Chromatographic Characterization and Process Performance of Column-Packed Anion Exchange Fibrous Adsorbents for High Throughput and High Capacity Bioseparations
Abstract
:1. Introduction
2. Experimental Materials and Methods
2.1. Materials
2.2. Physico-Chemical Characterization
2.2.1. Swelling and Porosity
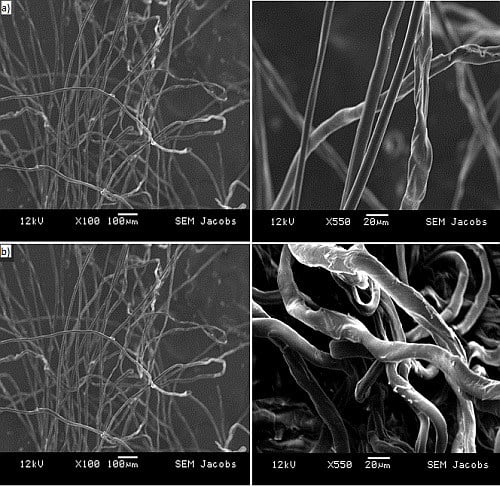
2.2.2. Microscopy
2.3. Functional Characterization
2.3.1. Column Packing
2.3.2. Pulse Experiments
2.3.3. Flow Permeability Experiments
2.3.4. Determination of the Ionic Capacity
2.3.5. Protein Adsorption under Static and Dynamic Conditions
3. Results and Discussion
3.1. Physico-Chemical Characterization of the Adsorptive Material


3.2. Functional Characterization
3.2.1. Packing Efficiency

3.2.2. Pulse Analysis to Determine Porosity Measurements

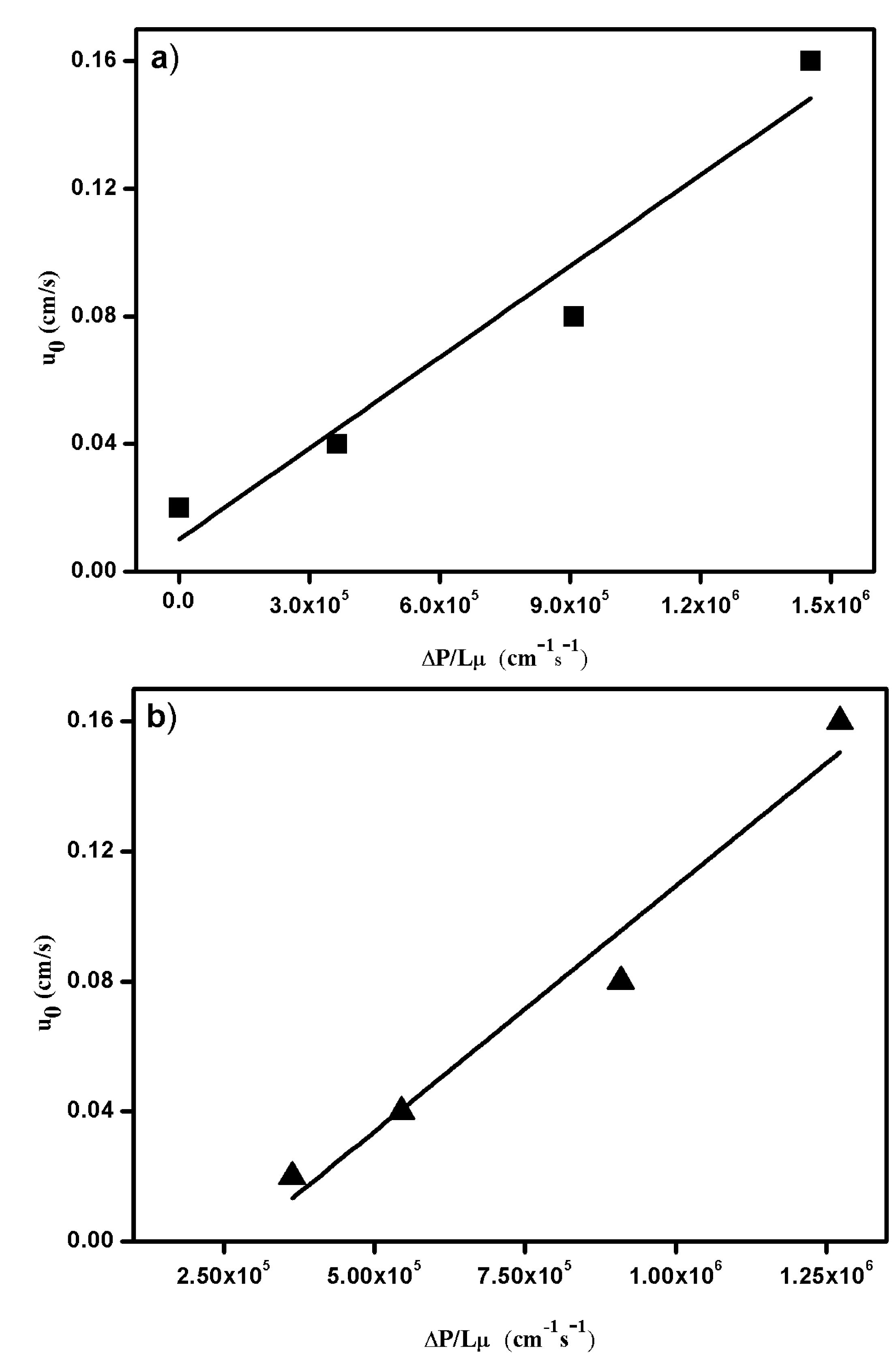
3.2.3. Pressure Drop Measurements
3.2.4. Total Ionic Capacity

3.3. Chromatography Performance

| Adsorbents | DBC at 10% Breakthrough (mg/mL) | |||
|---|---|---|---|---|
| 75 cm/h | 150 cm/h | 300 cm/h | 600 cm/h | |
| Q fibers | 76 ± 2 | 59 ± 2 | 53 ± 2 | 39 ± 2 |
| Q Sepharose FF | 82 ± 2 | 37 ± 2 | 32 ± 2 | 22 ± 2 |

3.4. Effect of Molecular Weight of the Sample on the DBC

4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dainiak, M.B.; Kumar, A.; Plieva, F.M.; Galaev, I.Y.; Mattiasson, B. Integrated isolation of antibody fragments from microbial cell culture fluids using supermacroporous cryogels. J. Chromatogr. A 2004, 1045, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Low, D.; O’Leary, R.; Pujar, N.S. Future of antibody purification. J. Chromatogr. B 2007, 848, 48–63. [Google Scholar] [CrossRef]
- Suzanne, S.F. Process economics of industrial monoclonal antibody manufacture. J. Chromatogr. B 2007, 848, 8–18. [Google Scholar] [CrossRef]
- Banik, R.; Santhiagu, A.; Kanari, B.; Sabarinath, C.; Upadhyay, S. Technological aspects of extractive fermentation using aqueous two-phase systems. World J. Microbiol. Biotechnol. 2003, 19, 337–398. [Google Scholar] [CrossRef]
- Gottschalk, U. Comprehensive Biotechnology; Academic Press: Waltham, MA, USA, 2011; Volume 3. [Google Scholar]
- Gottschalk, U. Bioseparation in antibody manufacturing: The good, the bad and the ugly. Biotechnol. Prog. 2008, 24, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, U. 3.57—Overview of downstream processing in the biomanufacturing industry. In Comprehensive Biotechnology, 2nd ed.; Moo-Young, M., Ed.; Academic Press: Waltham, MA, USA, 2011; pp. 669–682. [Google Scholar]
