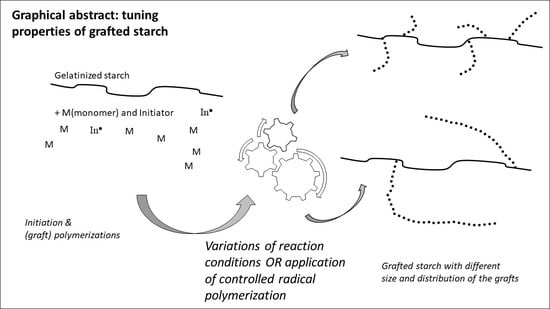
Free-Radical Graft Polymerization onto Starch as a Tool to Tune Properties in Relation to Potential Applications. A Review
Abstract
:1. Introduction
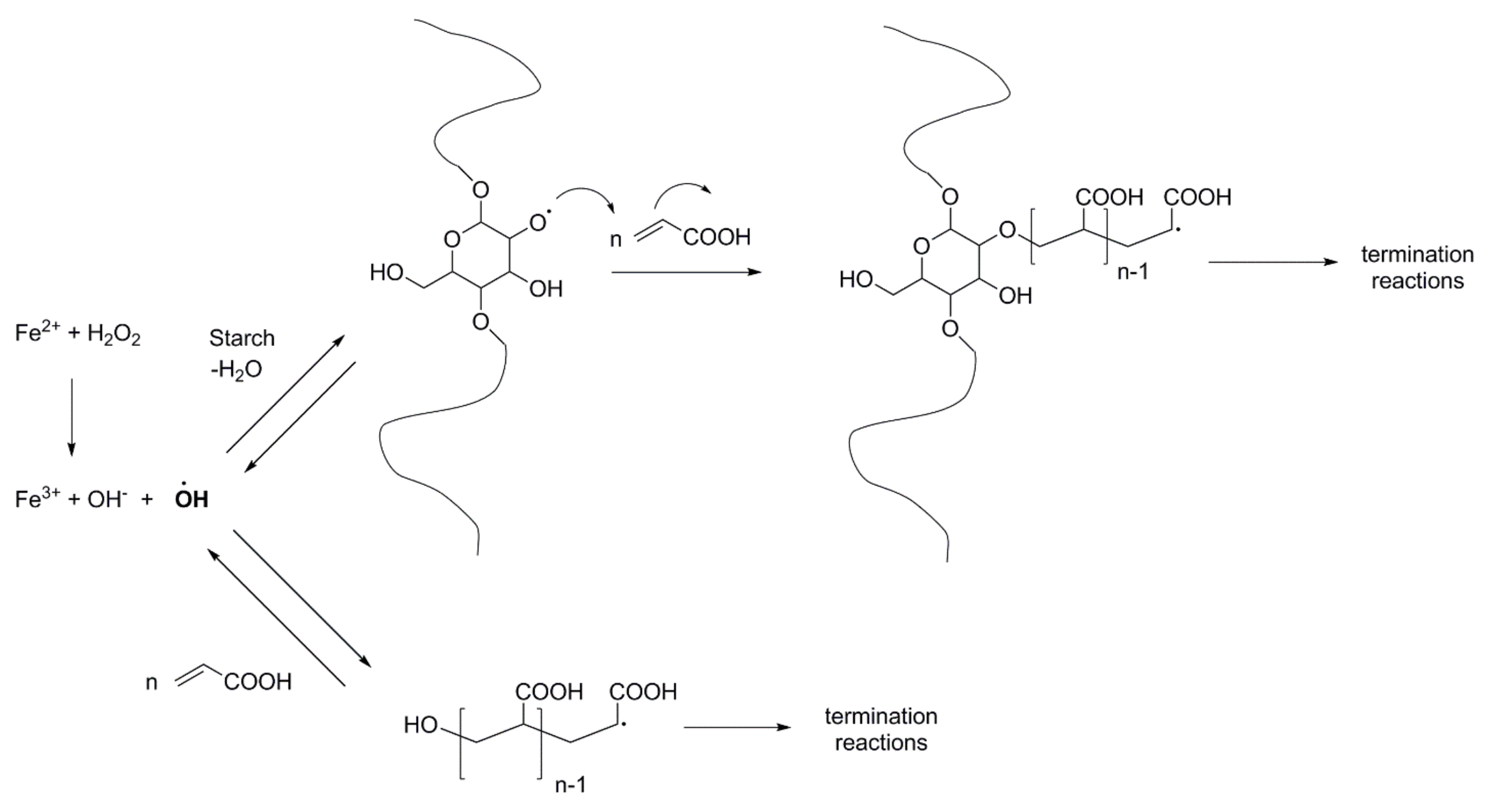
2. Starch Grafting: Reactions and Analyses
2.1. Reactions
2.2. Analytical Procedures and Aspects
3. Potential Applications and the Related Demands toward the Grafted Structure
3.1. Superabsorbents
3.2. Discussion of the Demands to Other Applications
3.3. Conclusions on Demands
4. How Variations in the Process Conditions Affect the Graft Structure
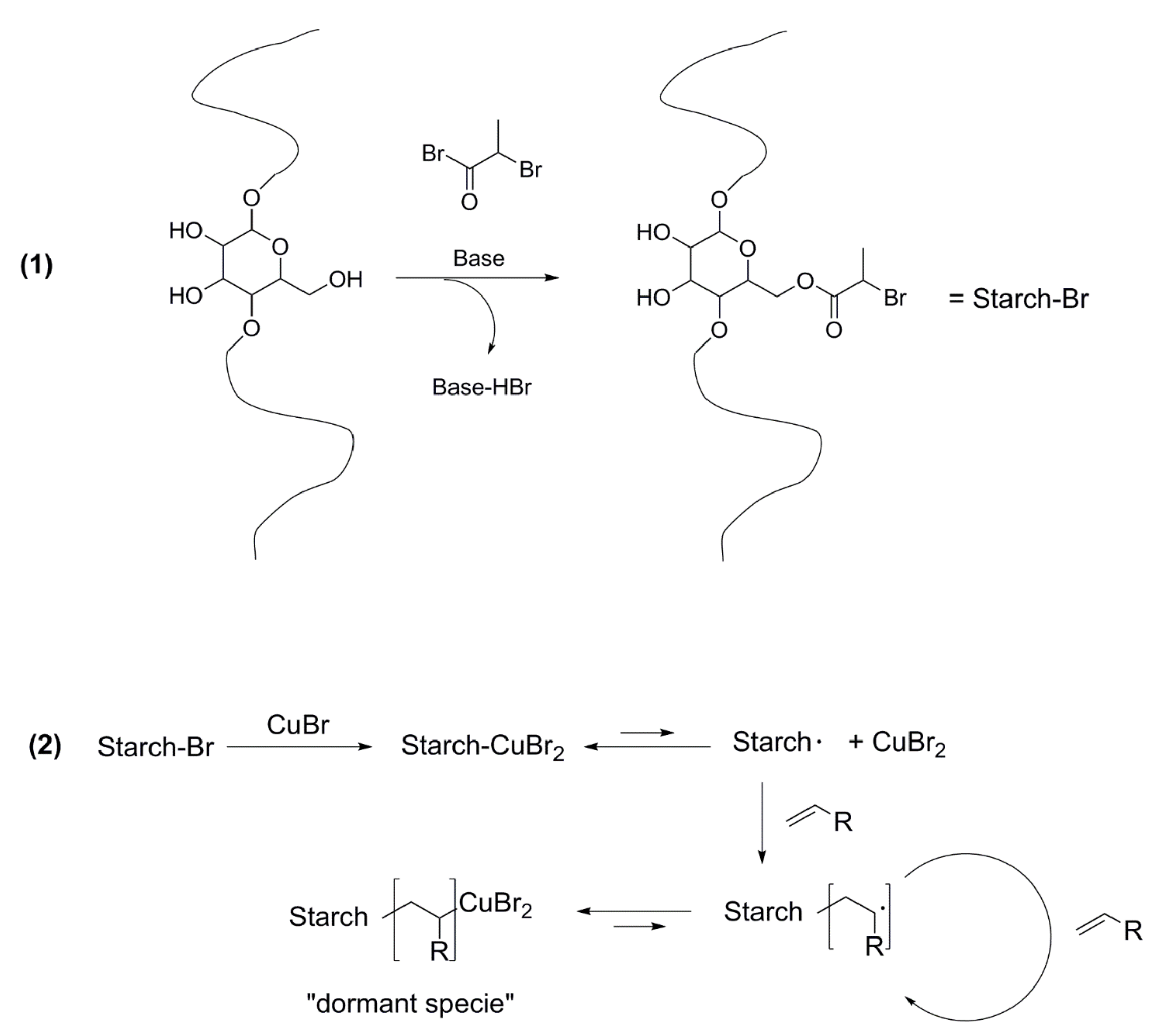
5. About the Current Status of Controlled Radical Polymerizations
5.1. New Methods to Control Radical Polymerization
5.2. CRP, Adding to or Competing with Traditional Free Radical Polymerization Processes
5.3. Future Prospects of CRP-Methods in Industrial Settings
5.4. CRP and Starch Grafting
6. Conclusions and Prospects
Author Contributions
Conflicts of Interest
References
- Fanta, G.F.; Doane, W.M. Grafted Starches, in Modified Starches: Properties and Uses; Wurzburg, O.B., Ed.; CRC Press: Boca Raton, FL, USA, 1986. [Google Scholar]
- Meimoun, J.; Wiatz, V.; Saint-Loup, R.; Julien Parcq, J.; Audrey Favrelle, A.; Bonnet, F.; Zinck, P. Modification of starch by graft copolymerization. Starch/Stärke 2018, 70. [Google Scholar] [CrossRef]
- Witono, J.R. New Materials by Grafting of Acrylic Acid onto Cassava Starch. Ph.D. Thesis, Chemical Engineering Dept., University of Groningen, Groningen, The Netherlands, 2012. [Google Scholar]
- Qiao, D.; Yu, L.; Bao, X.; Zhang, B.; Jiang, F. Understanding the microstructure and absorption rate of starch-based superabsorbent polymers prepared under high starch concentration. Carbohydr. Polym. 2017, 175, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Shang, X.; Liu, R.; Hu, J.; Liao, S. Synthesis and characterization of a novel amino modified starch and its adsorption properties for Cd(II) ions from aqueous solution. Carbohydr. Polym. 2011, 84, 430–438. [Google Scholar] [CrossRef]
- Mishra, S.; Mukul, A.; Sen, G.; Jha, U. Microwave assisted synthesis of polyacrylamide grafted starch (St-g-PAM) and its applicability as flocculant for water treatment. Int. J. Biol. Macromol. 2011, 48, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Ravve, A. Principles of Polymer Chemistry; Springer Science & Business Media: New York, NY, USA, 2013. [Google Scholar]
