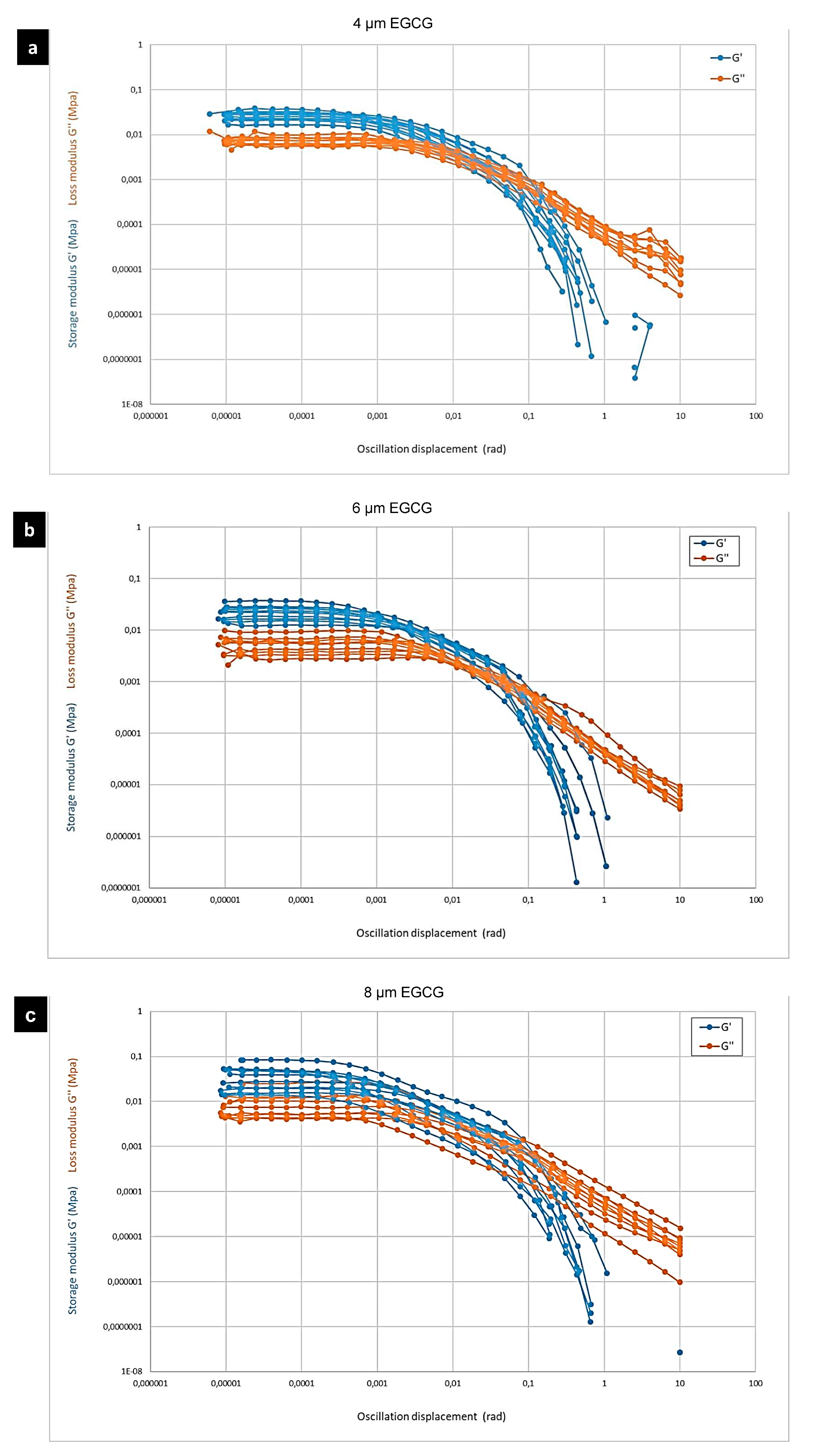
3.2. Rheological Properties of CPDB-Modified SA–Gelatine Hydrogel Enhanced with Different Concentrations of Epigallocatechin Gallate
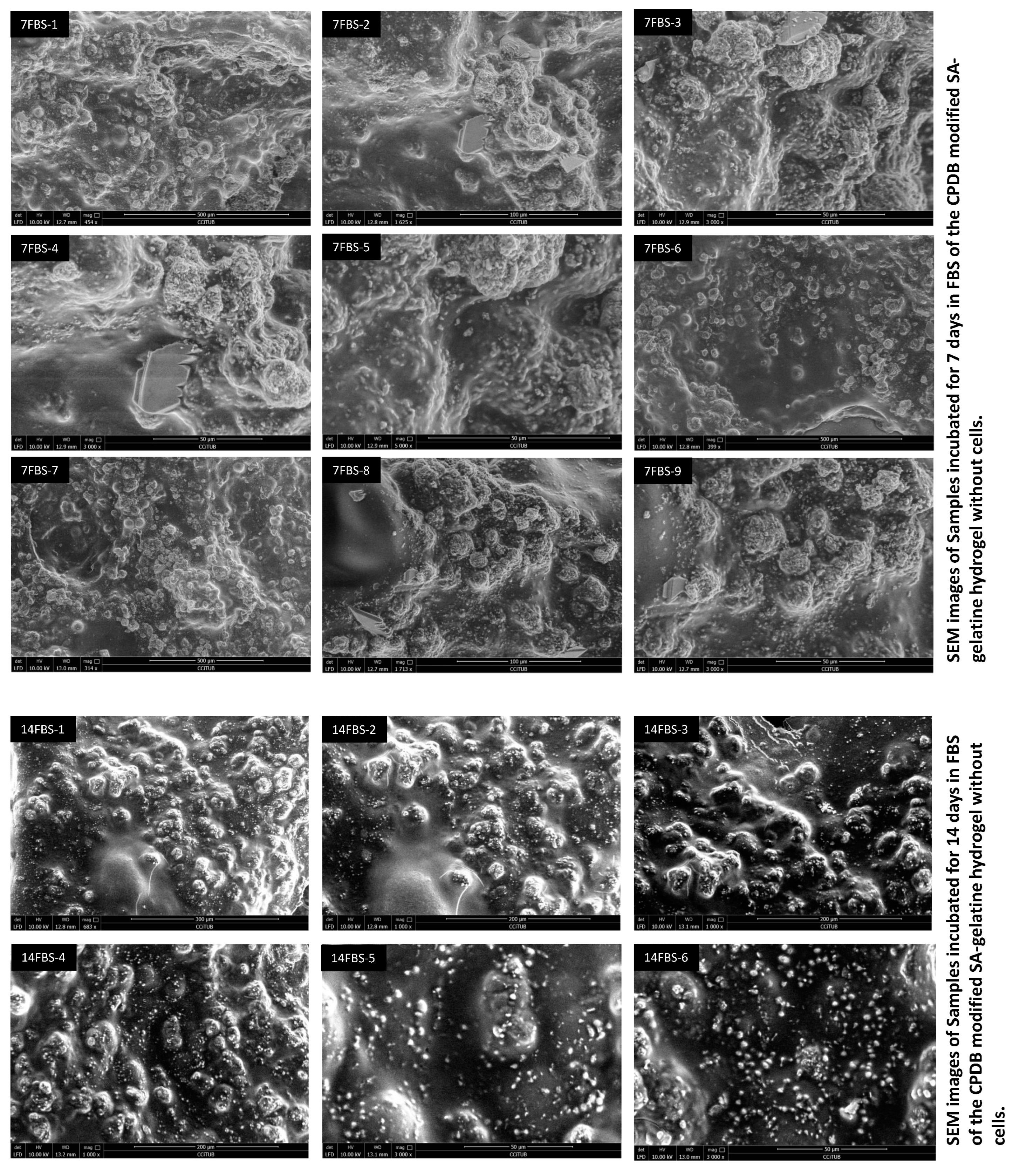
3.3. Mineralization of the 6% CPDB-Modified SA–Gelatine Hydrogel Incubated in Fetal Bovine Serum and Air

3.4. Mineralization Test of CPDB-Modified SA–Gelatine Encapsulating Osteosarcoma Cells Incubated in Fatal Bovine Serum and Air for 7 and 14 Days, Respectively
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wen, B.; Dai, Y.; Han, X.; Huo, F.; Xie, L.; Yu, M.; Wang, Y.; An, N.; Li, Z.; Guo, W. Biomineralization-inspired mineralized hydrogel promotes the repair and regeneration of dentin/bone hard tissue. NPJ Regen. Med. 2023, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Walsh, K.; Hoff, B.L.; Camci-Unal, G. Mineralization of Biomaterials for Bone Tissue Engineering. Bioengineering 2020, 7, 132. [Google Scholar] [CrossRef] [PubMed]
- Parra-Torrejón, B.; Jayawarna, V.; Rodrigo-Navarro, A.; Gonzalez-Valdivieso, J.; Dobre, O.; Ramírez-Rodríguez, G.B.; Salmeron-Sanchez, M.; Delgado-López, J.M. Bioinspired mineralization of engineered living materials to promote osteogenic differentiation. Biomater. Adv. 2023, 154, 213587. [Google Scholar] [CrossRef]
- Abdallah, Y.K.; Estévez, A.T. Biowelding 3D-Printed Biodigital Brick of Seashell-Based Biocomposite by Pleurotus ostreatus Mycelium. Biomimetics 2023, 8, 504. [Google Scholar] [CrossRef]
- Definition of Biomineralization. Available online: https://www.merriam-webster.com/dictionary/biomineralization (accessed on 28 September 2022).