- Przybycien, T.M.; Pujar, N.S.; Steele, L.M. Alternative bioseparation operations: Life beyond packed-bed chromatography. Curr. Opin. Biotechnol. 2004, 15, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Andrew, L. Process chromatography: Current constraints and future options for the adsorptive recovery of bioproducts. Curr. Opin. Biotechnol. 2002, 13, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Hubbuch, J.; Kula, M.R. Isolation and purification of biotechnological products. J. Non-Equilib. Thermodyn. 2007, 32, 99–127. [Google Scholar] [CrossRef]
- D’Souza, R.N.; Azedo, A.M.; Aires-Barros, R.; Krajnc, N.L.; Kramberger, P.; Carbajal, M.L.; Grasselli, M.; Meyer, R.; Fernández-Lahore, M. Emerging technologies for the integration and intensification of downstream bioprocesses. Pharm. Bioprocess. 2013, 1, 423–440. [Google Scholar] [CrossRef]
- Lightfoot, E.N.; Moscariello, J.S. Bioseparations. Biotechnol. Bioeng. 2004, 87, 259–273. [Google Scholar] [CrossRef] [PubMed]
- Guiochon, G. Preparative liquid chromatography. J. Chromatogr. A 2002, 965, 129–161. [Google Scholar] [CrossRef] [PubMed]
- Levison, P.R. Large-scale ion-exchange column chromatography of proteins. Comparison of different formats. J. Chromatogr. B 2003, 790, 17–33. [Google Scholar] [CrossRef]
- Ghosh, R. Protein separation using membrane chromatography: Opportunities and challenges. J. Chromatogr. A 2002, 952, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Charcosset, C. Review: Purification of proteins by membrane chromatography. J. Chem. Technol. Biotechnol. 1998, 71, 95–110. [Google Scholar]
- Thömmes, J.; Kula, M.R. Membrane chromatography? An integrative concept in the downstream processing of proteins. Biotechnol. Prog. 1995, 11, 357–367. [Google Scholar] [CrossRef]
- Boi, C.; Facchini, R.; Sorci, M.; Sarti, G.C. Characterisation of affinity membranes for IgG separation. Euromembrane 2006, 199, 544–546. [Google Scholar]
- Boi, C.; Dimartino, S.; Sarti, G.C. Performance of a new protein affinity membrane for the primary recovery of antibodies. Biotechnol. Prog. 2008, 24, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Gavara, P.R.; Cabrera, R.; Vennapusa, R.R.; Grasselli, M.; Fernandez-Lahore, M. Preparation, characterization, and process performance of composite fibrous adsorbents as cation exchangers for high throughput and high capacity bioseparations. J. Chromatogr. B 2012, 903, 14–22. [Google Scholar] [CrossRef]
- Singh, N.K.; Dsouza, R.N.; Grasselli, M.; Fernandez-Lahore, M. High capacity cryogel-type adsorbents for protein purification. J. Chromatogr. A 2014, 1355, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Hutten, I.M. Handbook of Nonwoven Filter Media; Butterworth-Heinemann: Burlington, MA, USA, 2007. [Google Scholar]
- Ruixia, L.; Hongxiao, G.J.T. Adsorption of fluoride, phosphate, and arsenate ions on a new type of ion exchange fiber. J. Colloid Interface Sci. 2002, 248, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Gong, B.L.; Li, X.Q.; Wang, F.R.; Xu, H.D.; Chang, X.J. Synthesis of polyacrylacylaminourea chelating fiber and properties of concentration and separation of trace metal ions from samples. Anal. Chim. Acta 2001, 427, 287–291. [Google Scholar] [CrossRef]