- Wu, H.; Liu, Z.; Yang, H.; Li, A. Evaluation of chain architectures and charge properties of various starch-based flocculants for flocculation of humic acid from water. Water Res. 2016, 96, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Noordergraaf, I.W.; Bronswijk, S.; Heeres, H.J. Graft copolymerization of acrylic acid onto starch. Improving the grafting performance by the development of dedicated dosage protocols. In Proceedings of the 10th European Congress of Chemical Engineering, Nice, France, 26 September–1 October 2015. [Google Scholar]
- Fanta, G.F.; Bagley, E.B. Starch, graft copolymers. In Encyclopedia of Polymer Science and Technology, Supplement 2; Mark, H.F., Bikalis, N.M., Eds.; Interscience: New York, NY, USA, 1977. [Google Scholar]
- Witono, J.R.; Noordergraaf, I.W.; Heeres, H.J.; Janssen, L.P.B.M. Graft Copolymerization of Acrylic Acid to Cassava Starch—Evaluation of the Influences of Process Parameters by an Experimental Design Method. Carbohydr. Polym. 2012, 90, 1522–1529. [Google Scholar] [CrossRef] [PubMed]
- Bayazeed, M.; Elzairy, M.R.; Hebeish, A. Synthesis and application of new thickeners. Part I: Preparation of poly(acrylic acid)-starch graft copolymer. Starch/Stärke 1989, 41, 233–236. [Google Scholar] [CrossRef]
- Hebeish, A.; Zahran, M.K.; El-Rafie, M.H.; El-Tahlawy, K.F. Preparation and characterization of poly(acrylic acid) polyblends. Polym. Polym. Compos. 1996, 2, 129–141. [Google Scholar]
- Witono, J.R.; Marsman, J.H.; Noordergraaf, I.W.; Heeres, H.J.; Janssen, L.P.B.M. Improved homopolymer separation to enable the application of 1H-NMR and HPLC for the determination of the reaction parameters in de graft copolymerization of acrylic acid onto starch. Carbohydr. Res. 2013, 370, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.B.; Jyothi, A.N. Cassava Starch-graft-Polymethacrylamide Copolymers as Flocculants and Textile Sizing Agents. Appl. Polym. Sci. 2014, 131, 39810. [Google Scholar] [CrossRef]
- Brockway, C.E. Efficiency and frequency of grafting of methyl methacrylate to granular corn starch. J. Polym. Sci. 1964, A2, 3721–3731. [Google Scholar] [CrossRef]
- Jyothi, A.N. Starch Graft Copolymers: Novel Applications in Industry. Compos. Interfaces 2010, 17, 165–174. [Google Scholar] [CrossRef]
