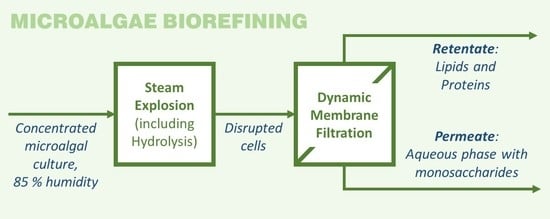
Steam Explosion and Vibrating Membrane Filtration to Improve the Processing Cost of Microalgae Cell Disruption and Fractionation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microalgae Samples
2.2. Steam Explosion
2.3. Filtration
2.4. Lipid Extraction
2.5. Analytical Techniques
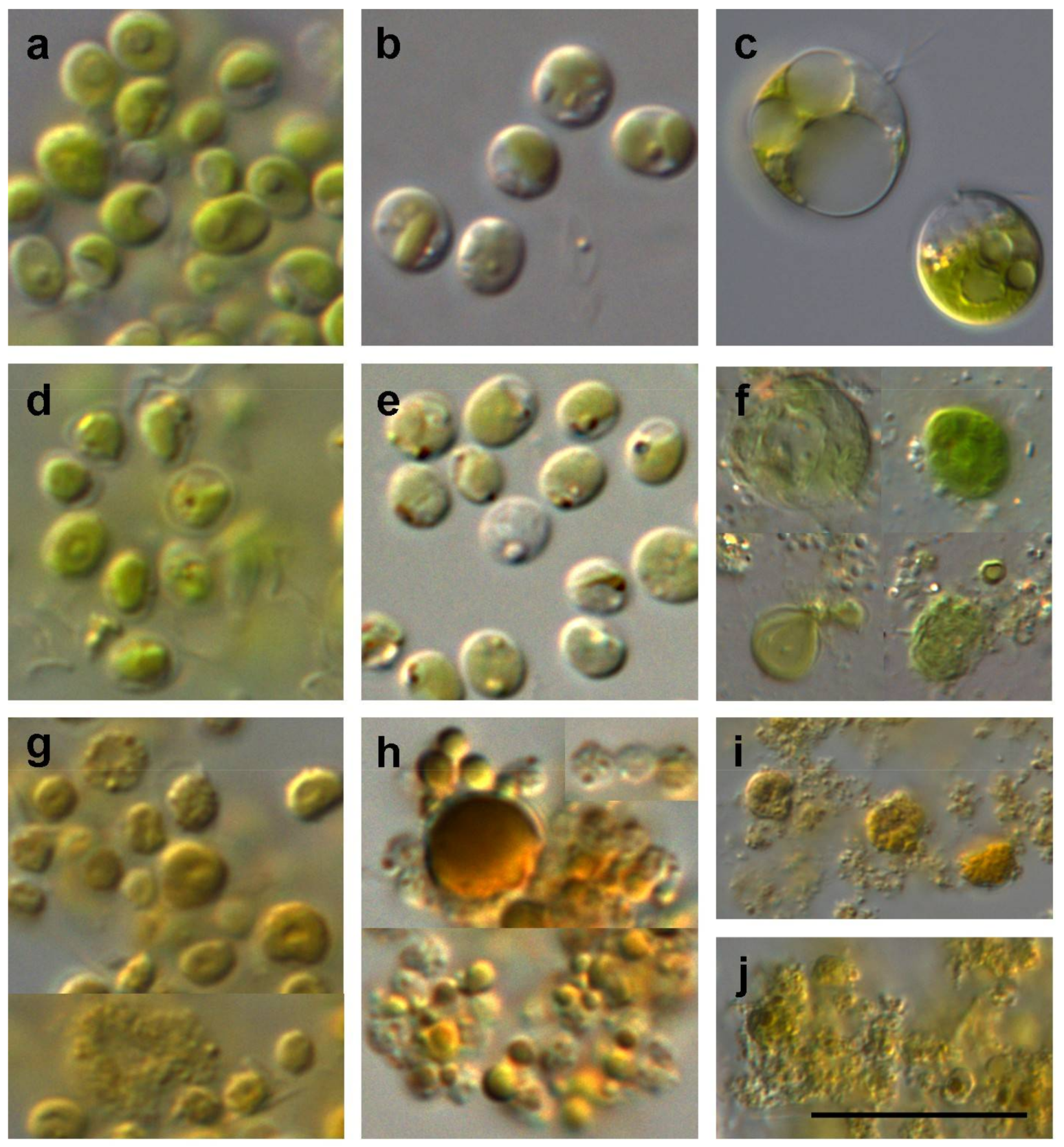