- Jaskari, T.; Vuorio, M.; Kontturi, K.; Manzanares, J.A.; Hirvonen, J. Ion-exchange fibers and drugs: An equilibrium study. J. Control. Release 2001, 70, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Wen, B.; Shan, X.Q.; Liu, R.X.; Tang, H.X. Preconcentration of trace elements in sea water with poly (acrylaminophosphonic-dithiocarbamate) chelating fiber for their determination by inductively coupled plasma mass spectrometry. Fresen. J. Anal. Chem. 1999, 363, 251–255. [Google Scholar] [CrossRef]
- Motobu, M.; Matsuo, S.; Wang, P.C.; Kataoka, H.; Matsumura, M. High renin productivity of rcho cells cultivated in radial-flow nonwoven fabric mat packed-bed reactor with increasing circulating flow rate. J. Ferment. Bioeng. 1997, 83, 443–450. [Google Scholar] [CrossRef]
- Chen, C.; Huang, Y.L.; Yang, S.T. A fibrous-bed bioreactor for continuous production of developmental endothelial locus-1 by osteosarcoma cells. J. Biotechnol. 2002, 97, 23–39. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.R.; Athalye, A.R. Mechanical and thermal-properties of glycidyl methacrylate grafted cotton cellulose. J. Appl. Polym. Sci. 1995, 57, 983–988. [Google Scholar] [CrossRef]
- Sokker, H.H.; Badawy, S.M.; Zayed, E.M.; Eldien, F.A.N.; Farag, A.M. Radiation-induced grafting of glycidyl methacrylate onto cotton fabric waste and its modification for anchoring hazardous wastes from their solutions. J. Hazard. Mater. 2009, 168, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.A.; Arthur, J.C. Radiation-initiated graft copolymerization of binary monomer mixtures containing acrylonitrile with cotton cellulose. J. Appl. Polym. Sci. 1970, 14, 3113–3128. [Google Scholar] [CrossRef]
- Brandt, S.; Goffe, R.A.; Kessler, S.; O-Conner, J.L.; Zale, S.E. Membrane based affin- ity technology for commercial scale purifications. Biotechnology 1988, 6, 779–782. [Google Scholar] [CrossRef]
- Boi, C.; Busini, V.; Salvalaglio, M.; Cavallotti, C.; Sarti, G.C. Understanding ligand-protein interactions in affinity membrane chromatography for antibody purification. J. Chromatogr. A 2009, 1216, 8687–8696. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.F.; Menkhaus, T.J.; Fong, H. Fabrication and bioseparation studies of adsorptive membranes/felts made from electrospun cellulose acetate nanofibers. J. Membr. Sci. 2008, 319, 176–184. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, H.Y.; Gurgel, P.V.; Carbonell, R.G. Polypropylene nonwoven fabrics with conformal grafting of poly(glycidyl methacrylate) for bioseparations. J. Membr. Sci. 2010, 364, 362–371. [Google Scholar] [CrossRef]