- Vinn, O. Occurrence, Formation and Function of Organic Sheets in the Mineral Tube Structures of Serpulidae (Polychaeta, Annelida). PLoS ONE 2013, 8, e75330. [Google Scholar] [CrossRef]
- Boskey, A.L. Biomineralization: Conflicts, challenges, and opportunities. J. Cell. Biochem. 1998, 30–31, 83–91. [Google Scholar] [CrossRef]
- Sarikaya, M. Biomimetics: Materials fabrication through biology. Proc. Natl. Acad. Sci. USA 1999, 96, 14183–14185. [Google Scholar] [CrossRef]
- Sherman, V.R.; Yang, W.; Meyers, M.A. The materials science of collagen. J. Mech. Behav. Biomed. Mater. 2015, 52, 22–50. [Google Scholar] [CrossRef]
- Yokoi, T.; Kawashita, M.; Ohtsuki, C. Biomimetic mineralization of calcium phosphates in polymeric hydrogels containing carboxyl groups. J. Asian Ceram. Soc. 2013, 1, 155–162. [Google Scholar] [CrossRef]
- Nie, L.; Li, X.; Wang, Z.; Hu, K.; Cai, R.; Li, P.; Han, Y.; Sun, M.; Yuan, H.; Suo, J.; et al. In vitro biomineralization on poly(vinyl alcohol)/biphasic calcium phosphate hydrogels. Bioinspired Biomim. Nanobiomater. 2020, 9, 122–128. [Google Scholar] [CrossRef]
- Onozato, H.; Watabe, N. Studies on fish scale formation and resorption. Cell Tissue Res. 1979, 201, 409–422. [Google Scholar] [CrossRef] [PubMed]
- Pepla, E.; Besharat, L.K.; Palaia, G.; Tenore, G.; Migliau, G. Nano-hydroxyapatite and its applications in preventive, restorative and regenerative dentistry: A review of literature. Ann. Stomatol. 2014, 5, 108–114. [Google Scholar] [CrossRef]
- Chen, Z.; Lv, Y. Gelatin/sodium alginate composite hydrogel with dynamic matrix stiffening ability for bone regeneration. Compos. Part B Eng. 2022, 243, 110162. [Google Scholar] [CrossRef]
- Yan, J.; Miao, Y.; Tan, H.; Zhou, T.; Ling, Z.; Chen, Y.; Xing, X.; Hu, X. Injectable alginate/hydroxyapatite gel scaffold combined with gelatin microspheres for drug delivery and bone tissue engineering. Mater. Sci. Eng. C 2016, 63, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Saiding, Q.; Cheng, L.; Zhang, L.; Wang, Z.; Wang, F.; Chen, X.; Chen, G.; Deng, L.; Cui, W. Capturing dynamic biological signals via bio-mimicking hydrogel for precise remodeling of soft tissue. Bioact. Mater. 2021, 6, 4506–4516. [Google Scholar] [CrossRef] [PubMed]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Hernández-González, A.C.; Téllez-Jurado, L.; Rodríguez-Lorenzo, L.M. Alginate hydrogels for bone tissue engineering, from injectables to bioprinting: A review. Carbohydr. Polym. 2020, 229, 115514. [Google Scholar] [CrossRef]
- Buwalda, S.J.; Boere, K.W.M.; Dijkstra, P.J.; Feijen, J.; Vermonden, T.; Hennink, W.E. Hydrogels in a historical perspective: From simple networks to smart materials. J. Control Release Off. J. Control Release Soc. 2014, 190, 254–273. [Google Scholar] [CrossRef]
- Dragan, E.S. Design and applications of interpenetrating polymer network hydrogels. A review. Chem. Eng. J. 2014, 243, 572–590. [Google Scholar] [CrossRef]
- Li, J.; Yan, J.F.; Wan, Q.Q.; Shen, M.J.; Ma, Y.X.; Gu, J.T.; Gao, P.; Tang, X.Y.; Yu, F.; Chen, J.H.; et al. Matrix stiffening by self-mineralizable guided bone regeneration. Acta Biomater. 2021, 125, 112–125. [Google Scholar] [CrossRef]
- Xu, Z.; Lam, M.T. Alginate Application for Heart and Cardiovascular Diseases. In Alginates and Their Biomedical Applications; Springer Series in Biomaterials Science and Engineering; Springer: Berlin/Heidelberg, Germany, 2017; pp. 185–212. [Google Scholar] [CrossRef]
- de Vos, P.; Lazarjani, H.A.; Poncelet, D.; Faas, M.M. Polymers in cell encapsulation from an enveloped cell perspective. Adv. Drug Deliv. Rev. 2014, 67–68, 15–34. [Google Scholar] [CrossRef]
- Pina, S.; Oliveira, J.M.; Reis, R.L. Natural-Based Nanocomposites for Bone Tissue Engineering and Regenerative Medicine: A Review. Adv. Mater. 2015, 27, 1143–1169. [Google Scholar] [CrossRef] [PubMed]
- Otterlei, M.; Østgaard, K.; Skjåk-Bræk, G.; Smidsrød, O.; Soon-Shiong, P.; Espevik, T. Induction of Cytokine Production from Human Monocytes Stimulated with Alginate. J. Immunother. 1991, 10, 286–291. [Google Scholar] [CrossRef]
- Becker, T.A.; Kipke, D.R. Flow properties of liquid calcium alginate polymer injected through medical microcatheters for endovascular embolization. J. Biomed. Mater. Res. 2002, 61, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Axpe, E.; Oyen, M. Applications of Alginate-Based Bioinks in 3D Bioprinting. Int. J. Mol. Sci. 2016, 17, 1976. [Google Scholar] [CrossRef] [PubMed]
- Rhim, J.-W. Physical and mechanical properties of water resistant sodium alginate films. LWT Food Sci. Technol. 2004, 37, 323–330. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Pujana, A.; Orive, G.; Pedraz, J.L.; Santos-Vizcaino, E.; Hernandez, R.M. Alginate Microcapsules for Drug Delivery. In Alginates and Their Biomedical Applications; Springer Series in Biomaterials Science and Engineering; Springer: Berlin/Heidelberg, Germany, 2017; pp. 67–100. [Google Scholar] [CrossRef]
- Osmokrovic, A.; Jancic, I.; Vunduk, J.; Petrovic, P.; Milenkovic, M.; Obradovic, B. Achieving high antimicrobial activity: Composite alginate hydrogel beads releasing activated charcoal with an immobilized active agent. Carbohydr. Polym. 2018, 196, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; Chen, D.; Shen, C.; Shen, J.; Zhao, H.; He, Y. Preparation of in situ forming and injectable alginate/mesoporous Sr-containing calcium silicate composite cement for bone repair. RSC Adv. 2017, 7, 23671–23679. [Google Scholar] [CrossRef]
- Szekalska, M.; Puciłowska, A.; Szymańska, E.; Ciosek, P.; Winnicka, K. Alginate: Current use and future perspectives in pharmaceutical and biomedical applications. Int. J. Polym. Sci. 2016, 2016, 7697031. [Google Scholar] [CrossRef]