2.5.1. Light Microscope
2.5.2. Dry Matter and Ash Content (TGA)
2.5.3. Total Lipid Extraction with Bligh and Dyer Method
2.5.4. Analytical Acid Hydrolysis
2.5.5. Monosaccharides Analysis
2.5.6. Protein Analysis
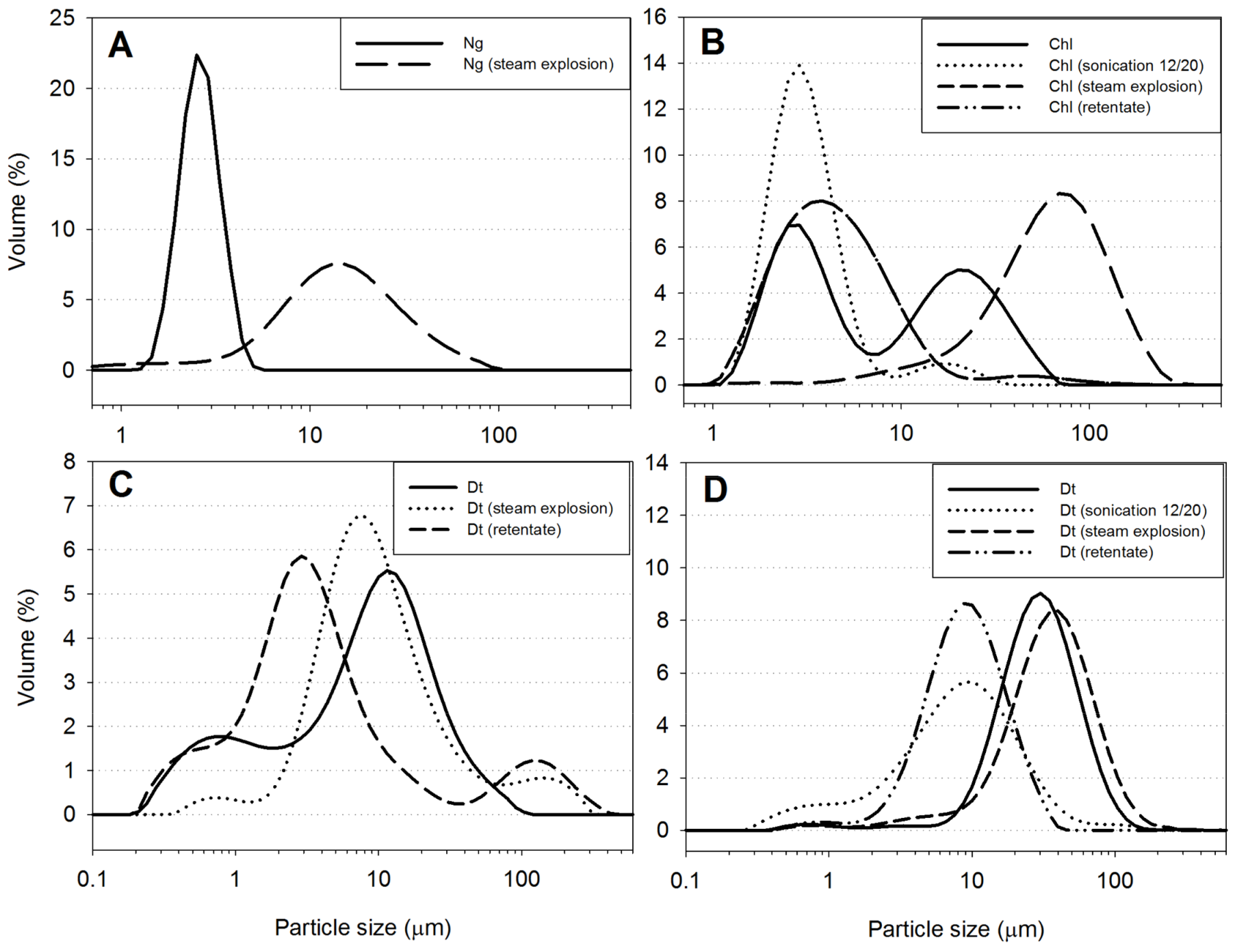
2.5.7. Particle Size Distribution
2.5.8. Optical Density
3. Results and Discussion
