The purpose of the work is the design of a separation unit for the production of 100 kg/day of hydrogen with a purity equal to 99.999% from a raw biogas reformer. A literature review shows that the common feed composition of the gas entering the PSA systems mainly comes from reforming of natural gas, which is different from the one here analyzed, due to higher presence of impurities. As discussed below, the inlet syngas composition strongly affects the final value of hydrogen purity, so it was necessary to design a new model of PSA/VPSA.
3.1. PSA Model Literature
This section reports modelling and experimental works on PSA, studying the effect of various parameters, such as bed size, bed configurations, pressure equalizations, feed composition, etc. Four-bed PSA, four-bed VPSA, and four-bed TSA are modelled by [
20], considering feed gas produced by the steam reforming of natural gas containing 75% of hydrogen. The purity required is in the range 99.9–99.99%. A comparison is made among the three different purification systems, trying to identify which is the best in terms of purity, recovery, and productivity. The VPSA system was selected as the most economical process to meet the fuel cell purity (99.99%), while the PSA would meet the requirements with a recovery as low as 10–15%, making this system uneconomical. Another work [
19] modelled a two- and four-bed PSA, considering as feed gas a syngas produced from coal gasification with low hydrogen concentration (38% H
2, 50% CO
2, 1% CH
4, 1% CO, 10% N
2). The work analyzes the different performances of two and four beds, varying each time a single parameter (carbon to zeolites ratio, pressure, temperature, adsorption time). Results showed that the four-bed PSA process was able to produce H
2 with a purity of 96–99.5% and a recovery of 71–85%, together with N
2 as the major impurity. The separation of hydrogen from a five component mixture (73.3% H
2, 16.6% CO
2, 3.5% CH
4, 2.9% CO, 3.7% N
2) by pressure swing adsorption was analyzed [
29]. A complete mathematical model that describes the dynamic behavior of a PSA unit is presented. This model is applied in the study of the behavior of both single column and four-column PSA processes with layered activated carbon/zeolite beds, and with an eight step cycle. The influence of feed flowrate, purge to feed ratio, and lengths of both adsorbent layers on the system performance is assessed. It is shown that the introduction of the zeolite layer improves both the purity and recovery of the process. A reduced model and a complete model were also compared. It was shown that the reduced model in which only micro-pore resistance was taken into account, and in which it was assumed thermal equilibrium between gas and solid phase, predicted with high accuracy the performances of the PSA. Moreover, [
21] studied a four-bed configuration with activated carbon and zeolites. The feed composition is a syngas with no N
2 (H
2 72.2%, CH
4 4.17%, CO 2.03%, CO
2 21.6%). The effect of linear velocity, adsorption time, purge quantity was investigated. The results showed an ultrapure hydrogen (99.999%) with a recovery of 66%. In [
30], a multicomponent breakthrough experiment to validate the mathematical model with a mixture of five components (H
2–CO
2–CO–CH
4–N
2) was performed. A ten-step VPSA experiment was performed with a feed composition of 79% H
2, 17% CO
2, 2.1% CH
4, 1.2% CO, and 0.7% N
2. It was demonstrated that the VPSA could reach high purity hydrogen (>99.99%), with recoveries as high as 75% at 5 bar, and a productivity of 160 mol
H2/kg
ads/day. The effect of pressure equalization was also investigated, which increases the recovery by 15% from one pressure equalization to three pressure equalizations. It was also demonstrated that higher feed pressure increases the productivity of the system, maintaining the same purity.