- Falkeborg, M.; Cheong, L.-Z.; Gianfico, C.; Sztukiel, K.M.; Kristensen, K.; Glasius, M.; Xu, X.; Guo, Z. Alginate oligosaccharides: Enzymatic preparation and antioxidant property evaluation. Food Chem. 2014, 164, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Cao, Y.; Xu, D.; Zhang, D.; Huang, Z. Influence of chitosan-sodium alginate pretreated with ultrasound on the enzyme activity, viscosity and structure of papain. J. Sci. Food Agric. 2016, 97, 1561–1566. [Google Scholar] [CrossRef]
- Burana-osot, J.; Hosoyama, S.; Nagamoto, Y.; Suzuki, S.; Linhardt, R.J.; Toida, T. Photolytic depolymerization of alginate. Carbohydr. Res. 2009, 344, 2023–2027. [Google Scholar] [CrossRef]
- Kelishomi, Z.H.; Goliaei, B.; Mahdavi, H.; Nikoofar, A.; Rahimi, M.; Moosavi-Movahedi, A.A.; Mamashli, F.; Bigdeli, B. Antioxidant activity of low molecular weight alginate produced by thermal treatment. Food Chem. 2016, 196, 897–902. [Google Scholar] [CrossRef]
- Coleman, R.J.; Jack, K.S.; Perrier, S.; Grøndahl, L. Hydroxyapatite Mineralization in the Presence of Anionic Polymers. Cryst. Growth Des. 2013, 13, 4252–4259. [Google Scholar] [CrossRef]
- Reakasame, S.; Boccaccini, A.R. Oxidized Alginate-Based Hydrogels for Tissue Engineering Applications: A Review. Biomacromolecules 2018, 19, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Bouhadir, K.H.; Lee, K.Y.; Alsberg, E.; Damm, K.L.; Anderson, K.W.; Mooney, D.J. Degradation of Partially Oxidized Alginate and Its Potential Application for Tissue Engineering. Biotechnol. Prog. 2001, 17, 945–950. [Google Scholar] [CrossRef]
- Gomez, C.G.; Rinaudo, M.; Villar, M.A. Oxidation of sodium alginate and characterization of the oxidized derivatives. Carbohydr. Polym. 2007, 67, 296–304. [Google Scholar] [CrossRef]
- Painter, T.; Larsen, B.; Sjövall, J.; Kääriäinen, L.; Rasmussen, S.E.; Sunde, E.; Sørensen, N.A. Formation of Hemiacetals between Neighbouring Hexuronic Acid Residues during the Periodate Oxidation of Alginate. Acta Chem. Scand. 1970, 24, 813–833. [Google Scholar] [CrossRef]
- Grant, G.T.; Morris, E.R.; Rees, D.A.; Smith, P.J.C.; Thom, D. Biological interactions between polysaccharides and divalent cations: The egg-box model. FEBS Lett. 1973, 32, 195–198. [Google Scholar] [CrossRef]
- Chan, L.W.; Jin, Y.; Heng, P.W.S. Cross-linking mechanisms of calcium and zinc in production of alginate microspheres. Int. J. Pharm. 2002, 242, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Mollah, M.Z.I.; Zahid, H.M.; Mahal, Z.; Faruque, M.R.I.; Khandaker, M.U. The Usages and Potential Uses of Alginate for Healthcare Applications. Front. Mol. Biosci. 2021, 8, 719972. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Gao, S.; Zhao, S.; Li, Y.; Cheng, L.; Li, J.; Yin, Y. Synthesis and characterization of disulfide-crosslinked alginate hydrogel scaffolds. Mater. Sci. Eng. C 2012, 32, 2153–2162. [Google Scholar] [CrossRef]
- Radhakrishnan, J.; Subramanian, A.; Krishnan, U.M.; Sethuraman, S. Injectable and 3D Bioprinted Polysaccharide Hydrogels: From Cartilage to Osteochondral Tissue Engineering. Biomacromolecules 2017, 18, 1–26. [Google Scholar] [CrossRef]
- Tønnesen, H.H.; Karlsen, J. Alginate in Drug Delivery Systems. Drug Dev. Ind. Pharm. 2002, 28, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Wright, B.; De Bank, P.A.; Luetchford, K.A.; Acosta, F.R.; Connon, C.J. Oxidized alginate hydrogels as niche environments for corneal epithelial cells. J. Biomed. Mater. Res. Part A 2013, 102, 3393–3400. [Google Scholar] [CrossRef]
- Zhou, H.; Xu, H.H.K. The fast release of stem cells from alginate-fibrin microbeads in injectable scaffolds for bone tissue engineering. Biomaterials 2011, 32, 7503–7513. [Google Scholar] [CrossRef]
- Baniasadi, H.; Mashayekhan, S.; Fadaoddini, S.; Haghirsharifzamini, Y. Design, fabrication and characterization of oxidized alginate–gelatin hydrogels for muscle tissue engineering applications. J. Biomater. Appl. 2016, 31, 152–161. [Google Scholar] [CrossRef]
- Sarker, B.; Singh, R.; Silva, R.; Roether, J.A.; Kaschta, J.; Detsch, R.; Schubert, D.W.; Cicha, I.; Boccaccini, A.R. Evaluation of Fibroblasts Adhesion and Proliferation on Alginate-Gelatin Crosslinked Hydrogel. PLoS ONE 2014, 9, e107952. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, H.-W. Emerging properties of hydrogels in tissue engineering. J. Tissue Eng. 2018, 9, 204173141876828. [Google Scholar] [CrossRef]
- Kuo, C.K.; Ma, P.X. Ionically crosslinked alginate hydrogels as scaffolds for tissue engineering: Part 1. Structure, gelation rate and mechanical properties. Biomaterials 2001, 22, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Lorenzo, L.M.; Saldaña, L.; Benito-Garzón, L.; García-Carrodeguas, R.; de Aza, S.; Vilaboa, N.; Román, J.S. Feasibility of ceramic-polymer composite cryogels as scaffolds for bone tissue engineering. J. Tissue Eng. Regen. Med. 2011, 6, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Téllez, D.; Téllez-Jurado, L.; Rodríguez-Lorenzo, L. Hydrogels for Cartilage Regeneration, from Polysaccharides to Hybrids. Polymers 2017, 9, 671. [Google Scholar] [CrossRef] [PubMed]
- El-Rashidy, A.A.; Roether, J.A.; Harhaus, L.; Kneser, U.; Boccaccini, A.R. Regenerating bone with bioactive glass scaffolds: A review of in vivo studies in bone defect models. Acta Biomater. 2017, 62, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Sarker, B.; Li, W.; Zheng, K.; Detsch, R.; Boccaccini, A.R. Designing Porous Bone Tissue Engineering Scaffolds with Enhanced Mechanical Properties from Composite Hydrogels Composed of Modified Alginate, Gelatin, and Bioactive Glass. ACS Biomater. Sci. Eng. 2016, 2, 2240–2254. [Google Scholar] [CrossRef] [PubMed]
- Fedorovich, N.E.; Alblas, J.; Hennink, W.E.; Öner, F.C.; Dhert, W.J.A. Organ printing: The future of bone regeneration? Trends Biotechnol. 2011, 29, 601–606. [Google Scholar] [CrossRef]
