Experimental Study on Feasibility of Enhanced Gas Recovery through CO2 Flooding in Tight Sandstone Gas Reservoirs
Abstract
:1. Introduction
2. Experimental Section
2.1. Cores
2.2. Fluid
2.3. Experimental Setup
2.4. Experimental Method
2.4.1. Measurement of Capillary Pressure
2.4.2. Measurement of CH4 Recovery and CO2 Storage Efficiency through CO2 Flooding
- (1)
- The short tight sandstone cores were added to the long-core holder in order, which was placed into the thermostat box. The displacement flow path was connected, according to the experimental device diagram presented in Figure 1.
- (2)
- The long-core was vacuum-pumped. The thermostat box was set to 110 °C and the long-core confining pressure was set to 30 MPa.
- (3)
- The irreducible water saturation status of the combined tight long-core was obtained under conditions of confining pressure and a displacement pressure drop. The simulated formation water was injected into the closed long-core holding system from intermediate container 2. Consequently, high-purity CH4 gas was injected into the system from intermediate container 1. In this process, the simulated formation water was displaced by CH4 until it did not flow out of the long-core outlet, which indicated that this procedure was over.
- (4)
- Pure CH4 was continuously injected into the closed long-core holding system with a constant pressure of 8 MPa. The tight sandstone long-core was fully saturated with CH4 and the outlet valve was closed during the entire saturation process. The CH4 saturation process was considered to be completed when the inlet pressure was stable for more than 12 h.
- (5)