3.2. PSA and VPSA Model
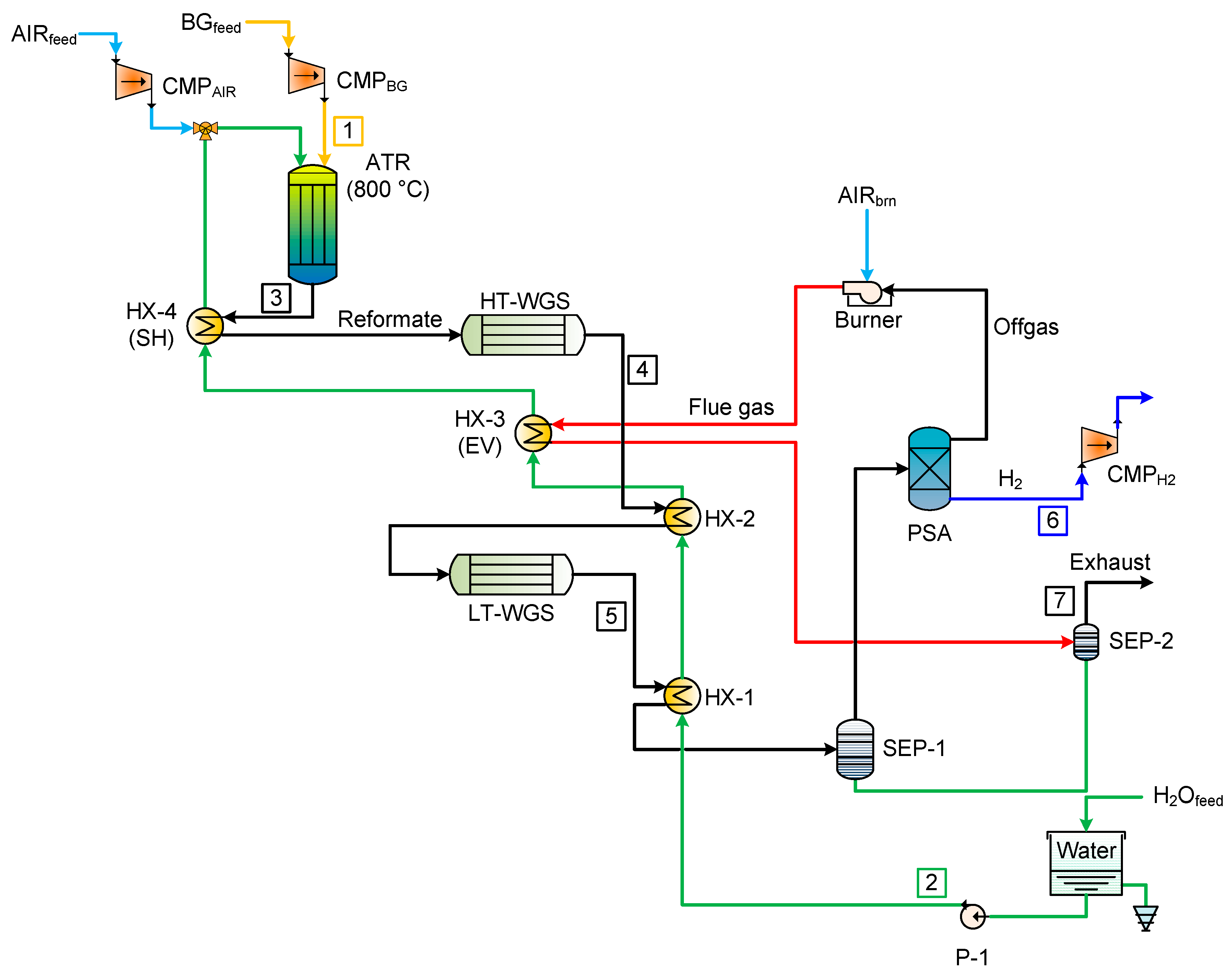
The software Aspen Adsorption has been used to model and simulate the performance of the systems. The flowsheet reported in
Figure 3 shows the components used in the simulation. The various references have been used to make a preliminary design of the PSA/VPSA, and to have sensitivity on the values and on the order of magnitude of the different parameters. The aim of this section was, hence, to design a system able to process 100 kg/day, delivering ultrapure hydrogen (99.999%). The simplest model of PSA (two-bed system) was firstly designed, and then, having a better understanding of the process and software used, it was possible to design four-bed PSA and then, finally, four-bed VPSA. In a H
2 PSA unit, a complex configuration involving a higher number of beds is fundamental to increase the recovery at the same purity. The multiple-bed solution is modelled with the “unibed” approach [
31] in Aspen Adsorption by means of the “interaction column”, which simulates any number of columns by storing the inlet stream properties (such as flowrate, composition, pressure,) and playing them back later according to the step sequence.