- Glass, J.E. Water-Soluble Polymers. Kirk-Othmer Encyclopedia of Chemical Technology, 4th ed.; Edward, J., Ed.; Wiley Online Library: New York, NY, USA, 2000; pp. 622–646. [Google Scholar]
- Weaver, M.O.; Montgomery, R.R.; Miller, L.D.; Sohns, V.E.; Fanta, G.F.; Doane, W.M. A practical process for the preparation of Superslurper, a starch-based polymer with a large capacity to absorb water. Starch/Stärke 1977, 29, 413–422. [Google Scholar] [CrossRef]
- Bucholz, F.L.; Graham, A.T. Modern Superabsorbent Polymer Technology; Wiley VCH: New York, NY, USA, 1998. [Google Scholar]
- Masuda, F.; Nishida, K.; Nakamura, A. Water Absorbing Resins. U.S. Patent 4,076,663, 28 February 1978. Sanyo Chemical Ind. [Google Scholar]
- Athawale, V.D. Lele: Recent trends in hydrogels based on starch-graft-acrylic acid: A review. Starch/Stärke 2001, 53, 7–13. [Google Scholar] [CrossRef]
- Klin, A.R.; Sanders, J. Low Molecular Weight Graft Copolymer. U.S. Patent 2008/0020961 Al, 24 January 2008. [Google Scholar]
- Kugler, S.; Spychaj, T.; Wilpiszewska, K.; Gor, K. Starch-Graft Copolymers of N-Vinylformamide and Acrylamide Modified with Montmorillonite Manufactured by Reactive Extrusion. J. Appl. Polym. Sci. 2013. [CrossRef]
- Qudsieh, I.Y.; Fakhru’l-Razi, A.; Kabbashi, N.A.; Mirghani, M.E.S.; Fandi, K.G.; Alam, M.Z.; Muyibi, S.A.; Nasef, M.M. Preparation and Characterization of a New Coagulant Based on the Sago Starch Biopolymer and Its application in Water Turbidity Removal. J. Appl. Polym. Sci. 2008, 109, 3140–3147. [Google Scholar] [CrossRef]
- Berndl, G. Untersuchungen an polymeren Waschmitteladditiven auf Basis von Propfcopolymeren ungesättigter Carbonsäuren auf Stärkeprodukte. Ph.D. Thesis, University of Regensburg, Regensburg, Germany, 1995. [Google Scholar]
- Antti Hamunen, R.; Anttila, M.; Nurmi, K. Thickening Comprising Aqueous Dispersion of Graft-Copolymerized Starch Agent. U.S. Patent 5,677,374, 14 October 1997. [Google Scholar]
- Witono, J.R.; Noordergraaf, I.W.; Heeres, H.J.; Janssen, L.P.B.M. Real-time viscosity monitoring during graft copolymerization of gelatinized starch. Plast. Res. Online 2017. [Google Scholar] [CrossRef]
- Schmidt, B.; Spychaj, T. Synthesis and properties of starch grafted poly (acrylamide-co-acrylic acid) copolymers. Prog. Environ. Sci. Technol. 2009, 2 Pt B, 1026–1031. [Google Scholar]