- The intermediate container 3, which was filled with high-purity CO2, was connected to the displacement device system. The pressures of the long-core inlet and outlet were 12 MPa and 8 MPa, respectively. This meant that the displacement differential pressure was 4 MPa. Each time that a 0.1 pore volume (PV) of CO2 was injected into the long-core, the pressure at each pressure point was recorded. Furthermore, the gas production at the outlet was also recorded and the gas contents of CH4 and CO2 were analyzed with the chromatography analyzer. The characteristics of CO2 migration and breakthrough of the front edge were monitored in real time. Additionally, the displacement efficiency of CO2 flooding CH4 was calculated. When the gas content of CO2 at the outlet exceeded 98%, the experiments were over.
- (6)
- Subsequent to each group of experiments, the tight sandstone long-core was vacuum-pumped and steps (2)–(5) were repeated.
3. Results and Discussion
3.1. CH4 Recovery through CO2 Flooding and CO2 Storage Efficiency under Constant Pressure Displacement
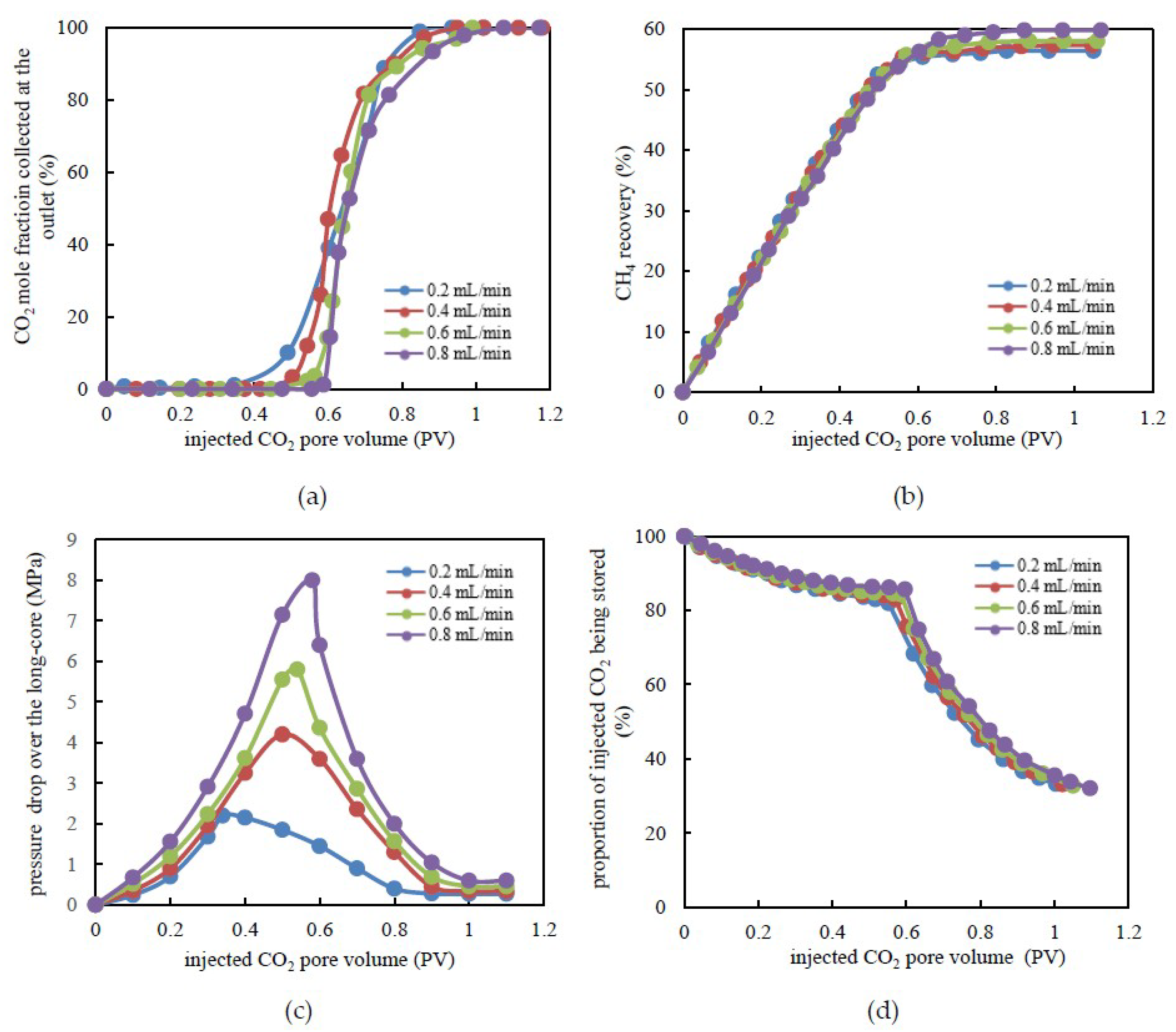
3.2. CH4 Recovery through CO2 Flooding and CO2 Storage Efficiency under Various Injection Rates
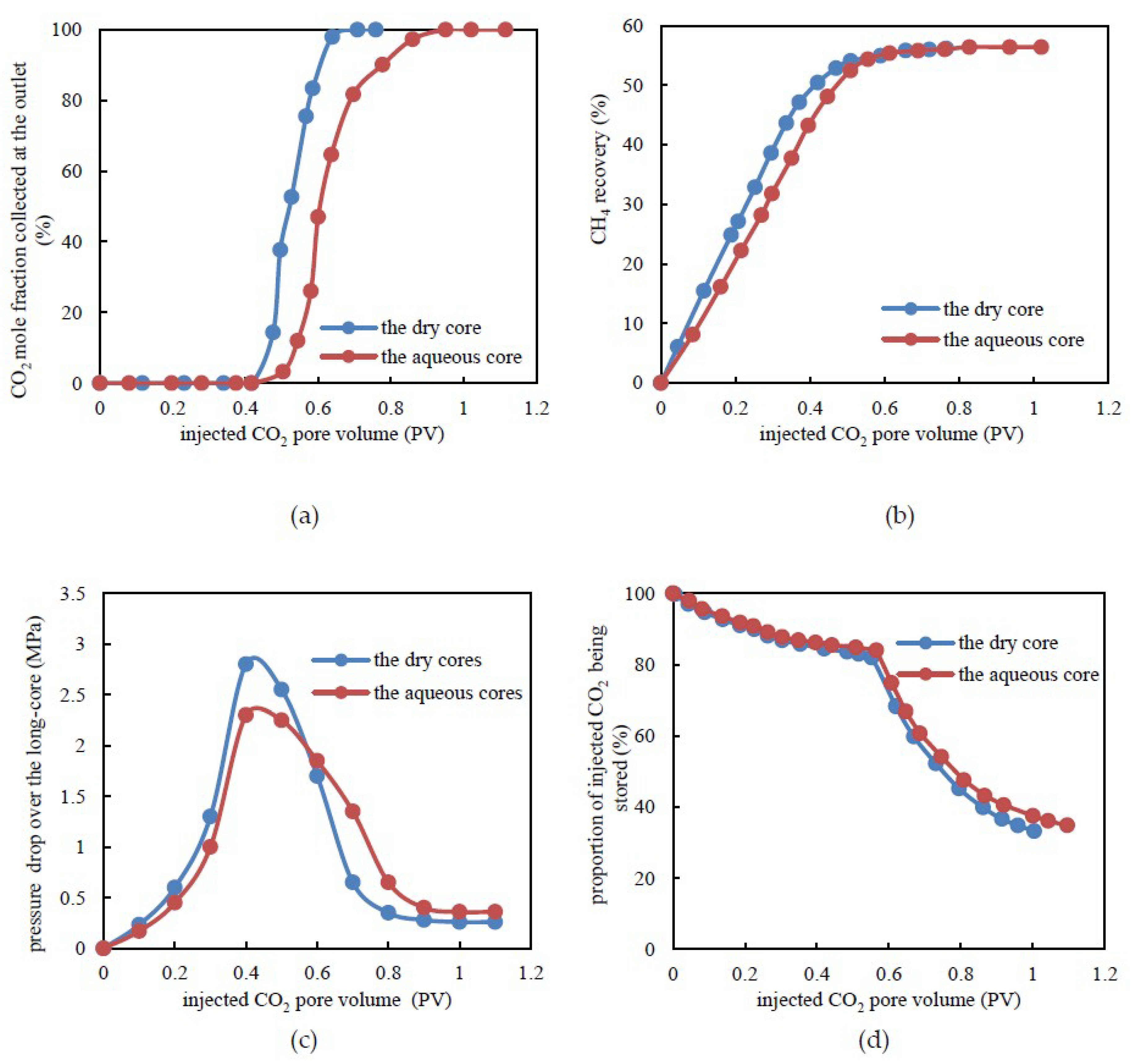
3.3. CH4 Recovery through CO2 Flooding and CO2 Storage Efficiency under Dry and Aqueous Cores
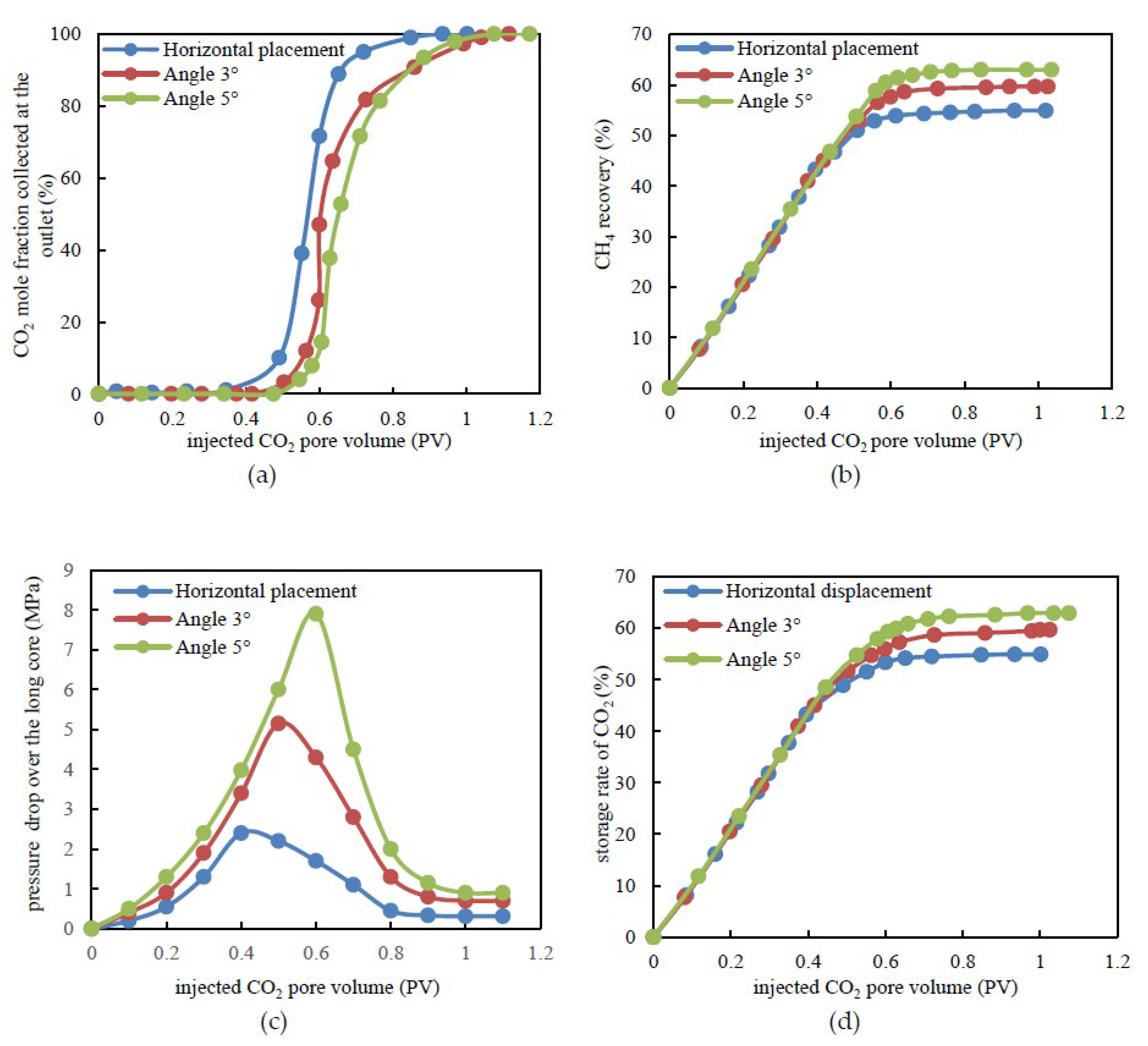
3.4. CH4 Recovery through CO2 Flooding and CO2 Storage Efficiency under Various Formation Dip Angle Conditions
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Peter, G.H.; Bath, O.B. Status report on miscible/immiscible gas flooding. J. Pet. Sci. Eng. 1989, 2, 103–117. [Google Scholar] [CrossRef]
- Santos, L.; Marcondes, F.; Sepehrnoori, K. A 3D compositional miscible gas flooding simulator with dispersion using Element-based Finite-Volume method. J. Pet. Sci. Eng. 2013, 112, 61–68. [Google Scholar] [CrossRef]