The sequence of steps is designed so that the whole PSA system provides constant feed, product, and off-gas flows, even though the system is made of multiple beds and not a continuous unit operation. The number of columns used for the simulation has been chosen according to references in which PSA systems have been designed to work in conditions similar in flowrate and composition to the one modelled in this work [
19,
20,
21]. Moreover, commercially available system for hydrogen production with the same size considers a four-column VPSA system [
32]. The process is generally referred to as Polybed [
33], and it can be applied to a large variety of PSA systems with any number of columns. The main features of the Polybed sequence are (i) stopping the adsorption step when a substantial part of the bed capacity remains unused near the product end for increasing the purity, and (ii) use of co-current depressurizations to greatly increase the recovery and performance of the system. The step sequence is taken from [
20].
The adsorbent layer materials are activated carbon and zeolite 5a, that have been proven, in literature, to have the best performance at investigated pressures and composition [
19]. The activated carbon mainly adsorbs CO
2, CH
4, and CO, and are placed at bottom of the column (feed inlet), while the zeolite layer adsorbs N
2 and CO, and it is placed at the top of the activated carbon. The relative length of the two layers is expressed by the “carbon-to-zeolite ratio”, and it is a key parameter inside the PSA system.
The geometry of the reactor, reported in
Table 5, has been set according to references showing configurations similar in size to the one designed in this work and to information about existing systems. The diameter of the column was set to 0.38 m, which made the gas velocity inside the reactor around 3 cm/s. The height of the PSA columns was set to 2 and 2.8 m for the two beds, while the four-bed configuration has a height of 2.8 meters, with a carbon-to-zeolite ratio equal to 50:50.
The parameters considered for the evaluation of the PSA/VPSA performances are
Pressure swing adsorption system is very complex, due to its cyclic nature, with multiple steps and interactions between beds undergoing different operations. For the maximization of the recovery with the purity constraint (99.999%), the following parameters have been manipulated during the simulations:
3 pressures (Feed, Product, Purge)
purge-to-feed ratio (P/F)
6 time steps
4 valve coefficients
gas velocity in the column
activated carbon-to-zeolites ratio
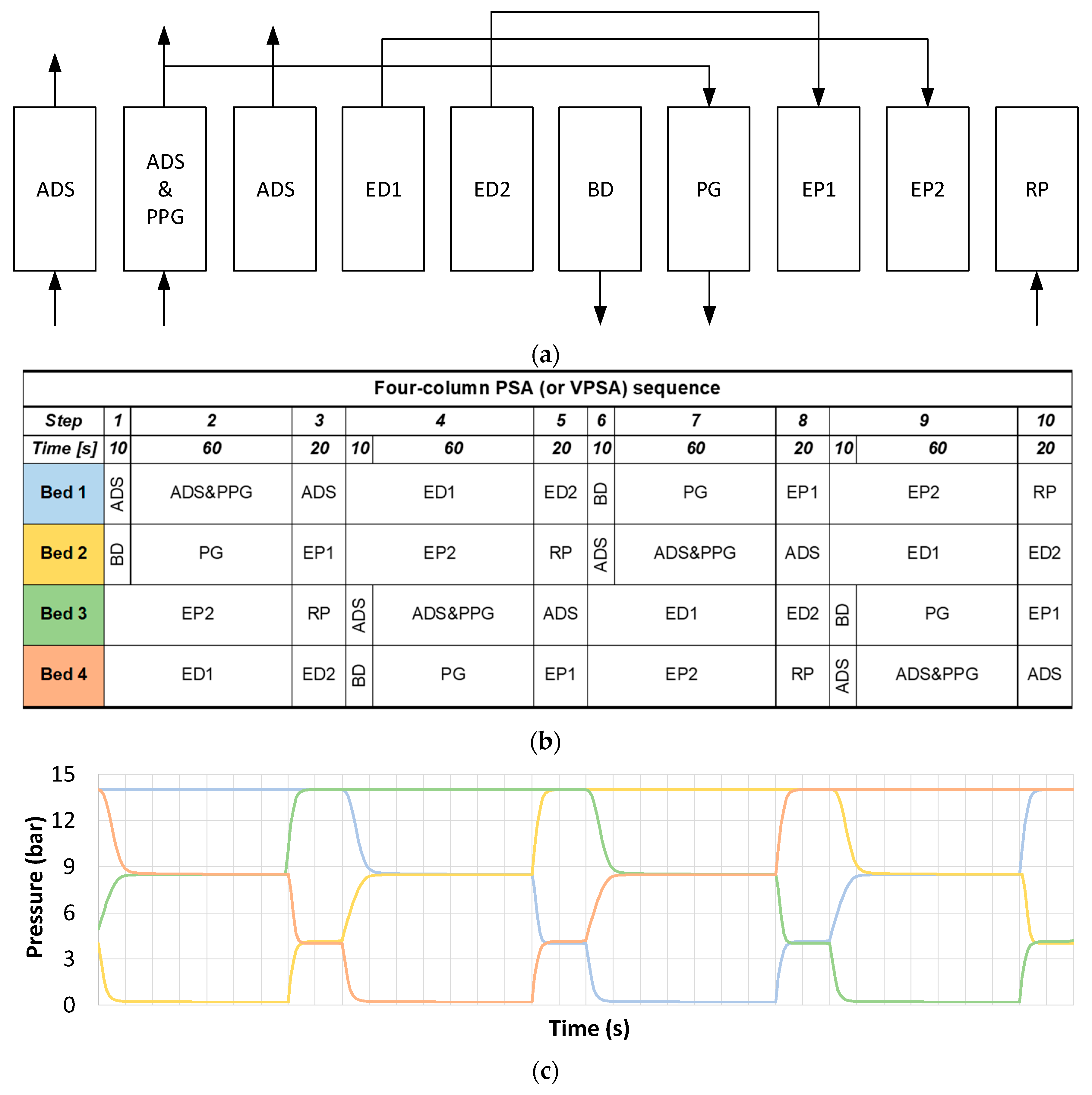
A typical cyclic sequence for the four-bed PSA or VPSA, reported in
Figure 4, consists of 10 steps:
- (1)
Adsorption (ADS): adsorption takes place in one column. Part of the H2-rich product stream is used to purge the other columns.
- (2)