3.1. Steam Explosion Treatment of Studied Strains
3.1.1. Cell Morphology
3.1.2. Particle Size Distribution
3.1.3. Lipid, Sugar, and Protein Contents
3.2. Fractionation of Steam Exploded Samples by Means of Membrane Filtration
3.2.1. Rejection
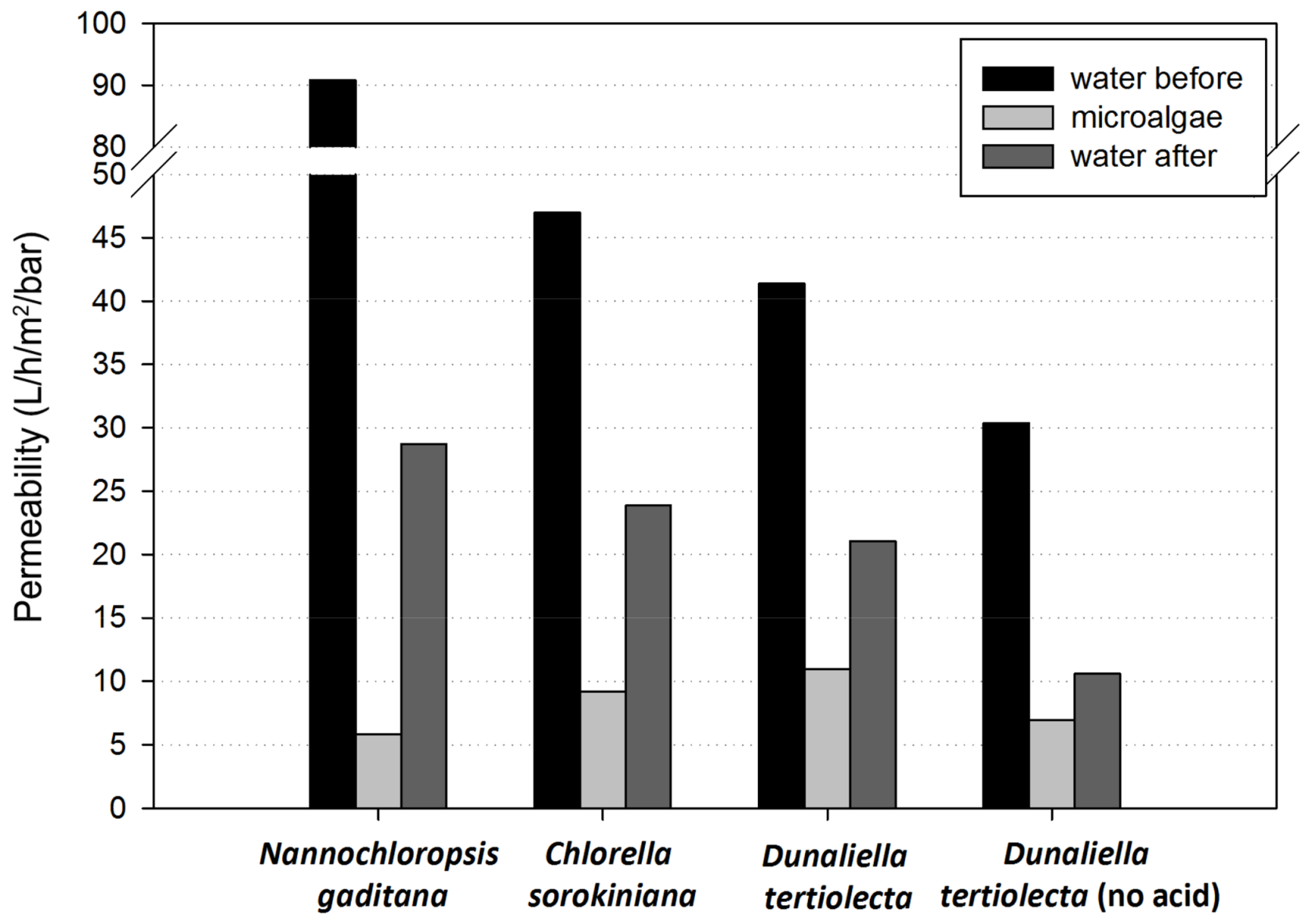
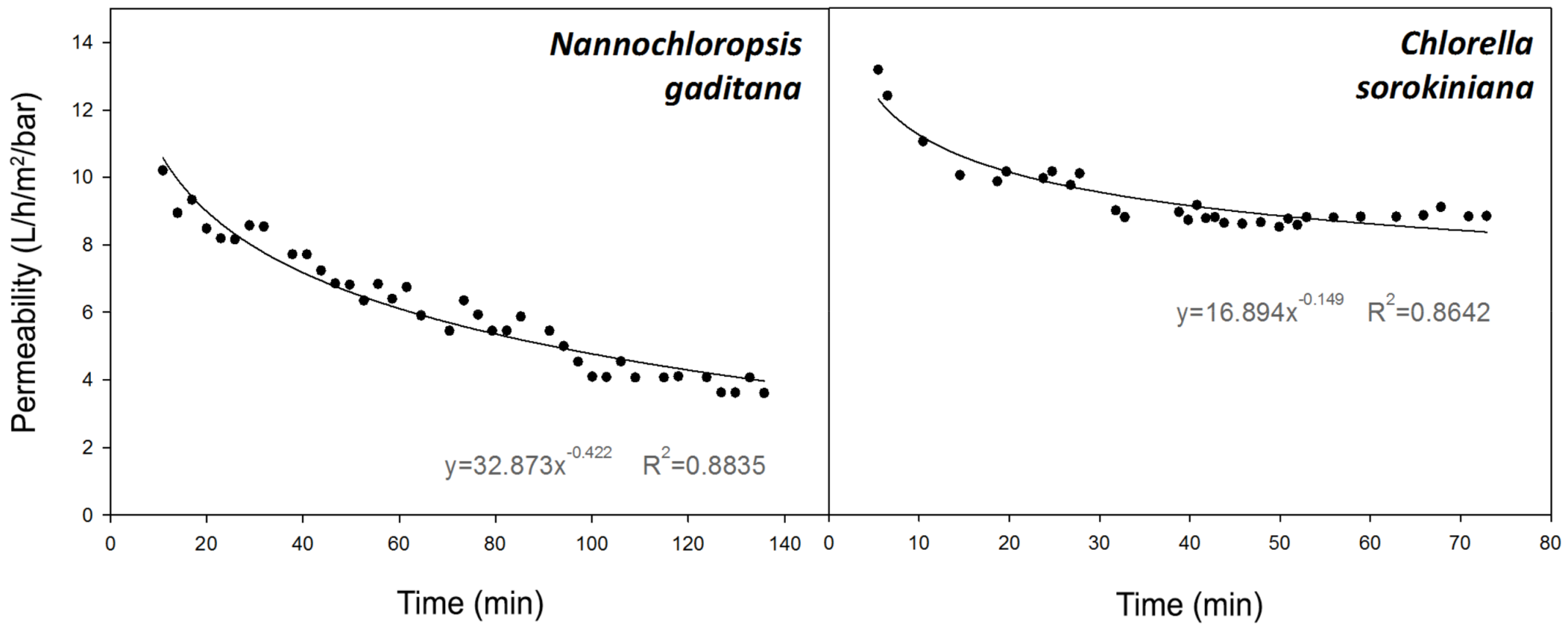
3.2.2. Permeability
3.2.3. Irreversible Fouling
4. Process Costs
4.1. Steam Explosion Costs
4.2. Dynamic Membrane Filtration Operational Costs
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, J.; Dunahay, T.; Benemann, J.; Roessler, P. A Look Back at the US Department of ENERGY’S Aquatic Species, Program: Biodiesel from Algae; National Renewable Energy Laboratory (NREL): Golden, CO, USA, 1998.