- Chen, M.; Wang, H.; Liu, Y.; Ma, L.; Wu, D.; Wang, S. Corrosion behavior study of oil casing steel on alternate injection air and foam liquid in air-foam flooding for enhance oil recovery. J. Pet. Sci. Eng. 2017, 165, 970–977. [Google Scholar] [CrossRef]
- Hosseini, S.; Alfi, M.; Nicot, J.; Nunez, L. Analysis of CO2 storage mechanisms at a CO2-EOR site, Cranfield, Mississippi. Greenhouse. Gases Sci. Eng. 2018, 27, 218–229. [Google Scholar] [CrossRef]
- Lai, F.; Li, Z.; Zhang, W.; Dong, H.; Kong, F.; Jiang, Z. Investigation of Pore Characteristics and Irreducible Water Saturation of Tight Reservoir Using Experimental and Theoretical Methods. Energy Fuels 2018, 32, 1–18. [Google Scholar] [CrossRef]
- Wang, H.; Ma, F.; Tong, X.; Liu, Z.; Zhang, X.; Wu, Z.; Li, D.; Wang, B.; Xie, Y.; Yang, L. Assessment of global unconventional oil and gas resources. Pet. Explor. Dev. 2016, 43, 850–862. [Google Scholar] [CrossRef]
- Guo, S.; Lyu, X.; Zhang, Y. Relationship between tight sandstone reservoir formation and hydrocarbon charging: A case study of a Jurassic reservoir in the eastern Kuqa Depression, Tarim Basin, NW China. J. Nat. Gas Eng. 2018, 52, 304–316. [Google Scholar] [CrossRef]
- Wang, F.; Liu, Y.; Hu, C.; Shen, A.; Liang, S.; Cai, B. A Simplified Physical Model Construction Method and Gas-Water Micro Scale Flow Simulation in Tight Sandstone Gas Reservoirs. Energies 2018, 11, 1559. [Google Scholar] [CrossRef]
- Gomez, A.; Briot, P.; Raynal, L.; Broutin, P.; Gimenez, M.; Soazic, M.; Cessat, P.; Saysset, S. ACACIA Project—Development of a Post-Combustion CO2 Capture Process. Case of the DMXTM Process. Oil Gas Sci. Technol. 2014, 69, 1121–1129. [Google Scholar] [CrossRef]