Adsorption and providing purge (ADS&PPG): part of the produced hydrogen is used to regenerate the bed undergoing the purge step.
- (3)
Adsorption (ADS): adsorption takes place in one column at low pressure. Part of the H2-rich product stream is used to purge the other columns.
- (4)
First depressurization (ED1): the column is co-currently depressurized to medium pressure level. The produced hydrogen is used to pressurize another column undergoing step (EP2).
- (5)
Second depressurization (ED2): pressure is further reduced by using the produced hydrogen to re-pressurize the column undergoing step (EP1).
- (6)
Blow down (BD): the column is counter-currently depressurized to the minimum pressure (atmospheric for PSA or sub-atmospheric for VPSA). The effluent gas is wasted.
- (7)
Purge (PG): the column is purged with H2 produced in step 2).
- (8)
First pressurization (EP1).
- (9)
Second pressurization (EP2).
- (10)
Feed pressurization (RP): the bed is co-currently pressurized with feed entering from the bottom of the column.
For each simulation, the time step has been optimized to meet the hydrogen purity requirement. The two-bed PSA sequence follows the same general concept, with the main difference of having only one pressure equalization (hence lower number of steps) and different steps timing.
The ideal Langmuir isotherm model can be extended to multicomponent systems by incorporating the effect of the other species on the adsorption process and on the adsorbent material. An extended Langmuir model is adopted, and the loading (defined as the number of moles of the
i-th component adsorbed over the mass of adsorbent), is expressed as follows:
where
pi is the partial pressure of the
i-th component.
The adsorption equilibrium parameters, for activated carbon and zeolites 5a as a function of operating pressure at 35 °C, were taken from [
19].
The pressure swing adsorption system have to be designed and optimized with different compositions of feed gases, which are a function of biogas feed and plant layout (SR or ATR). Stream results are reported in
Table 6. The composition differs between the steam reforming and the autothermal reforming, due to the oxidation of part of the methane with air in the autothermal reforming reactor. The difference in composition for the ATR case between landfill and anaerobic digestion biogas is almost negligible: for the same system pressure, hydrogen molar fraction for the former is around 2% lower than the second one, while CO
2 and N
2 differ by around 1%. On the other hand, differences in composition in the SR case are more evident. For the AD biogas, hydrogen molar fraction is 6% higher, carbon dioxide is present in slightly lower amount, while nitrogen is almost negligible: recovery is expected to be higher, thanks to lower impurities in the gas, especially nitrogen, which is the main cause of purity reduction.