- Zhu, L.; Ketola, T. Microalgae production as a biofuel feedstock: Risks and challenges. Int. J. Sustain. Dev. World 2012, 19, 268–274. [Google Scholar] [CrossRef]
- Zhu, L. Biorefinery as a promising approach to promote microalgae industry: An innovative framework. Renew. Sustain. Energy Rev. 2015, 41, 1376–1384. [Google Scholar] [CrossRef]
- Wijffels, R.H.; Barbosa, M.J.; Eppink, M.H.M. Microalgae for the production of bulk chemicals and biofuels. Biofuels Bioprod. Biorefin. 2010, 4, 287–295. [Google Scholar] [CrossRef]
- Aslam, A.; Thomas-Hall, S.R.; Mughal, T.A.; Schenk, P.M. Selection and adaptation of microalgae to growth in 100% unfiltered coal-fired flue gas. Bioresour. Technol. 2017, 233, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Su, Y. Process effect of microalgal-carbon dioxide fixation and biomass production: A review. Renew. Sustain. Energy Rev. 2014, 31, 121–132. [Google Scholar] [CrossRef]
- Gupta, P.L.; Lee, S.M.; Choi, H.J. Integration of microalgal cultivation system for wastewater remediation and sustainable biomass production. World J. Microbiol. Biotechnol. 2016, 32, 139. [Google Scholar] [CrossRef] [PubMed]
- Suresh Kumar, K.; Dahms, H.U.; Won, E.J.; Lee, J.S.; Shin, K.H. Microalgae—A promising tool for heavy metal remediation. Ecotoxicol. Environ. Saf. 2015, 113, 329–352. [Google Scholar] [CrossRef] [PubMed]
- Lupatini, A.L.; Colla, L.M.; Canan, C.; Colla, E. Potential application of microalga spirulina platensis as a protein source. J. Sci. Food Agric. 2017, 97, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Sanchez Rizza, L.; Sanz Smachetti, M.E.; Do Nascimento, M.; Salerno, G.L.; Curatti, L. Bioprospecting for native microalgae as an alternative source of sugars for the production of bioethanol. Algal Res. 2017, 22, 140–147. [Google Scholar] [CrossRef]
- Reyes, F.A.; Mendiola, J.A.; Ibañez, E.; Del Valle, J.M. Astaxanthin extraction from haematococcus pluvialis using CO2-expanded ethanol. J. Supercrit. Fluids 2014, 92, 75–83. [Google Scholar] [CrossRef]
- Uquiche, E.; Antilaf, I.; Millao, S. Enhancement of pigment extraction from B. braunii pretreated using CO2 rapid depressurization. Braz. J. Microbiol. 2016, 47, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Cuello, J.L.; Hoshino, T.; Kuwahara, S.; Brown, C.L. Scale-up-bioreactor design and culture optimization. In Biotechnology for Biofuel Production and Optimization; Elsevier: Amsterdam, The Netherlands, 2016; pp. 497–511. [Google Scholar]
- Thomassen, G.; Egiguren Vila, U.; Van Dael, M.; Lemmens, B.; Van Passel, S. A techno-economic assessment of an algal-based biorefinery. Clean Technol. Environ. Policy 2016, 18, 1849–1862. [Google Scholar] [CrossRef]
- Ersahin, M.E.; Ozgun, H.; Dereli, R.K.; Ozturk, I.; Roest, K.; van Lier, J.B. A review on dynamic membrane filtration: Materials, applications and future perspectives. Bioresour. Technol. 2012, 122, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Hapońska, M.; Clavero, E.; Salvadó, J.; Torras, C. Application of ABS membranes in dynamic filtration for Chlorella sorokiniana dewatering. Biomass Bioenergy 2016, 111, 224–231. [Google Scholar] [CrossRef]
- Kim, J.; Yoo, G.; Lee, H.; Lim, J.; Kim, K.; Kim, C.W.; Park, M.S.; Yang, J.W. Methods of downstream processing for the production of biodiesel from microalgae. Biotechnol. Adv. 2013, 31, 862–876. [Google Scholar] [CrossRef] [PubMed]