- Li, C.H.; Ladisch, C.M.; Yang, Y.Q.; Hendrickson, R.; Keim, C.; Mosier, N.; Ladisch, M.R. Optimal packing characteristics of rolled, continuous stationary-phase columns. Biotechnol. Prog. 2002, 18, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Hamaker, K.; Rau, S.L.; Hendrickson, R.; Liu, J.; Ladisch, C.M.; Ladisch, M.R. Rolled stationary phases: Dimensionally structured textile adsorbents for rapid liquid chromatography of proteins. Ind. Eng. Chem. Res. 1999, 38, 865–872. [Google Scholar] [CrossRef]
- Shukla, S.R.; Athalye, A.R. Graft-copolymerization of glycidyl methacrylate onto cotton cellulose. J. Appl. Polym. Sci. 1994, 54, 279–288. [Google Scholar] [CrossRef]
- King, J.K.; Pinto, N.G. Short fibrous supports for preparative chromatographic separations of biomolecules. J. Chromatogr. 1992, 609, 61–68. [Google Scholar] [CrossRef]
- Singh, A.; Pinto, N.G. Polymeric short-fiber chromatographic supports for downstream processing of biomolecules. React. Polym. 1995, 24, 229–242. [Google Scholar] [CrossRef]
- Bondar, Y.; Kim, H.J.; Yoon, S.H.; Lim, Y.J. Synthesis of cation-exchange adsorbent for anchoring metal ions by modification of poly(glycidyl methacrylate) chains grafted onto polypropylene fabric. React. Funct. Polym. 2004, 58, 43–51. [Google Scholar] [CrossRef]
- Ma, Z.; Ramakrishna, S. Electrospun regenerated cellulose nanofiber affinity membrane functionalized with protein a/g for IgG purification. J. Membr. Sci. 2008, 319, 23–28. [Google Scholar] [CrossRef]
- Kaur, S.; Ma, Z.; Gopal, R.; Singh, G.; Ramakrishna, S.; Matsuura, T. Plasma-induced graft copolymerization of poly(methacrylic acid) on electrospun poly(vinylidene fluoride) nanofiber membrane. Langmuir 2007, 23, 13085–13092. [Google Scholar] [CrossRef] [PubMed]
- Blessing, T.; Remy, J.S.; Behr, J.P. Template oligomerization of DNA-bound cations produces calibrated nanometric particles. J. Am. Chem. Soc. 1998, 120, 8519–8520. [Google Scholar] [CrossRef]
- Nakamae, K.; Nizuka, T.; Miyata, T.; Furukawa, M.; Nishino, T.; Kato, K.; Inoue, T.; Hoffman, A.S.; Kanzaki, Y. Lysozyme loading and release from hydrogels carrying pendant phosphate groups. J. Biomater. Sci. Polym. Ed. 1997, 9, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Jung, T.; Kamm, W.; Breitenbach, A.; Klebe, G.; Kissel, T. Loading of tetanus toxoid to biodegradable nanoparticles from branched poly(sulfobutyl-polyvinyl alcohol)-g-(lactide-co-glycolide) nanoparticles by protein adsorption: A mechanistic study. Pharm. Res. 2002, 19, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Lahore, M.; Mariano, G. Composite Material. U.S. Patent No. 20,130,112,623, 9 May 2013. [Google Scholar]
- Bibi, N.S.; Gavara, P.R.; Espinosa, S.L.S.; Grasselli, M.; Fernandez-Lahore, M. Synthesis and performance of 3d-megaporous structures for enzyme immobilization and protein capture. Biotechnol. Prog. 2011, 27, 1329–1338. [Google Scholar] [CrossRef] [PubMed]