- Djordjevic, S.; Nikolic, L.; Kovacevic, S.; Miljkovic, M.; Djordjevic, D. Effect of various initiators on molar mass determination of hydrolyzed potato starch-acryl-amide graft copolymers. Chem. Ind. Chem. Eng. Q. 2013, 19, 493–503. [Google Scholar] [CrossRef]
- Willet, J.L.; Finkenstadt, V.L. Comparison of Cationic and Unmodified Starches in Reactive Extrusion of Starch–Polyacrylamide Graft Copolymers. J. Polym. Environ. 2009, 17, 248–253. [Google Scholar] [CrossRef]
- Ganzeveld, K.J. The Counter-Rotating Twin Screw Extruder as a Polymerization Reactor. Ph.D. Thesis, University of Groningen, Groningen, The Netherlands, 1992. [Google Scholar]
- De Graaf, R.A. The Use of Twin Screw Extruders as Starch Modification Reactors. Ph.D. Thesis, University of Groningen, Groningen, The Netherlands, 1996. [Google Scholar]
- Destarac, M. Controlled Radical Polymerization: Industrial Stakes, Obstacles and Achievements. Macromol. J. 2010, 4, 165–179. [Google Scholar] [CrossRef]
- Tsarevsky, N.V.; Matyjaszewski, K. “Green” Atom Transfer Radical Polymerization: From Process Design to Preparation of Well-Defined Environmentally Friendly Polymeric Materials. Chem. Rev. 2007, 107, 2270–2299. [Google Scholar] [CrossRef] [PubMed]
- Matyjaszewski, K. Atom Transfer Radical Polymerization (ATRP): Current Status and Future Perspectives. Macromolecules 2012, 45, 4015–4039. [Google Scholar] [CrossRef]
- White, D.; Coca, S.; O’Dywer, J.B. Pigment Dispersions Containing Dispersants Prepared by Controlled Radical Polymerization Having Hydrophilic and Hydrophobic Segments. U.S. Patent 6642301 B2, 4 November 2003. [Google Scholar]
- Taton, D.; Destarac, M.; Zard, S.Z. Macromolecular Design by Interchange of Xanthates: Background, design, scope and applications. In Handbook of RAFT Polymerization; Barner-Kowollik, C., Ed.; Wiley-VCH: Weinheim, Germany, 2008; p. 373. [Google Scholar]
- Shen, Y.; Tang, H.; Ding, S. Catalyst separation in atom transfer radical polymerization. Prog. Polym. Sci. 2004, 29, 1053–1078. [Google Scholar] [CrossRef]
- Perrier, S. 50th Anniversary Perspective: RAFT Polymerization A. User Guide. Macromolecules 2017, 50, 7433–7447. [Google Scholar] [CrossRef]
- Hawkett, B.; Such, C.; Nguyen, D.N.; Farrugia, J.; McKinnon, O. Polymer Product and Interfacial Polymerisation Process Using Raft Agent. WO Patent 06/037161, 2006. [Google Scholar]
- Such, C.H.; Rizzardo, E.; Serelis, A.K.; Hawkett, B.S.; Gilbert, R.G.; Ferguson, C.J.; Hughes, R. Aqueous Dispersion of Polymer Particles. WO Patent 03/055919, 10 July 2003. [Google Scholar]