- Shi, Y.; Jia, Y.; Pan, W.; Huang, L.; Yan, J.; Zheng, R. Potential evaluation on CO2-EGR in tight and low-permeability reservoirs. Nat. Gas Ind. B 2017, 37, 62–69. [Google Scholar] [CrossRef]
- Littke, R.; Krooss, B.; Merkel, A.; Gensterblum, Y. Gas saturation and CO2 enhancement potential of coalbed methane reservoirs as a function of depth. AAPG Bull. 2014, 98, 395–420. [Google Scholar] [CrossRef]
- Liang, W.; Wu, D.; Zhao, Y. Experimental study of coalbeds methane replacement by carbon dioxide. Chin. J. Rock Mech. Eng. 2010, 29, 665–673. (In Chinese) [Google Scholar]
- Zeng, Q.; Wang, Z.; Liu, L.; Ye, J. Modeling CH4 Displacement by CO2 in Deformed Coalbeds during Enhanced Coalbed Methane Recovery. Energy Fuels 2018, 32, 1942–1955. [Google Scholar] [CrossRef]
- Dang, Y.; Zhao, L.; Lu, X.; Xu, J.; Sang, P.; Guo, S.; Zhu, H.; Guo, W. Molecular simulation of CO2/CH4 adsorption in brown coal: Effect of oxygen-, nitrogen-, and sulfur-containing functional groups. Appl. Surf. Sci. 2017, 423, 33–42. [Google Scholar] [CrossRef]
- Murray, R.; Derek, B.; Ferus, I.; Cui, X.; Cory, T.; Mike, C. A Laboratory Study of CO2 Interactions within Shale and Tight Sand Cores-Duvernay, Montney and Wolfcamp Formations. In Proceedings of the SPE Canana Unconventional Resources Conference, Calgary, AB, Canada, 13–14 March 2018. [Google Scholar] [CrossRef]
- Xu, C.; Cai, J.; Yu, Y.; Yan, K.; Li, X. Effect of pressure on methane recovery from natural gas hydrates by methane-carbon dioxide replacement. Appl. Energy 2018, 217, 527–536. [Google Scholar] [CrossRef]
- Stanfield, P. Use of low- and High-IFT fluid Systems in experimental and numerical modeling of Systems that mimic CO2 storage in deep saline Formations. J. Pet. Sci. Eng. 2015, 129, 97–109. [Google Scholar] [CrossRef] [Green Version]