- Plieva, F.M.; Savina, I.N.; Deraz, S.; Andersson, J.; Galaev, I.Y.; Mattiasson, B. Characterization of supermacroporous monolithic polyacrylamide based matrices designed for chromatography of bioparticles. J. Chromatogr. B 2004, 807, 129–137. [Google Scholar] [CrossRef]
- Lendero, N.; Vidič, J.; Brne, P.; Frankovič, V.; Štrancar, A.; Podgornik, A. Characterization of ion exchange stationary phases via ph transition profiles. J. Chromatogr. A 2008, 1185, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Lendero, N.; Vidic, J.; Brne, P.; Podgornik, A.; Strancar, A. Simple method for determining the amount of ion-exchange groups on chromatographic supports. J. Chromatogr. A 2005, 1065, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Boschetti, E.J.L.C. Enhanced diffusion chromatography and related sorbents for biopurification. In Bioseparation and Bioprocessing; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2008. [Google Scholar]
- Bibi, N.S.; Singh, N.K.; Dsouza, R.N.; Aasim, M.; Fernandez-Lahore, M. Synthesis and performance of megaporous immobilized metal-ion affinity cryogels for recombinant protein capture and purification. J. Chromatogr. A 2013, 1272, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Schure, M.R.; Maier, R.S. How does column packing microstructure affect column efficiency in liquid chromatography? Role Theory Chromatogr. 2006, 1126, 58–69. [Google Scholar]
- Guiochon, G.; Sarker, M. Consolidation of the packing material in chromatographic columns under dynamic axial compression. I. Fundamental study. J. Chromatogr. A 1995, 704, 247–268. [Google Scholar] [CrossRef]
- Koh, J.-H.; Broyles, B.S.; Guan-Sajonz, H.; Hu, M.Z.C.; Guiochon, G. Consolidation and column performance of several packing materials for liquid chromatography in a dynamic axial compression column. J. Chromatogr. A 1998, 813, 223–238. [Google Scholar] [CrossRef]
- Herigstad, M.O.; Gurgel, P.V.; Carbonell, R.G. Transport and binding characterization of a novel hybrid particle impregnated membrane material for bioseparations. Biotechnol. Prog. 2011, 27, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.Y.; Shi, Q.H.; Sun, Y. Novel biporous polymeric stationary phase for high-speed protein chromatography. J. Chromatogr. A 2004, 1061, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Sun, Y. Fabrication and characterization of a novel biporous spherical adsorbent for protein chromatography. Chromatographia 2003, 57, 29–35. [Google Scholar] [CrossRef]
- Arnold, F.H.; Blanch, H.W.; Wilke, C.R. Analysis of affinity separations ii: The characterization of affinity columns by pulse techniques. Chem. Eng. J. 1985, 30, B25–B36. [Google Scholar] [CrossRef]