- Destarac, M.; Deroo, S.; Lannibois-Dreán, H.; Sénéchal, A.; Bzducha, W. MADIX Technology: From Innovative Concepts to Industrialization of Block Copolymers for Emulsion Stabilization. In Controlled/Living Radical Polymerization: Progress in RAFT, DT, NMP and OMRP; American Chemical Society: Washington, DC, USA, 2009; p. 347. [Google Scholar]
- Wever, D.A.Z.; Polgar, L.M.; Stuart, M.C.A.; Picchioni, F.; Broekhuis, A. Polymer Molecular Architecture as a Tool for Controlling the Rheological Properties of Aqueous Polyacrylamide Solutions for Enhanced Oil Recovery. Ind. Eng. Chem. Res. 2013, 52, 16993–17005. [Google Scholar] [CrossRef]
- Klemm, B.; Raffa, P.; Picchioni, F.; van Mastrigt, F. Star-Like Branched Polyacrylamides by RAFT polymerisation—Part I: Synthesis and Characterisation. Ind. Eng. Chem. Res. 2017. under review. [Google Scholar]
- Tan, J.; Sun, H.; Yu, M.; Sumerlin, B.S.; Zhang, L. Photo-PISA: Shedding Light on Polymerization-Induced Self-Assembly. ACS Macro Lett. 2015, 4, 1249–1253. [Google Scholar] [CrossRef]
- Xu, S.; Ng, G.; Xu, J.; Kuchel, R.P.; Yeow, J.; Boyer, C. 2-(Methylthio)ethyl Methacrylate: A Versatile Monomer for Stimuli Responsiveness and Polymerization-Induced Self-Assembly in the Presence of Air. ACS Macro Lett. 2017, 6, 1237–1244. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Davis, T.P. Handbook of Radical Polymerization; John Wiley and Sons Inc.: New York, NY, USA, 2012. [Google Scholar]
- Yan, J.; Pan, X.; Schmitt, M.; Wang, Z.; Bockstaller, M.R.; Matyjaszewski, K. Enhancing Initiation Efficiency in Metal-Free Surface-Initiated Atom Transfer Radical Polymerization (SI-ATRP). ACS Macro Lett. 2016, 5, 661–665. [Google Scholar] [CrossRef]
- Treat, N.J.; Sprafke, H.; Kramer, J.W.; Clark, P.G. Metal-Free Atom Transfer Radical Polymerization. J. Am. Chem. Soc. 2014, 136, 16096–16101. [Google Scholar] [CrossRef] [PubMed]
- Simakova, A.; Averick, S.E.; Konkolewicz, D.; Matyjaszewski, K. Aqueous ARGET ATRP. Macromolecules 2012, 45, 6371–6379. [Google Scholar] [CrossRef]
- Konkolewicz, D.; Magenau, A.J.D.; Averick, S.E.; Simakova, A.; He, H.; Matyjaszewski, K. ICAR ATRP with ppm Cu catalyst in Water. Macromolecules 2012, 45, 4461–4468. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Pan, X.; Fu, L.; Lathwal, S.; Olszewski, M.; Yan, J.; Enciso, A.E.; Wang, Z.; Xia, H.; Matyjaszewski, K. Ultrasonication-Induced Aqueous Atom Transfer Radical Polymerization. ACS Macro Lett. 2018, 7, 275–280. [Google Scholar] [CrossRef]