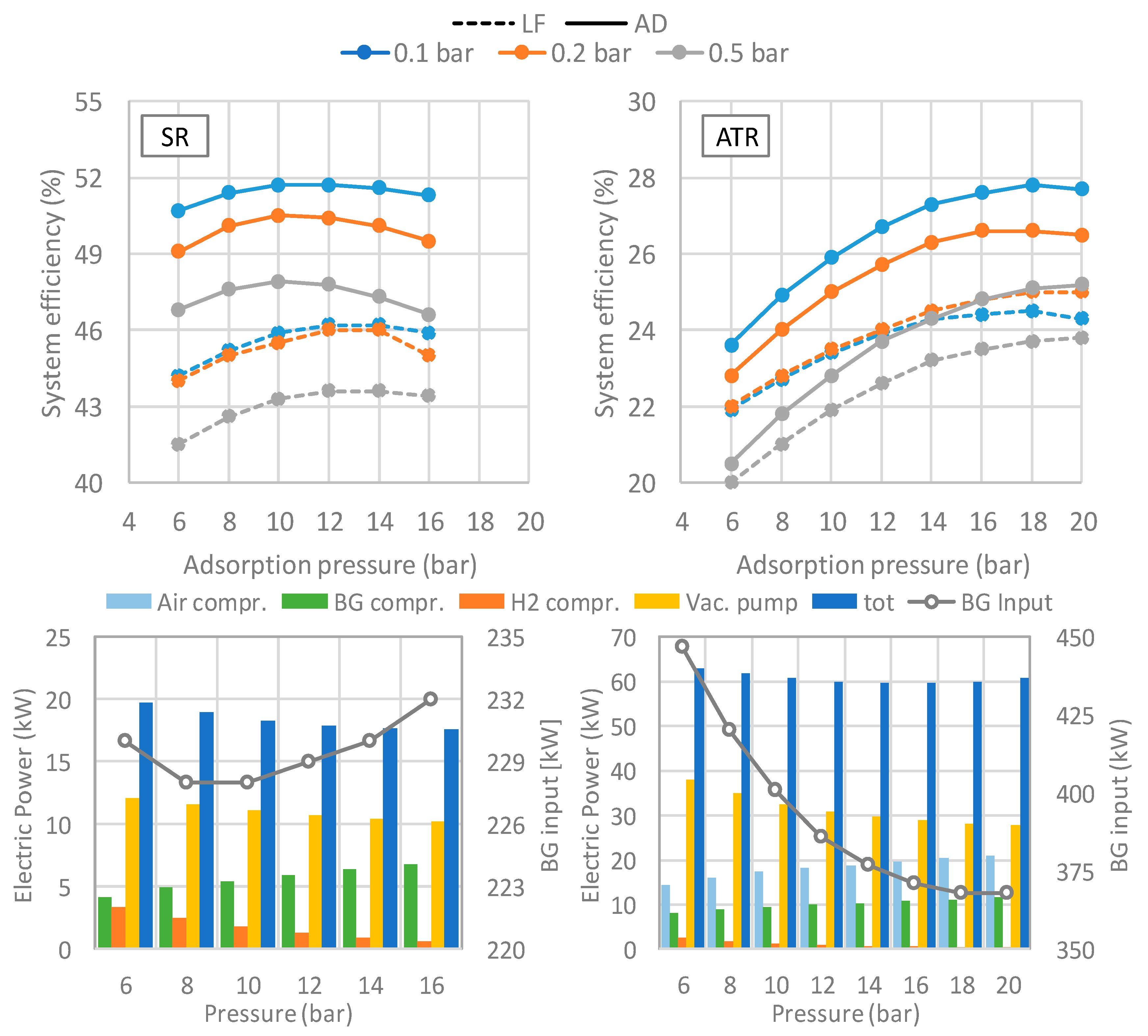
The effect of pressure on methane conversion into hydrogen shows two opposite trends for SR and ATR. For the SR layout, the molar fraction of hydrogen decreases with increasing pressure, while methane content increases: steam reforming reaction increases the number of moles, hence the equilibrium shifts towards the reactants when the pressure is increased. On the contrary, hydrogen content in the ATR layout increases at increasing system pressure.