- Lin, H.-R.; Yeh, Y.-J. Porous alginate/hydroxyapatite composite scaffolds for bone tissue engineering: Preparation, characterization, andin vitro studies. J. Biomed. Mater. Res. 2004, 71B, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Sarker, A.; Amirian, J.; Min, Y.K.; Lee, B.T. HAp granules encapsulated oxidized alginate–gelatin–biphasic calcium phosphate hydrogel for bone regeneration. Int. J. Biol. Macromol. 2015, 81, 898–911. [Google Scholar] [CrossRef] [PubMed]
- Perez, R.A.; Kim, H.-W. Core-shell designed scaffolds of alginate/alpha-tricalcium phosphate for the loading and delivery of biological proteins. J. Biomed. Mater. Res. Part A 2012, 101A, 1103–1112. [Google Scholar] [CrossRef]
- Ishikawa, K.; Takagi, S.; Chow, L.C.; Ishikawa, Y. Properties and mechanisms of fast-setting calcium phosphate cements. J. Mater. Sci. Mater. Med. 1995, 6, 528–533. [Google Scholar] [CrossRef]
- Lee, G.-S.; Park, J.-H.; Won, J.-E.; Shin, U.S.; Kim, H.-W. Alginate combined calcium phosphate cements: Mechanical properties and in vitro rat bone marrow stromal cell responses. J. Mater. Sci. Mater. Med. 2011, 22, 1257–1268. [Google Scholar] [CrossRef] [PubMed]
- Derakhshanfar, S.; Mbeleck, R.; Xu, K.; Zhang, X.; Zhong, W.; Xing, M. 3D bioprinting for biomedical devices and tissue engineering: A review of recent trends and advances. Bioact. Mater. 2018, 3, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.; Blokzijl, M.M.; Levato, R.; Visser, C.W.; Castilho, M.; Hennink, W.E.; Vermonden, T.; Malda, J. Assessing bioink shape fidelity to aid material development in 3D bioprinting. Biofabrication 2017, 10, 014102. [Google Scholar] [CrossRef] [PubMed]
- Paxton, N.; Smolan, W.; Böck, T.; Melchels, F.; Groll, J.; Jungst, T. Proposal to assess printability of bioinks for extrusion-based bioprinting and evaluation of rheological properties governing bioprintability. Biofabrication 2017, 9, 044107. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.H.Y.; Naficy, S.; Yue, Z.; Kapsa, R.; Quigley, A.; Moulton, S.E.; Wallace, G.G. Bio-ink properties and printability for extrusion printing living cells. Biomater. Sci. 2013, 1, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Freeman, F.E.; Kelly, D.J. Tuning Alginate Bioink Stiffness and Composition for Controlled Growth Factor Delivery and to Spatially Direct MSC Fate within Bioprinted Tissues. Sci. Rep. 2017, 7, 17042. [Google Scholar] [CrossRef]
- Park, J.; Lee, S.J.; Lee, H.; Park, S.A.; Lee, J.Y. Three dimensional cell printing with sulfated alginate for improved bone morphogenetic protein-2 delivery and osteogenesis in bone tissue engineering. Carbohydr. Polym. 2018, 196, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Izadifar, M.; Babyn, P.; Kelly, M.E.; Chapman, D.; Chen, X. Bioprinting Pattern-Dependent Electrical/Mechanical Behavior of Cardiac Alginate Implants: Characterization and Ex Vivo Phase-Contrast Microtomography Assessment. Tissue Eng. Part C Methods 2017, 23, 548–564. [Google Scholar] [CrossRef] [PubMed]
- Giuseppe, M.D.; Law, N.; Webb, B.; Macrae, R.A.; Liew, L.J.; Sercombe, T.B.; Dilley, R.J.; Doyle, B.J. Mechanical behaviour of alginate-gelatine hydrogels for 3D bioprinting. J. Mech. Behav. Biomed. Mater. 2018, 79, 150–157. [Google Scholar] [CrossRef]
- Palmer, I.; Nelson, J.; Wolfgang Schatton Dunne, N.J.; Buchanan, F.; Clarke, S.A. Biocompatibility of calcium phosphate bone cement with optimised mechanical properties: An in vivo study. J. Mater. Sci. Mater. Med. 2016, 27, 191. [Google Scholar] [CrossRef]
- Busch, A.; Jäger, M.; Mayer, C.; Sowislok, A. Functionalization of Synthetic Bone Substitutes. Int. J. Mol. Sci. 2021, 22, 4412. [Google Scholar] [CrossRef] [PubMed]
- You, F.; Chen, X.; Cooper, D.M.L.; Chang, T.; Eames, B.F. Homogeneous hydroxyapatite/alginate composite hydrogel promotes calcified cartilage matrix deposition with potential for three-dimensional bioprinting. Biofabrication 2018, 11, 015015. [Google Scholar] [CrossRef] [PubMed]
- Russo, L.; Taraballi, F.; Lupo, C.; Poveda, A.; Jiménez-Barbero, J.; Sandri, M.; Tampieri, A.; Nicotra, F.; Cipolla, L. Carbonate hydroxyapatite functionalization: A comparative study towards (bio)molecules fixation. Interface Focus 2014, 4, 20130040. [Google Scholar] [CrossRef] [PubMed]
- An, Y.H.; Draughn, R.A. (Eds.) Mechanical Testing of Bone and the Bone-Implant Interface, 1st ed.; CRC Press: Boca Raton, FL, USA, 1999. [Google Scholar] [CrossRef]
- Pautke, C.; Schieker, M.; Tischer, T.; Kolk, A.; Neth, P.; Mutschler, W.; Milz, S. Characterization of osteosarcoma cell lines MG-63, Saos-2 and U-2 OS in comparison to human osteoblasts. Anticancer Res. 2004, 24, 3743–3748. [Google Scholar] [PubMed]
- Jouyandeh, M.; Vahabi, H.; Rabiee, N.; Rabiee, M.; Bagherzadeh, M.; Saeb, M.R. Green composites in bone tissue engineering. Emergent Mater. 2021, 5, 603–620. [Google Scholar] [CrossRef]
- Pesode, P.; Barve, S.; Wankhede, S.V.; Ahmad, A. Sustainable Materials and Technologies for Biomedical Applications. Adv. Mater. Sci. Eng. 2023, 2023, 6682892. [Google Scholar] [CrossRef]
- Lacroix, J.; Lao, J.; Jallot, E. Green and safe in situ templating of bioactive glass scaffolds for bone tissue engineering. J. Mater. Chem. B 2013, 1, 1782. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, W.; Kong, M.; Li, Z.; Yang, T.; Wang, Q.; Teng, W. Self-healing hybrid hydrogels with sustained bioactive components release for guided bone regeneration. J. Nanobiotechnology 2023, 21, 62. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Deng, Z.; Li, L. Biomineralized Materials as Model Systems for Structural Composites: 3D Architecture. Adv. Mater. 2022, 34, 2106259. [Google Scholar] [CrossRef]
- Beatty, D.N.; Williams, S.L.; Srubar, W.V. Biomineralized Materials for Sustainable and Durable Construction. Annu. Rev. Mater. Res. 2022, 52, 411–439. [Google Scholar] [CrossRef]