- Darcis, M.; Class, H.; Flemisch, B.; Helmig, R. Sequential Model Coupling for Feasibility Studies of CO2 Storage in Deep Saline Aquifers Couplage séquentiel des modèles numériques appliqué aux études de faisabilité du stockage de CO2 en aquifère salin profond. Oil Gas Sci. Technol. 2011, 66, 93–103. [Google Scholar] [CrossRef]
- Tian, W.; Li, A.; Ren, X.; Josephine, Y. The threshold pressure gradient effect in the tight sandstone gas reservoirs with high water saturation. Fuel 2018, 226, 221–229. [Google Scholar] [CrossRef]
- Kubus, P. CCS and CO2-storage possibilities in Hungary. In Proceedings of the SPE International Conference on CO2 Capture, Storage, and Utilization, New Orleans, LA, USA, 10–12 November 2010. [Google Scholar] [CrossRef]
- Birkedal, K.; Hauge, L.; Graue, A.; Ersland, G. Transport Mechanisms for CO2-CH4 Exchange and Safe CO2 Storage in Hydrate-Bearing Sandstone. Energies 2015, 8, 4073–4095. [Google Scholar] [CrossRef]
- Vandeweijer, V.; Meer, B.; Hofstee, C. Monitoring the CO2, injection site: K12-B. Energy Procedia 2011, 4, 5471–5478. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Xue, F.; Wang, Y.; Ren, B.; Zhang, L.; Ren, S. CO2 foam flooding for improved oil recovery: Reservoir simulation models and influencing factors. J. Pet. Sci. Eng. 2015, 133, 838–850. [Google Scholar] [CrossRef]
- Fakhr, M.; Rashidi, A.; Giti, A.; Akbarnejad, M.; Masoud, Z. Parametric Study for the Growth of Single-walled Carbon Nanotubes over Co-Mo/Mgo in Fluidized Bed Reactor by CCVD Method. J. Pet. Sci. Eng. 2010, 4, 28–34. [Google Scholar] [CrossRef]
- Bottero, S.; Hassanizadeh, S.; Kleingeld, P. From Local Measurements to an Upscaled Capillary Pressure–Saturation Curve. Transp. Porous Media 2011, 88, 271–291. [Google Scholar] [CrossRef] [Green Version]