- Nurra, C.; Clavero, E.; Salvadó, J.; Torras, C. Vibrating membrane filtration as improved technology for microalgae dewatering. Bioresour. Technol. 2014, 157, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, N.; Vandanjon, L.; Jaouen, P.; Quéméneur, F. Membrane technology for the continuous separation microalgae/culture medium: Compared performances of cross-flow microfiltration and ultrafiltration. Aquac. Eng. 1999, 20, 191–208. [Google Scholar] [CrossRef]
- Zhao, F.; Chu, H.; Zhang, Y.; Jiang, S.; Yu, Z.; Zhou, X.; Zhao, J. Increasing the vibration frequency to mitigate reversible and irreversible membrane fouling using an axial vibration membrane in microalgae harvesting. J. Membr. Sci. 2017, 529, 215–223. [Google Scholar] [CrossRef]
- Cheng, J.; Huang, R.; Li, T.; Zhou, J.; Cen, K. Physicochemical characterization of wet microalgal cells disrupted with instant catapult steam explosion for lipid extraction. Bioresour. Technol. 2015, 191, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Grimi, N.; Dubois, A.; Marchal, L.; Jubeau, S.; Lebovka, N.I.; Vorobiev, E. Selective extraction from Microalgae nannochloropsis sp. Using different methods of cell disruption. Bioresour. Technol. 2014, 153, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Lorente, E.; Hapońska, M.; Clavero, E.; Torras, C.; Salvadó, J. Microalgae fractionation using steam explosion, dynamic and tangential cross-flow membrane filtration. Bioresour. Technol. 2017, 237, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Mendes-Pinto, M.M.; Raposo, M.F.J.; Bowen, J.; Young, A.J.; Morais, R. Evaluation of different cell disruption processes on encysted cells of haematococcus pluvialis: Effects on astaxanthin recovery and implications for bio-availability. J. Appl. Phycol. 2001, 13, 19–24. [Google Scholar] [CrossRef]
- Nurra, C.; Torras, C.; Clavero, E.; Ríos, S.; Rey, M.; Lorente, E.; Farriol, X.; Salvadó, J. Biorefinery concept in a microalgae pilot plant. Culturing, dynamic filtration and steam explosion fractionation. Bioresour. Technol. 2014, 163, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Yap, B.H.J.; Dumsday, G.J.; Scales, P.J.; Martin, G.J.O. Energy evaluation of algal cell disruption by high pressure homogenisation. Bioresour. Technol. 2015, 184, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Safi, C. Microalgae Biorefinery: Proposition of a Fractionation Process; Université de Toulouse: Toulouse, France, 2013. [Google Scholar]
- Lorente, E.; Farriol, X.; Salvadó, J. Steam explosion as a fractionation step in biofuel production from microalgae. Fuel Process. Technol. 2015, 131, 93–98. [Google Scholar] [CrossRef]
- Lee, A.K.; Lewis, D.M.; Ashman, P.J. Disruption of microalgal cells for the extraction of lipids for biofuels: Processes and specific energy requirements. Biomass Bioenergy 2012, 46, 89–101. [Google Scholar] [CrossRef]
- Heitz, M.; Capek-Ménard, E.; Koeberle, P.G.; Gagné, J.; Chornet, E.; Overend, R.P.; Taylor, J.D.; Yu, E. Fractionation of populus tremuloides at the pilot plant scale: Optimization of steam pretreatment conditions using the stake II technology. Bioresour. Technol. 1991, 35, 23–32. [Google Scholar] [CrossRef]
- Verwijst, T.; Baggerman, J.; Liebermann, F.; van Rijn, C.J.M. High-frequency flow reversal for continuous microfiltration of milk with microsieves. J. Membr. Sci. 2015, 494, 121–129. [Google Scholar] [CrossRef]
- Malmali, M.; Stickel, J.J.; Wickramasinghe, S.R. Sugar concentration and detoxification of clarified biomass hydrolysate by nanofiltration. Sep. Purif. Technol. 2014, 132, 655–665. [Google Scholar] [CrossRef]