- Reinhardt, O.; Ihmann, S.; Ahlhelm, M.; Gelinsky, M. 3D bioprinting of mineralizing cyanobacteria as novel approach for the fabrication of living building materials. Front. Bioeng. Biotechnol. 2023, 11, 1145177. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Marra, K.G. Injectable, Biodegradable Hydrogels for Tissue Engineering Applications. Materials 2010, 3, 1746–1767. [Google Scholar] [CrossRef]
- Tran, N.Q.; Joung, Y.K.; Lih, E.; Park, K.M.; Park, K.D. Supramolecular Hydrogels Exhibiting Fast In Situ Gel Forming and Adjustable Degradation Properties. Biomacromolecules 2010, 11, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Taaca, L.M.; Prieto, E.; Vasquez, M.R. Current Trends in Biomedical Hydrogels: From Traditional Crosslinking to Plasma-Assisted Synthesis. Polymers 2022, 14, 2560. [Google Scholar] [CrossRef] [PubMed]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Ponticiello, M.S.; Schinagl, R.M.; Kadiyala, S.; Barry, F. Gelatin-based resorbable sponge as a carrier matrix for human mesenchymal stem cells in cartilage regeneration therapy. J. Biomed. Mater. Res. 2000, 52, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Pesákovă, V.; Stol, M.; Adam, M. Comparison of the influence of gelatine and collagen substrates on growth of chondrocytes. Folia Biol. 1990, 36, 264–270. [Google Scholar]
- Kim, A.Y.; Kim, Y.; Lee, S.H.; Yoon, Y.; Kim, W.-H.; Kweon, O.-K. Effect of Gelatin on Osteogenic Cell Sheet Formation Using Canine Adipose-Derived Mesenchymal Stem Cells. Cell Transplant. 2017, 26, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Zhang, H.; Chen, W. Differential physical, rheological, and biological properties of rapid in situ gelable hydrogels composed of oxidized alginate and gelatin derived from marine or porcine sources. J. Mater. Sci. Mater. Med. 2009, 20, 1263–1271. [Google Scholar] [CrossRef]
- Boguń, M.; Rabiej, S. The influence of fiber formation conditions on the structure and properties of nanocomposite alginate fibers containing tricalcium phosphate or montmorillonite. Polym. Compos. 2009, 31, 1321–1331. [Google Scholar] [CrossRef]
- Sakai, S.; Yamaguchi, S.; Takei, T.; Kawakami, K. Oxidized Alginate-Cross-Linked Alginate/Gelatin Hydrogel Fibers for Fabricating Tubular Constructs with Layered Smooth Muscle Cells and Endothelial Cells in Collagen Gels. Biomacromolecules 2008, 9, 2036–2041. [Google Scholar] [CrossRef] [PubMed]
- Kong, H. Designing alginate hydrogels to maintain viability of immobilized cells. Biomaterials 2003, 24, 4023–4029. [Google Scholar] [CrossRef] [PubMed]
- Boontheekul, T.; Kong, H.-J.; Mooney, D.J. Controlling alginate gel degradation utilizing partial oxidation and bimodal molecular weight distribution. Biomaterials 2005, 26, 2455–2465. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.J.; Kaigler, D.; Kim, K.; Mooney, D.J. Controlling Rigidity and Degradation of Alginate Hydrogels via Molecular Weight Distribution. Biomacromolecules 2004, 5, 1720–1727. [Google Scholar] [CrossRef] [PubMed]
- Boanini, E.; Rubini, K.; Panzavolta, S.; Bigi, A. Chemico-physical characterization of gelatin films modified with oxidized alginate. Acta Biomater. 2010, 6, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Łabowska, M.B.; Cierluk, K.; Jankowska, A.M.; Kulbacka, J.; Detyna, J.; Michalak, I. A Review on the Adaption of Alginate-Gelatin Hydrogels for 3D Cultures and Bioprinting. Materials 2021, 14, 858. [Google Scholar] [CrossRef]
- Tareq, A.; Hussein, M. Sodium Alginate-Gelatin Cross-Linked Microspheres for Releasing Diltiazem Hcl. Sci. J. Univ. Zakho 2016, 4, 226–235. [Google Scholar] [CrossRef]
- Xu, H.H.; Wang, P.; Wang, L.; Bao, C.; Chen, Q.; Weir, M.D.; Chow, L.C.; Zhao, L.; Zhou, X.; Reynolds, M.A. Calcium phosphate cements for bone engineering and their biological properties. Bone Res. 2017, 5, 17056. [Google Scholar] [CrossRef] [PubMed]
- Fernández, E.; Vlad, M.D.; Gel, M.M.; López, J.; Torres, R.; Cauich, J.V.; Bohner, M. Modulation of porosity in apatitic cements by the use of α-tricalcium phosphate—Calcium sulphate dihydrate mixtures. Biomaterials 2005, 26, 3395–3404. [Google Scholar] [CrossRef]
- del Real, R.P.; Wolke, J.G.C.; Vallet-Regí, M.; Jansen, J.A. A new method to produce macropores in calcium phosphate cements. Biomaterials 2002, 23, 3673–3680. [Google Scholar] [CrossRef]
- An, J.; Wolke, J.G.; Jansen, J.A.; Leeuwenburgh, S.C. Influence of polymeric additives on the cohesion and mechanical properties of calcium phosphate cements. J. Mater. Sci. Mater. Med. 2016, 27, 58. [Google Scholar] [CrossRef]
- Hesaraki, S.; Zamanian, A.; Moztarzadeh, F. The influence of the acidic component of the gas-foaming porogen used in preparing an injectable porous calcium phosphate cement on its properties: Acetic acid versus citric acid. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 86B, 208–216. [Google Scholar] [CrossRef]
- Ginebra, M.P.; Driessens, F.C.M.; Planell, J.A. Effect of the particle size on the micro and nanostructural features of a calcium phosphate cement: A kinetic analysis. Biomaterials 2004, 25, 3453–3462. [Google Scholar] [CrossRef] [PubMed]
- Félix Lanao, R.P.; Leeuwenburgh, S.C.G.; Wolke, J.G.C.; Jansen, J.A. Bone response to fast-degrading, injectable calcium phosphate cements containing PLGA microparticles. Biomaterials 2011, 32, 8839–8847. [Google Scholar] [CrossRef] [PubMed]