- Xiao, C.; Lu, D.; Xu, S.; Huang, L. Tunable synthesis of starch-poly(vinyl acetate) bioconjugate. Starch/Staerke 2011, 63, 209–216. [Google Scholar] [CrossRef]
- Lu, D.R.; Xiao, C.M.; Xu, S.J.; Ye, Y.F. Tailor-made starch-based conjugates containing well-defined poly(vinyl acetate) and its derivative poly(vinyl alcohol). eXPRESS Polym. Lett. 2011, 5, 535–544. [Google Scholar] [CrossRef] [Green Version]
- Lu, D.; Xiao, C.; Sun, F. Controlled grafting of poly(vinylacetate) onto starch via RAFT polymerization. J. Appl. Polym. Sci. 2012, 124, 3450–3455. [Google Scholar] [CrossRef]
| Grafting percentage | GP% | Weight of grafted polymer with respect to the original weight of starch × 100% |
| Graft efficiency | GE% | Weight of the grafted polymer divided by the total weight of polymer formed × 100% |
| Graft size | MWw | Average molecular weight of the grafts, Dalton |
| Graft spacing * | NAGU | Average number of anhydroglucose unit (AGU) between two graft attachments |
| Fe/AGU | GP% | <Grafted Chain Size> (MWw) | 1/Graft Frequency (NAGU) |
|---|---|---|---|
| Moles of Fe2+ per number of AGU-groups | wt% of grafted polymer | Average MW of acrylic acid grafts | Average number of AGU-groups between two grafts |
| 1:97 | 11% | 132,000 | 7400 |
| 1:218 | 13% | 590,000 | 28,000 |
| Application Target/Property | General Features | Graft Size | Spacing |
|---|---|---|---|
| Viscosifier (thickener) | To generate high viscosity with minimal dosage, starch is a major contributor to properties | Long | Open |
| Metal ion absorbent | High binding capacity, functionality is in the grafts -> Good GP is more important but easy access is wanted | Long (high GP) | Open enough to allow easy entrance of metal ions. |
| Flocculants | Molecules with good access to e.g., clay or coal particles, ionic charge may also be important, Homopolymer maybe tolerable. | Long | Open |
| Detergent co-builder | ‘Small’ molecules for low viscosity | Short | Tight |
| Superabsorbent * | High capacity and good access | Not too short * | Open * |
| Sizing agent | A mixture of grafted starch and homopolymer can be applied | Smaller complete molecules (starch + grafts) perform better | |