- Kumar, K.; Das, D. Growth characteristics of chlorella sorokiniana in airlift and bubble column photobioreactors. Bioresour. Technol. 2012, 116, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Chua, E.T.; Schenk, P.M. A biorefinery for nannochloropsis: Induction, harvesting, and extraction of epa-rich oil and high-value protein. Bioresour. Technol. 2017, 244, 1416–1424. [Google Scholar] [CrossRef] [PubMed]
- Francavilla, M.; Kamaterou, P.; Intini, S.; Monteleone, M.; Zabaniotou, A. Cascading microalgae biorefinery: Fast pyrolysis of dunaliella tertiolecta lipid extracted-residue. Algal Res. 2015, 11, 184–193. [Google Scholar] [CrossRef]
- Scholz, M.J.; Weiss, T.L.; Jinkerson, R.E.; Jing, J.; Roth, R.; Goodenough, U.; Posewitz, M.C.; Gerken, H.G. Ultrastructure and composition of the nannochloropsis gaditana cell wall. Eukaryot. Cell 2014, 13, 1450–1464. [Google Scholar] [CrossRef] [PubMed]
- Takeda, H. Taxonomical assignment of chlorococal algae from their cell wall composition. Phytochemistry 1993, 34, 1053–1055. [Google Scholar] [CrossRef]
- Kodner, R.B.; Summons, R.E.; Knoll, A.H. Phylogenetic investigation of the aliphatic, non-hydrolyzable biopolymer algaenan, with a focus on green algae. Org. Geochem. 2009, 40, 854–862. [Google Scholar] [CrossRef] [Green Version]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Phys. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Templeton, D.W.; Quinn, M.; Van Wychen, S.; Hyman, D.; Laurens, L.M.L. Separation and quantification of microalgal carbohydrates. J. Chromatogr. A 2012, 1270, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Barbarino, E.; Lourenço, S.O. An evaluation of methods for extraction and quantification of protein from marine macro- and microalgae. J. Appl. Phycol. 2005, 17, 447–460. [Google Scholar] [CrossRef]
- Hoffman, J.; Pate, R.C.; Drennen, T.; Quinn, J.C. Techno-economic assessment of open microalgae production systems. Algal Res. 2017, 23, 51–57. [Google Scholar] [CrossRef]
- t Lam, G.P.; Vermuë, M.H.; Eppink, M.H.M.; Wijffels, R.H.; van den Berg, C. Multi-product microalgae biorefineries: From concept towards reality. Trends Biotechnol. 2017, 36, 216–277. [Google Scholar] [CrossRef] [PubMed]
- Sui, W.; Chen, H. Multi-stage energy analysis of steam explosion process. Chem. Eng. Sci. 2014, 116, 254–262. [Google Scholar] [CrossRef]
- Carrere, H.; Antonopoulou, G.; Affes, R.; Passos, F.; Battimelli, A.; Lyberatos, G.; Ferrer, I. Review of feedstock pretreatment strategies for improved anaerobic digestion: From lab-scale research to full-scale application. Bioresour. Technol. 2016, 199, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Günerken, E.; D’Hondt, E.; Eppink, M.H.M.; Garcia-Gonzalez, L.; Elst, K.; Wijffels, R.H. Cell disruption for microalgae biorefineries. Biotechnol. Adv. 2015, 33, 243–260. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Yeh, K.-L.; Aisyah, R.; Lee, D.-J.; Chang, J.-S. Cultivation, photobioreactor design and harvesting of microalgae for biodiesel production: A critical review. Bioresour. Technol. 2011, 102, 71–81. [Google Scholar] [CrossRef] [PubMed]
- New Logic Research, Inc. Vsep Products. Available online: http://www.vsep.com/products/index.html (accessed on 23 October 2017).
- Ministerio de Energía, Turismo y Agenda Digital de España. Energía y Emisiones. Available online: http://www.minetad.gob.es/es-ES/IndicadoresyEstadisticas/DatosEstadisticos/IV.%20Energ%C3%ADa%20y%20emisiones/IV_12.pdf (accessed on 23 October 2017).
- New Logic Research, Inc. Vsep Case Studies and Application Notes. Available online: http://www.vsep.com/downloads/case_studies_application_notes.html (accessed on 23 October 2017).