- Ilie, A.; Ghiţulică, C.; Andronescu, E.; Cucuruz, A.; Ficai, A. New composite materials based on alginate and hydroxyapatite as potential carriers for ascorbic acid. Int. J. Pharm. 2016, 510, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Hillig, W.B.; Choi, Y.; Murtha, S.; Natravali, N.; Ajayan, P. An open-pored gelatin/hydroxyapatite composite as a potential bone substitute. J. Mater. Sci. Mater. Med. 2007, 19, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Devina, N.; Eriwati, Y.K.; Santosa, A.S. The purity and viscosity of sodium alginate extracted from Sargassum brown seaweed species as a basic ingredient in dental alginate impression material. J. Phys. Conf. Ser. 2018, 1073, 052012. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Zhang, X.; Rahman, S.E.; Su, S.; Wei, J.; Ning, F.; Hu, Z.; Martínez-Zaguilán, R.; Sennoune, S.R.; et al. 3D printed agar/ calcium alginate hydrogels with high shape fidelity and tailorable mechanical properties. Polymer 2021, 214, 123238. [Google Scholar] [CrossRef]
- Kreller, T.; Distler, T.; Heid, S.; Gerth, S.; Detsch, R.; Boccaccini, A.R. Physico-chemical modification of gelatine for the improvement of 3D printability of oxidized alginate-gelatine hydrogels towards cartilage tissue engineering. Mater. Des. 2021, 208, 109877. [Google Scholar] [CrossRef]
- Bociaga, D.; Bartniak, M.; Grabarczyk, J.; Przybyszewska, K. Sodium Alginate/Gelatine Hydrogels for Direct Bioprinting—The Effect of Composition Selection and Applied Solvents on the Bioink Properties. Materials 2019, 12, 2669. [Google Scholar] [CrossRef]
- Zhang, N.; Zhao, D.; Liu, N.; Wu, Y.; Yang, J.; Wang, Y.; Xie, H.; Ji, Y.; Zhou, C.; Zhuang, J.; et al. Assessment of the degradation rates and effectiveness of different coated Mg-Zn-Ca alloy scaffolds for in vivo repair of critical-size bone defects. J. Mater. Sci. Mater. Med. 2018, 29, 138. [Google Scholar] [CrossRef]
- Kim, J.-A.; Lim, J.; Naren, R.; Yun, H.; Park, E.K. Effect of the biodegradation rate controlled by pore structures in magnesium phosphate ceramic scaffolds on bone tissue regeneration in vivo. Acta Biomater. 2016, 44, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Rego, J.M.; Mendoza-Cerezo, L.; Macías-García, A.; Carrasco-Amador, J.P.; Marcos-Romero, A.C. Methodology for characterizing the printability of hydrogels. Int. J. Bioprinting 2023, 9, 667. [Google Scholar] [CrossRef] [PubMed]
- Pettignano, A.; Häring, M.; Bernardi, L.; Tanchoux, N.; Quignard, F.; Díaz, D.D. Self-healing alginate–gelatin biohydrogels based on dynamic covalent chemistry: Elucidation of key parameters. Mater. Chem. Front. 2017, 1, 73–79. [Google Scholar] [CrossRef]
- Cohen, B.; Panker, M.; Zuckerman, E.; Foox, M.; Zilberman, M. Effect of calcium phosphate-based fillers on the structure and bonding strength of novel gelatin–alginate bioadhesives. J. Biomater. Appl. 2013, 28, 1366–1375. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.-S.; Wang, J.-C.; Lai, P.-L.; Liu, S.-M.; Chen, Y.-S.; Chen, W.-C.; Hung, C.-C. Biodegradable Hydrogel Beads Combined with Calcium Phosphate Bone Cement for Bone Repair: In Vitro and In Vivo Characterization. Polymers 2022, 14, 505. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, X.; Neufurth, M.; Tolba, E.; Schepler, H.; Xiao, S.; Schröder, H.C.; Müller, W.E.G. Biomimetic Alginate/Gelatin Cross-Linked Hydrogels Supplemented with Polyphosphate for Wound Healing Applications. Molecules 2020, 25, 5210. [Google Scholar] [CrossRef] [PubMed]
- Gurikov, P.; Smirnova, I. Non-Conventional Methods for Gelation of Alginate. Gels 2018, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Orshesh, Z.; Hesaraki, S.; Khanlarkhani, A. Blooming gelatin: An individual additive for enhancing nanoapatite precipitation, physical properties, and osteoblastic responses of nanostructured macroporous calcium phosphate bone cements. Int. J. Nanomed. 2017, 12, 745–758. [Google Scholar] [CrossRef]
- Nguyen, T.-P.; Lee, B.-T. Fabrication of oxidized alginate-gelatin-BCP hydrogels and evaluation of the microstructure, material properties and biocompatibility for bone tissue regeneration. J. Biomater. Appl. 2011, 27, 311–321. [Google Scholar] [CrossRef]
- Yao, Y.; Yang, Y.; Ye, Q.; Cao, S.; Zhang, X.; Zhao, K.; Jian, Y. Effects of pore size and porosity on cytocompatibility and osteogenic differentiation of porous titanium. J. Mater. Sci. Mater. Med. 2021, 32, 72. [Google Scholar] [CrossRef] [PubMed]
- Herrada-Manchón, H.; Fernández, M.; Aguilar, E. Essential Guide to Hydrogel Rheology in Extrusion 3D Printing: How to Measure It and Why It Matters? Gels 2023, 9, 517. [Google Scholar] [CrossRef]
- Maciel, B.R.; Baki, K.; Oelschlaeger, C.; Willenbacher, N. The Influence of Rheological and Wetting Properties of Hydrogel-Based Bio-Inks on Extrusion-Based Bioprinting. Chem. Ing. Tech. 2022, 94, 393–401. [Google Scholar] [CrossRef]
- Anwar, F.; Amin, K.F.; Hoque, M.E. Tools and techniques for characterizing sustainable hydrogels. In Sustainable Hydrogels; Elsevier: Amsterdam, The Netherlands, 2023; pp. 47–77. [Google Scholar] [CrossRef]
- Sen, K.S.; Duarte Campos, D.F.; Köpf, M.; Blaeser, A.; Fischer, H. The Effect of Addition of Calcium Phosphate Particles to Hydrogel-Based Composite Materials on Stiffness and Differentiation of Mesenchymal Stromal Cells toward Osteogenesis. Adv. Healthc. Mater. 2018, 7, 1800343. [Google Scholar] [CrossRef] [PubMed]
- Nagle, D.G.; Ferreira, D.; Zhou, Y.-D. Epigallocatechin-3-gallate (EGCG): Chemical and biomedical perspectives. Phytochemistry 2006, 67, 1849–1855. [Google Scholar] [CrossRef] [PubMed]
- Du, G.-J.; Zhang, Z.; Wen, X.-D.; Yu, C.; Calway, T.; Yuan, C.-S.; Wang, C.-Z. Epigallocatechin Gallate (EGCG) is the most effective cancer chemopreventive polyphenol in green tea. Nutrients 2012, 4, 1679–1691. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Deng, J.; Man, Y.; Qu, Y. Green Tea Extracts Epigallocatechin-3-gallate for Different Treatments. BioMed Res. Int. 2017, 2017, 5615647. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, X.; Feng, C.; Zhu, S.; Li, Y.; Guo, Z.; Chen, X. Disinfection properties of the tea polyphenol epigallocatechin gallate in the presence of calcium ions. AQUA—Water Infrastruct. Ecosyst. Soc. 2021, 70, 731–740. [Google Scholar] [CrossRef]