- Bird, R.B.; Stewart, W.E.; Lightfoot, E.N. Transport Phenomena; Wiley: New York, NY, USA, 2002; Volume 2. [Google Scholar]
- Keener, R.N., Ш; Fernandez, E.J.; Maneval, J.E.; Hart, R.A. Advancement in the modeling of pressure-flow for the guidance of development and scale-up of commercial-scale biopharmaceutical chromatography. J. Chromatogr. A 2008, 1190, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Du, K.-F.; Yan, M.; Wang, Q.-Y.; Song, H. Preparation and characterization of novel macroporous cellulose beads regenerated from ionic liquid for fast chromatography. J. Chromatogr. A 2010, 1217, 1298–1304. [Google Scholar] [CrossRef] [PubMed]
- Amersham Biosciences. Bioprocess media. In Sepharose Fast Flow Ion Exchangers; Amersham Biosciences: Uppsala, Sweden, 2003. [Google Scholar]
- Garcia, M.C.; Marina, M.L.; Torre, M. Perfusion chromatography: An emergent technique for the analysis of food proteins. J. Chromatogr. A 2000, 880, 169–187. [Google Scholar] [CrossRef] [PubMed]
- Iberer, G.; Hahn, R.; Jungbauer, A. Monoliths as stationary phases for separating biopolymers—Fourth-generation chromatography sorbents. LC GC N. Am. 1999, 17, 998–1005. [Google Scholar]
- Gutsche, R.; Bunke, G. Modelling the liquid-phase adsorption in packed beds at low reynolds numbers: An improved hydrodynamic model. Chem. Eng. Sci. 2008, 63, 4203–4217. [Google Scholar] [CrossRef]
- Afeyan, N.B.; Fulton, S.P.; Regnier, F.E. Perfusion chromatography packing materials for proteins and peptides. J. Chromatogr. 1991, 544, 267–279. [Google Scholar] [CrossRef]
- Svec, F.; Frechet, J.M.J. Molded rigid monolithic porous polymers: An inexpensive, efficient, and versatile alternative to beads for the design of materials for numerous applications. Ind. Eng. Chem. Res. 1999, 38, 34–48. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gavara, P.R.; Bibi, N.S.; Sanchez, M.L.; Grasselli, M.; Fernandez-Lahore, M. Chromatographic Characterization and Process Performance of Column-Packed Anion Exchange Fibrous Adsorbents for High Throughput and High Capacity Bioseparations. Processes 2015, 3, 204-221. https://doi.org/10.3390/pr3010204
Gavara PR, Bibi NS, Sanchez ML, Grasselli M, Fernandez-Lahore M. Chromatographic Characterization and Process Performance of Column-Packed Anion Exchange Fibrous Adsorbents for High Throughput and High Capacity Bioseparations. Processes. 2015; 3(1):204-221. https://doi.org/10.3390/pr3010204
Chicago/Turabian StyleGavara, Poondi Rajesh, Noor Shad Bibi, Mirna Lorena Sanchez, Mariano Grasselli, and Marcelo Fernandez-Lahore. 2015. "Chromatographic Characterization and Process Performance of Column-Packed Anion Exchange Fibrous Adsorbents for High Throughput and High Capacity Bioseparations" Processes 3, no. 1: 204-221. https://doi.org/10.3390/pr3010204