- Dassey, A.J.; Theegala, C.S. Harvesting economics and strategies using centrifugation for cost effective separation of microalgae cells for biodiesel applications. Bioresour. Technol. 2013, 128, 241–245. [Google Scholar] [CrossRef] [PubMed]
| Lipids | Sugar | Protein | ||||
|---|---|---|---|---|---|---|
| Bligh & Dyer | Hexane | Hot NaOH | Solubilization | |||
| Nannochloropsis gaditana | Untreated | 22.2% (0.4) | 2.1% (0.3) | 18.8% (0.8) | 17.3% (0.8) | 1.4% (0.1) |
| Steam exploded | 22.3% (0.1) | 17.6%(0.2) | 12.9% (0.6) | 8.4% (0.6) | 9.1% (0.4) | |
| Chlorella sorokiniana | Untreated | 13.0% (0.2) | 0.6% (0.0) | 23.5% (1.3) | 19.2% (0.3) | 2.2% (0.0) |
| Steam exploded | 11.8% (0.1) | 4.8% (0.2) | 18.6% (0.9) | 9.2% (0.1) | 10.7% (0.1) | |
| Dunaliella tertiolecta (I) | Untreated | 26.6% (0.8) | 2.8% (0.7) | 26.1% (2.2) | 14.5% (0.5) | 12.0% (0.3) |
| Steam exploded | 29.7% (3.2) | 10.6% (0.1) | 19.2% (0.8) | 2.6% (0.0) | 5.1% (0.3) | |
| Dunaliella tertiolecta (II) | Untreated | 11.4% (1.2) | 1.6% (0.1) | 25.8% (2.4) | 10.5% (0.0) | 5.9% (0.1) |
| Steam exploded No acid | 11.9% (0.1) | 2.1% (0.0) | 8.6% (0.6) | 4.8% (0.1) | 4.4% (0.4) | |
| Nannochloropsis gaditana | Chlorella sorokiniana | |||||
| Steam Exploded Sample | Retentate | Permeate | Steam Exploded Sample | Retentate | Permeate | |
| Total weight (g) | 6000 | 2400 | 3600 | 6000 | 2240 | 3760 |
| DAF percentage | 5.1 (0.1) | 10.1 (0.2) | 1.8 (0.05) | 2.7 (0.02) | 5.8 (0.08) | 0.9 (0.01) |
| Lipid (g/L) | 9.2 (0.3) | 22.7 (0.5) | 0.07 (0.01) | 1.3 (0.05) | 3.9 (0.09) | 0.05 (0.01) |
| Sugar (g/L) | 6.8 (0.3) | 5.9 (0.2) | 6.0 (0.2) | 5.1 (0.1) | 5.2 (0.2) | 4.9 (0.1) |
| Protein (g/L) | 4.7 (0.2) | 5.65 (0.15) | n.d. | 2.92 (0.04) | 4.8 (0.14) | n.d. |
| Dunaliella tertiolecta | Dunaliella tertiolecta (No Acid) | |||||
| Steam Exploded Sample | Retentate | Permeate | Steam Exploded Sample | Retentate | Permeate | |
| Total weight (g) | 6000 | 2290 | 3710 | 6000 | 2400 | 3600 |
| DAF percentage | 1.7 (0.01) | 3.2 (0.04) | 0.9 (0.01) | 1.6 (0.01) | 3.0 (0.02) | 0.7 (0.01) |
| Lipid (g/L) | 1.8 (0.02) | 3.5 (0.08) | 0.07 (0.01) | 0.34 (0.03) | 1.3 (0.01) | 0.07 (0.01) |
| Sugar (g/L) | 3.3 (0.2) | 2.9 (0.1) | 3.2 (0.2) | 1.4 (0.1) | 1.3 (0.1) | 1.2 (0.1) |
| Protein (g/L) | 0.89 (0.05) | 1.46 (0.06) | n.d. | 0.71 (0.06) | 1.13 (0.07) | n.d. |
| OD750nm | ||
| Blank | Permeate | |
| Nannochloropsis gaditana | 0.081 (0.001) | 0.091 (0.003) |
| Chlorella sorokiniana | 0.081 (0.001) | 0.101 (0.002) |
| Dunaliella tertiolecta | 0.083 (0.001) | 0.085 (0.001) |
| Dunaliella tertiolecta (no acid) | 0.083 (0.001) | 0.083 (0.000) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorente, E.; Hapońska, M.; Clavero, E.; Torras, C.; Salvadó, J. Steam Explosion and Vibrating Membrane Filtration to Improve the Processing Cost of Microalgae Cell Disruption and Fractionation. Processes 2018, 6, 28. https://doi.org/10.3390/pr6040028
Lorente E, Hapońska M, Clavero E, Torras C, Salvadó J. Steam Explosion and Vibrating Membrane Filtration to Improve the Processing Cost of Microalgae Cell Disruption and Fractionation. Processes. 2018; 6(4):28. https://doi.org/10.3390/pr6040028
Chicago/Turabian StyleLorente, Esther, Monika Hapońska, Ester Clavero, Carles Torras, and Joan Salvadó. 2018. "Steam Explosion and Vibrating Membrane Filtration to Improve the Processing Cost of Microalgae Cell Disruption and Fractionation" Processes 6, no. 4: 28. https://doi.org/10.3390/pr6040028