- Gao, B.; Honda, Y.; Yamada, Y.; Tanaka, T.; Takeda, Y.; Nambu, T.; Baba, S. Utility of Thermal Cross-Linking in Stabilizing Hydrogels with Beta-Tricalcium Phosphate and/or Epigallocatechin Gallate for Use in Bone Regeneration Therapy. Polymers 2021, 14, 40. [Google Scholar] [CrossRef]
- Dai, D.; Wang, J.; Xie, H.; Zhang, C. An epigallocatechin gallate–amorphous calcium phosphate nanocomposite for caries prevention and demineralized enamel restoration. Mater. Today Bio 2023, 21, 100715. [Google Scholar] [CrossRef]
- Pietryga, K.; Panaite, A.; Pamuła, E. Composite scaffolds enriched with calcium carbonate microparticles loaded with epigallocatechin gallate for bone tissue regeneration. Eng. Biomater. 2022, 25, 12–21. [Google Scholar] [CrossRef]
- Jo, Y.; Sarkar, N.; Bose, S. In vitrobiological evaluation of epigallocatechin gallate (EGCG) release from three-dimensional printed (3DP) calcium phosphate bone scaffolds. J. Mater. Chem. B 2023, 11, 5503–5513. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Jiao, Z.; Guo, S. Effect of Epigallocatechin Gallate on the Properties of Gelatin. Int. J. Food Prop. 2014, 17, 2119–2130. [Google Scholar] [CrossRef]
- Dou, L.; Li, B.; Zhang, K.; Chu, X.; Hou, H. Physical properties and antioxidant activity of gelatin-sodium alginate edible films with tea polyphenols. Int. J. Biol. Macromol. 2018, 118, 1377–1383. [Google Scholar] [CrossRef] [PubMed]
- Vetsch, J.R.; Paulsen, S.J.; Müller, R.; Hofmann, S. Effect of fetal bovine serum on mineralization in silk fibroin scaffolds. Acta Biomater. 2015, 13, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Villasante, A.; Robinson, S.T.; Cohen, A.R.; Lock, R.; Guo, X.E.; Vunjak-Novakovic, G. Human Serum Enhances Biomimicry of Engineered Tissue Models of Bone and Cancer. Front. Bioeng. Biotechnol. 2021, 9, 658472. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, B.; Pazarceviren, A.E.; Tezcaner, A.; Evis, Z. Historical development of simulated body fluids used in biomedical applications: A review. Microchem. J. 2020, 155, 104713. [Google Scholar] [CrossRef]
- van der Valk, J. Fetal bovine serum (FBS): Past-present-future. ALTEX 2018, 35, 99–118. [Google Scholar] [CrossRef]
- Sangeetha, K.; Thamizhavel, A.; Girija, E.K. Effect of gelatin on the in situ formation of Alginate/Hydroxyapatite nanocomposite. Mater. Lett. 2013, 91, 27–30. [Google Scholar] [CrossRef]
- Tomić, S.L.; Nikodinović-Runić, J.; Vukomanović, M.; Babić, M.M.; Vuković, J.S. Novel Hydrogel Scaffolds Based on Alginate, Gelatin, 2-Hydroxyethyl Methacrylate, and Hydroxyapatite. Polymers 2021, 13, 932. [Google Scholar] [CrossRef]
- Pham Minh, D.; Rio, S.; Sharrock, P.; Sebei, H.; Lyczko, N.; Tran, N.D.; Raii, M.; Nzihou, A. Hydroxyapatite starting from calcium carbonate and orthophosphoric acid: Synthesis, characterization, and applications. J. Mater. Sci. 2014, 49, 4261–4269. [Google Scholar] [CrossRef]
- Sans, J.; Arnau, M.; Sanz, V.; Turon, P.; Alemán, C. Hydroxyapatite-based biphasic catalysts with plasticity properties and its potential in carbon dioxide fixation. Chem. Eng. J. 2022, 433, 133512. [Google Scholar] [CrossRef]
- Kameo, Y.; Sakano, N.; Adachi, T. Theoretical concept of cortical to cancellous bone transformation. Bone Rep. 2020, 12, 100260. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Liu, H.; Ding, Z.; Xiao, L.; Qiang, L.ü.; Kaplan, D.L. Simulation of Cortical and Cancellous Bone to Accelerate Tissue Regeneration. Adv. Funct. Mater. 2023, 33, 2301839. [Google Scholar] [CrossRef] [PubMed]
- Mohd Pu’ad, N.A.S.; Koshy, P.; Abdullah, H.Z.; Idris, M.I.; Lee, T.C. Syntheses of hydroxyapatite from natural sources. Heliyon 2019, 5, e01588. [Google Scholar] [CrossRef] [PubMed]
- Sawada, M.; Sridhar, K.; Kanda, Y.; Yamanaka, S. Pure hydroxyapatite synthesis originating from amorphous calcium carbonate. Sci. Rep. 2021, 11, 11546. [Google Scholar] [CrossRef] [PubMed]
- Mohd Pu’ad, N.A.S.; Alipal, J.; Abdullah, H.Z.; Idris, M.I.; Lee, T.C. Synthesis of eggshell derived hydroxyapatite via chemical precipitation and calcination method. Mater. Today Proc. 2021, 42, 172–177. [Google Scholar] [CrossRef]
- Javadinejad, H.R.; Ebrahimi-Kahrizsangi, R. Thermal and kinetic study of hydroxyapatite formation by solid-state reaction. Int. J. Chem. Kinet. 2020, 53, 583–595. [Google Scholar] [CrossRef]
- Takagi, S.; Chow, L.C.; Ishikawa, K. Formation of hydroxyapatite in new calcium phosphate cements. Biomaterials 1998, 19, 1593–1599. [Google Scholar] [CrossRef]
- Lotsari, A.; Rajasekharan, A.K.; Halvarsson, M.; Andersson, M. Transformation of amorphous calcium phosphate to bone-like apatite. Nat. Commun. 2018, 9, 4170. [Google Scholar] [CrossRef]
- Wu, Y.X.; Choi, E.J.; Vu, A.A.; Jiang, P.; Ali, S.N.; Patel, R.M.; Landman, J.; Clayman, R.V. Comparison of Ureteral Stent Biomaterials: Encrustation Profile in Lithogenic Artificial Urine Models. ACS Omega 2023, 8, 29003–29011. [Google Scholar] [CrossRef] [PubMed]
- Doostmohammadi, A.; Monshi, A.; Salehi, R.; Fathi, M.H.; Seyedjafari, E.; Shafiee, A.; Soleimani, M. Cytotoxicity evaluation of 63s bioactive glass and bone-derived hydroxyapatite particles using human bone-marrow stem cells. Biomed. Pap. Fac. Med. Palacký Univ. Olomouc Czech Repub. 2011, 155, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Branzoi, I.V.; Iordoc, M.; Branzoi, F.; Vasilescu-Mirea, R.; Sbarcea, G. Influence of diamond-like carbon coating on the corrosion resistance of the NITINOL shape memory alloy. Surf. Interface Anal. 2010, 42, 502–509. [Google Scholar] [CrossRef]
- Lee, D.Y.; Lee, S.Y.; Yun, S.H.; Jeong, J.W.; Kim, J.H.; Kim, H.W.; Choi, J.S.; Kim, G.-D.; Joo, S.T.; Choi, I.; et al. Review of the Current Research on Fetal Bovine Serum and the Development of Cultured Meat. Food Sci. Anim. Resour. 2022, 42, 775–799. [Google Scholar] [CrossRef] [PubMed]