3.4. VPSA Results
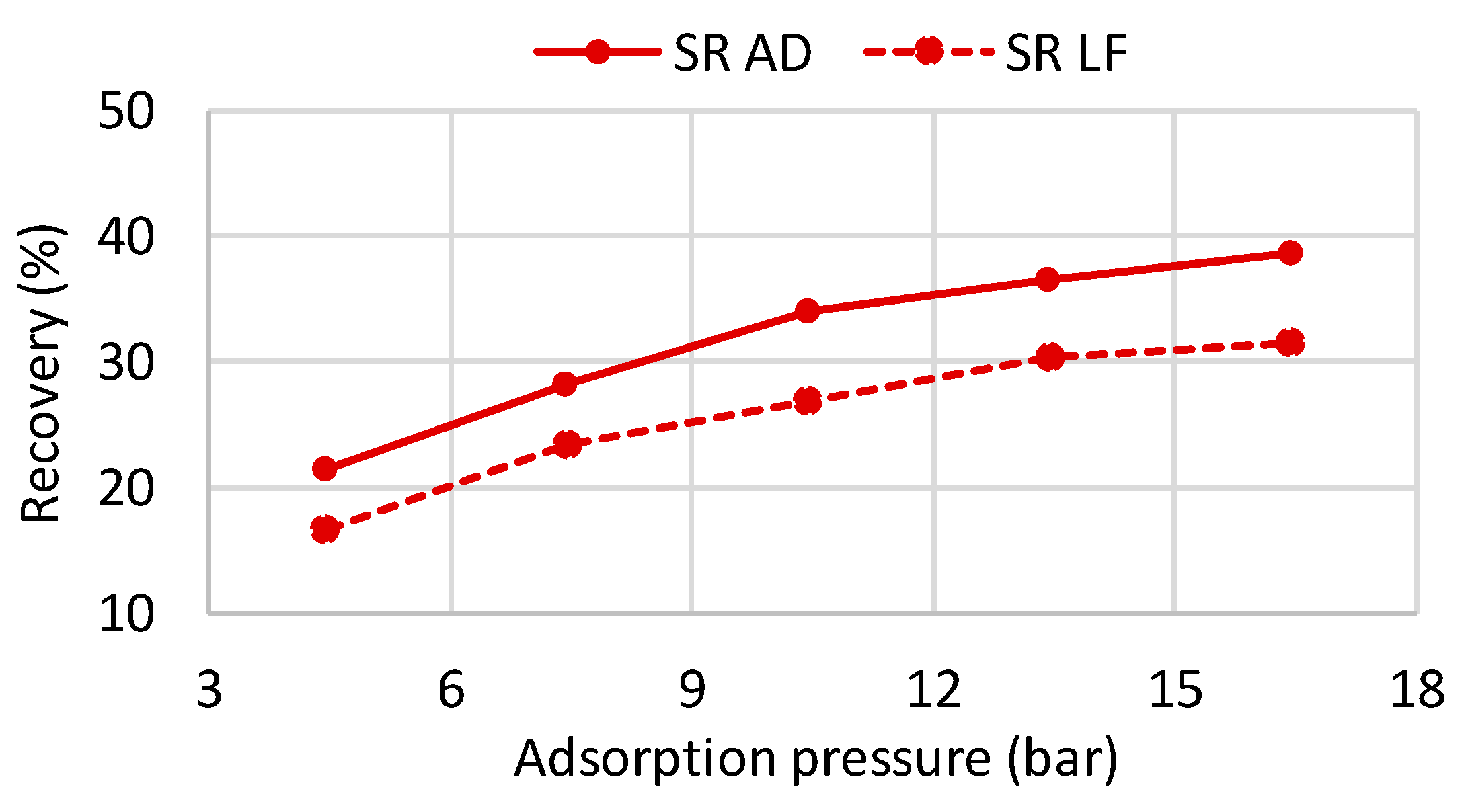
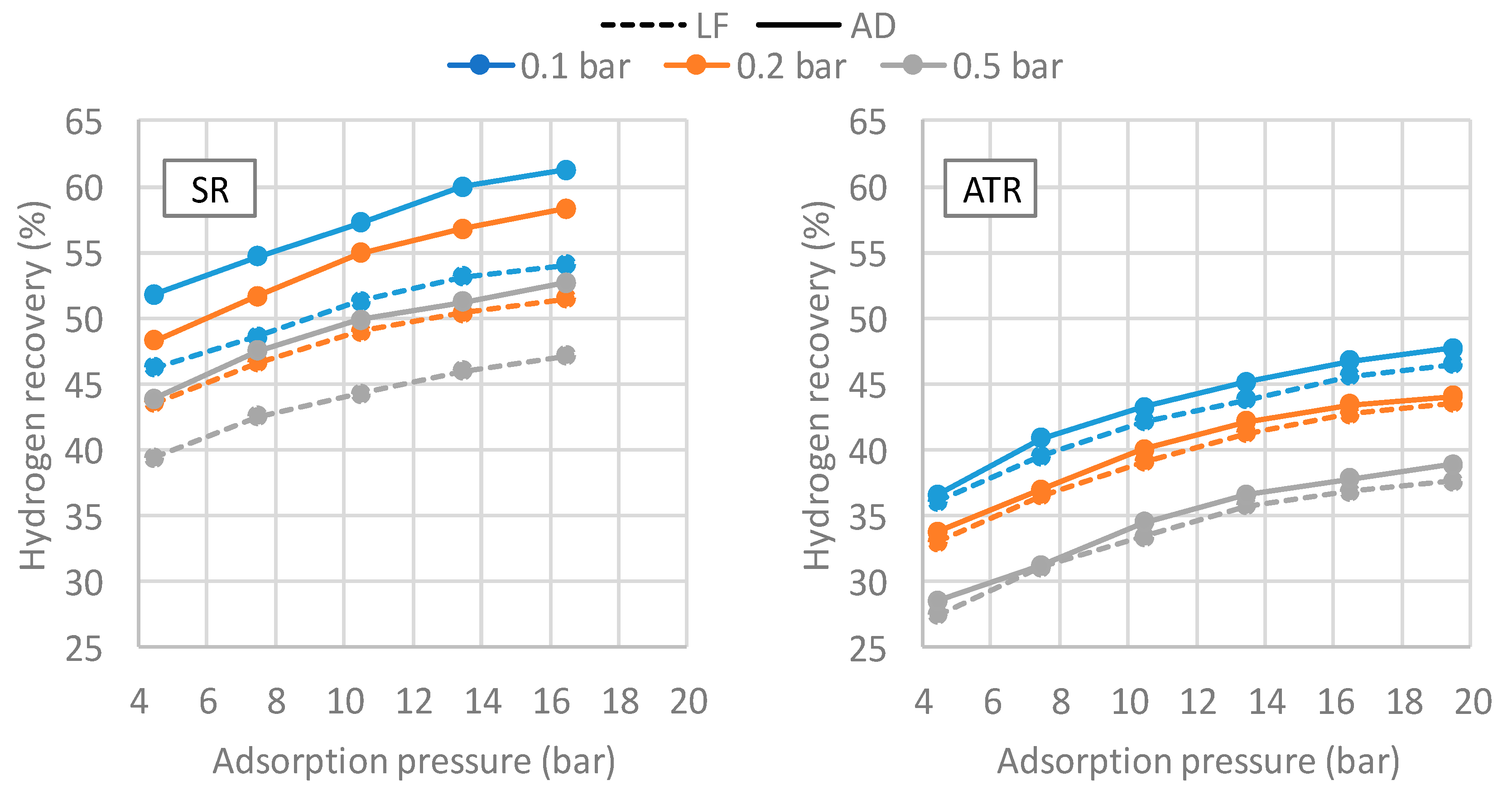
Figure 6 shows the recoveries of the VPSA system for the four different compositions analyzed. The reported pressures take into account also the pressure drops in the plant upstream in the VPSA. The three curves represent three different vacuum levels during the purge step: 0.1, 0.2, and 0.5 bar. The recoveries curve for all the cases reported here are calculated at a purity level equal to 99.999% (5.0), according to the requirements of the BIONICO.
The resulting recovery–pressure curves are then implemented in the overall system model. As it is possible to notice, the highest recovery is always obtained for the lowest vacuum pressure (0.1 bar). The gas composition is the parameter that mostly affects the performance of the system. In particular, the higher the hydrogen content in the feed stream, the higher the recovery, since lower impurities have to be purged when regenerating the column. The two recoveries for the syngas produced by the autothermal plant layout are almost identical, since the composition of the feed gas is very similar as previously described: their recoveries, however, are significantly lower with respect to the SR cases, as the high amount of nitrogen and carbon dioxide requires a large quantity of hydrogen for purging the impurity-filled column. For all the cases, nitrogen represents the main impurity in the product stream, while the amount of carbon dioxide, carbon monoxide, and methane is almost negligible.