- Zou, C.; Zhu, R.; Wu, S.; Yang, Z.; Tao, S.; Yuan, X.; Hou, L.; Yang, H.; Xu, C.; Li, D.; et al. Type, characteristics, genesis and prospects of conventional and unconventional hydrocarbon accumulation: Taking tight gas in China as an instance. Acta Pet. Sin. 2012, 33, 173–187. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, C.; Zhang, Z.; Qin, R.; Yu, J. Comparative experimental study of the core irreducible water saturation of tight gas reservoir. Nat. Gas Geosci. 2016, 27, 352–358. [Google Scholar] [CrossRef]
- Hu, H.; Cheng, Y. Modeling by computational fluid dynamics simulation of pipeline corrosion in CO2-containing oil-water two phase flow. J. Pet. Sci. Eng. 2016, 146, 134–141. [Google Scholar] [CrossRef]



| K+ | Na+ | Ca2+ | Mg2+ | Sr2+ | Cl− | SO42− | CO32− | HCO3− | OH− | Total Salinity |
|---|---|---|---|---|---|---|---|---|---|---|
| (mg/L) | ||||||||||
| 1541 | 15,221 | 12,344 | 2539 | 650 | 59,774 | 744 | none | 257 | none | 102,000 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Liu, Y.; Hu, C.; Wang, Y.; Shen, A.; Liang, S. Experimental Study on Feasibility of Enhanced Gas Recovery through CO2 Flooding in Tight Sandstone Gas Reservoirs. Processes 2018, 6, 214. https://doi.org/10.3390/pr6110214
Wang F, Liu Y, Hu C, Wang Y, Shen A, Liang S. Experimental Study on Feasibility of Enhanced Gas Recovery through CO2 Flooding in Tight Sandstone Gas Reservoirs. Processes. 2018; 6(11):214. https://doi.org/10.3390/pr6110214
Chicago/Turabian StyleWang, Fengjiao, Yikun Liu, Chaoyang Hu, Yongping Wang, Anqi Shen, and Shuang Liang. 2018. "Experimental Study on Feasibility of Enhanced Gas Recovery through CO2 Flooding in Tight Sandstone Gas Reservoirs" Processes 6, no. 11: 214. https://doi.org/10.3390/pr6110214