- Murray, E.; Provvedini, D.; Curran, D.; Catherwood, B.; Sussman, H.; Manolagas, S. Characterization of a human osteoblastic osteosarcoma cell line (SAOS-2) with high bone alkaline phosphatase activity. J. Bone Miner. Res. 2009, 2, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Saldaña, L.; Bensiamar, F.; Bore, A.; Vilaboa, N. In search of representative models of human bone-forming cells for cytocompatibility studies. Acta Biomater. 2011, 7, 4210–4221. [Google Scholar] [CrossRef]
- Rodan, S.B.; Imai, Y.; Thiede, M.A.; Wesolowski, G.; Thompson, D.; Bar-Shavit, Z.; Shull, S.; Mann, K.; Rodan, G.A. Characterization of a human osteosarcoma cell line (Saos-2) with osteoblastic properties. Cancer Res. 1987, 47, 4961–4966. [Google Scholar] [PubMed]
- Fernandes, R.J.; Harkey, M.A.; Weis, M.; Askew, J.W.; Eyre, D.R. The post-translational phenotype of collagen synthesized by SAOS-2 osteosarcoma cells. Bone 2007, 40, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Bilbe, G.; Roberts, E.; Birch, M.; Evans, D.B. PCR phenotyping of cytokines, growth factors and their receptors and bone matrix proteins in human osteoblast-like cell lines. Bone 1996, 19, 437–445. [Google Scholar] [CrossRef]
- Kolan, K.C.R.; Semon, J.A.; Bromet, B.; Day, D.E.; Leu, M.C. Bioprinting with human stem cells-laden alginate-gelatin bioink and bioactive glass for tissue engineering. Int. J. Bioprinting 2019, 5, 3. [Google Scholar] [CrossRef]
- Neufurth, M.; Wang, X.; Schröder, H.C.; Feng, Q.; Diehl-Seifert, B.; Ziebart, T.; Steffen, R.; Wang, S.; Müller, W.E.G. Engineering a morphogenetically active hydrogel for bioprinting of bioartificial tissue derived from human osteoblast-like SaOS-2 cells. Biomaterials 2014, 35, 8810–8819. [Google Scholar] [CrossRef]
- Hu, C.; He, S.; Lee, Y.J.; He, Y.; Kong, E.M.; Li, H.; Anastasio, M.A.; Popescu, G. Live-dead assay on unlabeled cells using phase imaging with computational specificity. Nat. Commun. 2022, 13, 713. [Google Scholar] [CrossRef]
- Serbanescu, M. Evaluation of cytotoxic activity and anticancer potential of indigenous rosemary (Rosmarinus officinalis L.) and oregano (Origanum vulgare L.) dry extracts on mg-63 bone osteosarcoma human cell line. Rom. J. Morphol. Embryol. 2021, 62, 525. [Google Scholar]
- Měřička, P.; Janoušek, L.; Benda, A.; Lainková, R.; Sabó, J.; Dalecká, M.; Prokšová, P.; Salmay, M.; Špunda, R.; Pecha, O.; et al. Cell Viability Assessment Using Fluorescence Vital Dyes and Confocal Microscopy in Evaluating Freezing and Thawing Protocols Used in Cryopreservation of Allogeneic Venous Grafts. Int. J. Mol. Sci. 2021, 22, 10653. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.E.G.; Schröder, H.C.; Tolba, E.; Diehl-Seifert, B.; Wang, X. Mineralization of bone-related SaOS-2 cells under physiological hypoxic conditions. FEBS J. 2015, 283, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Sanginario, V.; Ginebra, M.P.; Tanner, K.E.; Planell, J.A.; Ambrosio, L. Biodegradable and semi-biodegradable composite hydrogels as bone substitutes: Morphology and mechanical characterization. J. Mater. Sci. Mater. Med. 2006, 17, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yang, K.; Yu, F.; Chen, X.; Wu, S.; Zhu, Z. Preparation of novel bilayer hydrogels by combination of irradiation and freeze-thawing and their physical and biological properties. Polym. Int. 2009, 58, 1291–1298. [Google Scholar] [CrossRef]
- Xu, N.; Xu, J.; Zheng, X.; Hui, J. Preparation of Injectable Composite Hydrogels by Blending Poloxamers with Calcium Carbonate-Crosslinked Sodium Alginate. ChemistryOpen 2020, 9, 451–458. [Google Scholar] [CrossRef]
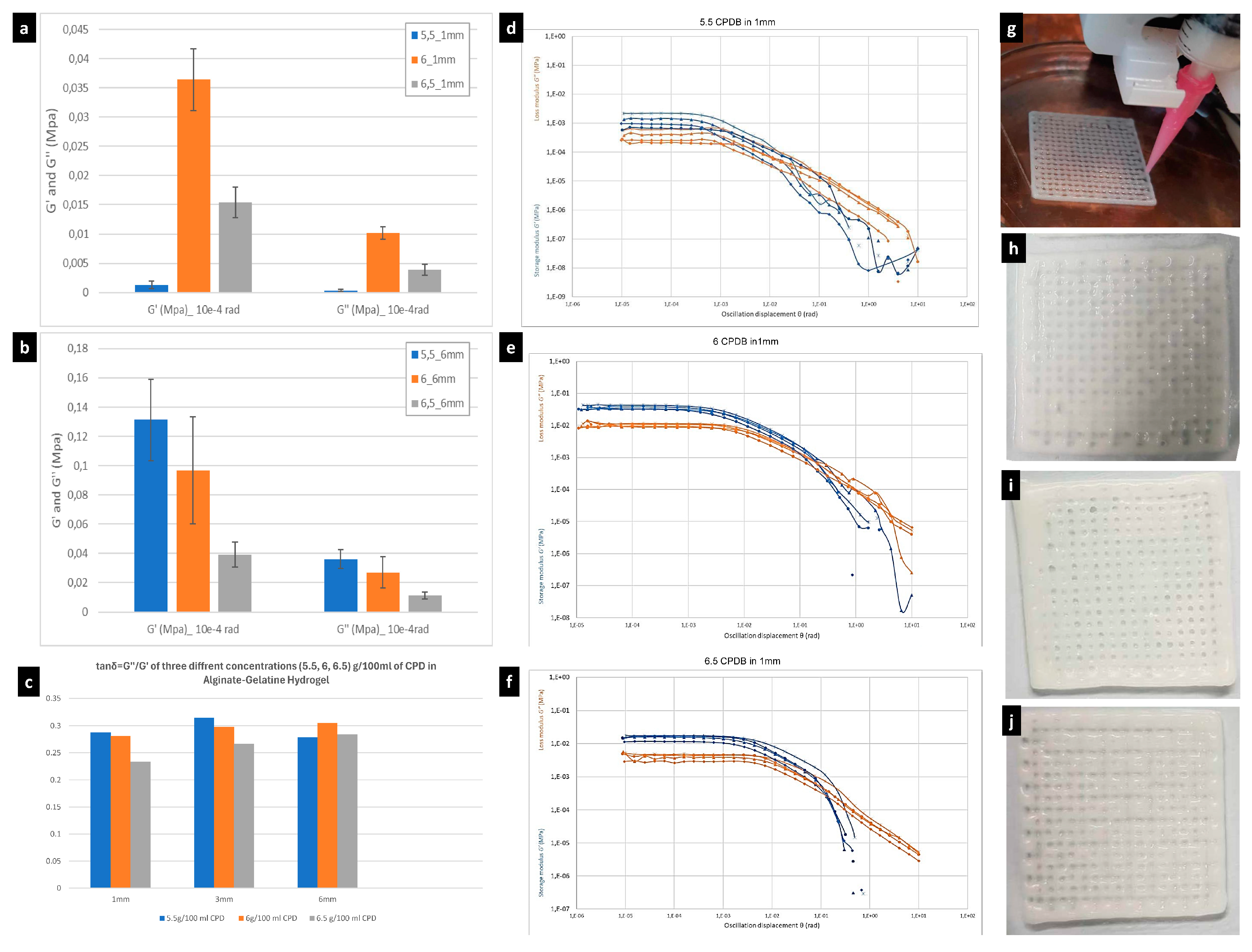


| Sample | G′ (MPa) 10−4 rad | G″ (MPa) 10−4 rad | tanδ = G″/G′ |
|---|---|---|---|
| 5.5 g/100 mL CPD in 1 mm | 0.00143814 | 0.000414447 | 0.288 |
| 6 g/100 mL CPD in 1 mm | 0.0384162 | 0.0108194 | 0.281 |
| 6.5 g/100 mL CPD in 1 mm | 0.0156726 | 0.00367397 | 0.234 |
| 5.5 g/100 mL CPD in 3 mm | 0.110418 | 0.0348274 | 0.315 |
| 6 g/100 mL CPD in 3 mm | 0.0162533 | 0.0048435 | 0.298 |
| 6.5 g/100 mL CPD in 3 mm | 0.0223588 | 0.00598952 | 0.267 |
| 5.5 g/100 mL CPD in 6 mm | 0.148103 | 0.0413324 | 0.279 |
| 6 g/100 mL CPD in 6 mm | 0.089164 | 0.027222 | 0.305 |
| 6.5 g/100 mL CPD in 6 mm | 0.0410406 | 0.0116671 | 0.284 |
| Concentration (µm) | G′ (MPa) 10−4 rad | G″ (MPa) 10−4 rad | tanδ = G″/G′ |
|---|---|---|---|
| 4 EGCG | 0.0275944 | 0.00809548 | 0.2933 |
| 6 EGCG | 0.0216016 | 0.00548179 | 0.2537 |
| 8 EGCG | 0.0277438 | 0.00765127 | 0.2757 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estevez, A.T.; Abdallah, Y.K. Biomimetic Approach for Enhanced Mechanical Properties and Stability of Self-Mineralized Calcium Phosphate Dibasic–Sodium Alginate–Gelatine Hydrogel as Bone Replacement and Structural Building Material. Processes 2024, 12, 944. https://doi.org/10.3390/pr12050944
Estevez AT, Abdallah YK. Biomimetic Approach for Enhanced Mechanical Properties and Stability of Self-Mineralized Calcium Phosphate Dibasic–Sodium Alginate–Gelatine Hydrogel as Bone Replacement and Structural Building Material. Processes. 2024; 12(5):944. https://doi.org/10.3390/pr12050944
Chicago/Turabian StyleEstevez, Alberto T., and Yomna K. Abdallah. 2024. "Biomimetic Approach for Enhanced Mechanical Properties and Stability of Self-Mineralized Calcium Phosphate Dibasic–Sodium Alginate–Gelatine Hydrogel as Bone Replacement and Structural Building Material" Processes 12, no. 5: 944. https://doi.org/10.3390/pr12050944
APA StyleEstevez, A. T., & Abdallah, Y. K. (2024). Biomimetic Approach for Enhanced Mechanical Properties and Stability of Self-Mineralized Calcium Phosphate Dibasic–Sodium Alginate–Gelatine Hydrogel as Bone Replacement and Structural Building Material. Processes, 12(5), 944. https://doi.org/10.3390/pr12050944