| Case 1: Grafting of methyl methacrylate with Fenton’s initiator combined with ascorbic acid. Oxidized, but un-gelatinized starch, was grafted in a stirred lab-scale batch reactor at 20–40 °C [16]. | |||
| Initiator: 3 components HPOX/Fe2+/Ascorbic acid, relative dosage | Monomer, relative dosage | NAGU | MWw |
| 10/1/0 | 100 | 2700 | 410,000 Dalton |
| 100/1/10 | 100 | 330 | 45,000 |
| 10/1/0 | 50 | 3500 | 280,000 |
| 100/1/10 | 50 | 350 | 24,000 |
| 100/1/10 | 20 | 530 | 18,000 |
| 100/1/10 | 10 | 3500 | 58,000 |
| Case 2: Grafting of acrylamide onto cationic corn starch in a continuous extruder reactor, with ammonium peroxide as the initiator and water solvent. Starch was not pre-gelatinized but conditions in the reactor will cause in-situ gelatinization, since temperature in the reactor was 90 °C. Reactions are very fast since residence time is 3 min, with a feed rate of starch of 50–68 g/min. Enzymatic degradation of starch and GPC were used to analyze the grafts [31]. | |||
| Initiator dosage (AP) | Monomer/Starch | NAGU | MWw |
| 3.9 × 10−3 mol/kg feed | 0.8 mol/mol | 3500 | 374,000 Dalton |
| 7.8 | 0.8 | 2700 | 284,000 |
| 15.5 | 0.8 | 2600 | 269,000 |
| 3.9 | 1.8 | 2000 | 520,000 |
| 7.8 | 1.8 | 2100 | 465,000 |
| 15.5 | 1.8 | 1200 | 253,000 |
| Case 3: Grafting of acrylamide onto gelatinized cassava starch in batch, with Cerium Ammonium Nitrate initiator. Conditions for these runs: 55 °C, 120 min reaction time, 10 gm starch [15]. | |||
| Initiator dosage | Monomer added | NAGU | MWw |
| 0.44 g/L | 20 gm | 30,000 | 200,000 Dalton |
| 0.88 | 20 | 12,600 | 240,000 |
| 0.66 | 15 | 22,300 | 310,000 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noordergraaf, I.-W.; Fourie, T.K.; Raffa, P. Free-Radical Graft Polymerization onto Starch as a Tool to Tune Properties in Relation to Potential Applications. A Review. Processes 2018, 6, 31. https://doi.org/10.3390/pr6040031
Noordergraaf I-W, Fourie TK, Raffa P. Free-Radical Graft Polymerization onto Starch as a Tool to Tune Properties in Relation to Potential Applications. A Review. Processes. 2018; 6(4):31. https://doi.org/10.3390/pr6040031
Chicago/Turabian StyleNoordergraaf, Inge-Willem, Tori. K. Fourie, and Patrizio Raffa. 2018. "Free-Radical Graft Polymerization onto Starch as a Tool to Tune Properties in Relation to Potential Applications. A Review" Processes 6, no. 4: 31. https://doi.org/10.3390/pr6040031