Treatment of Oil-Contaminated Water by Modified Polysilicate Aluminum Ferric Sulfate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthetic Ship Oil-Contaminated Water
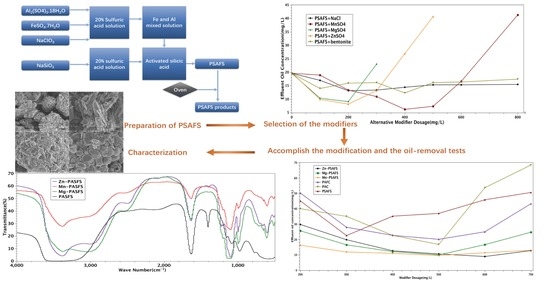
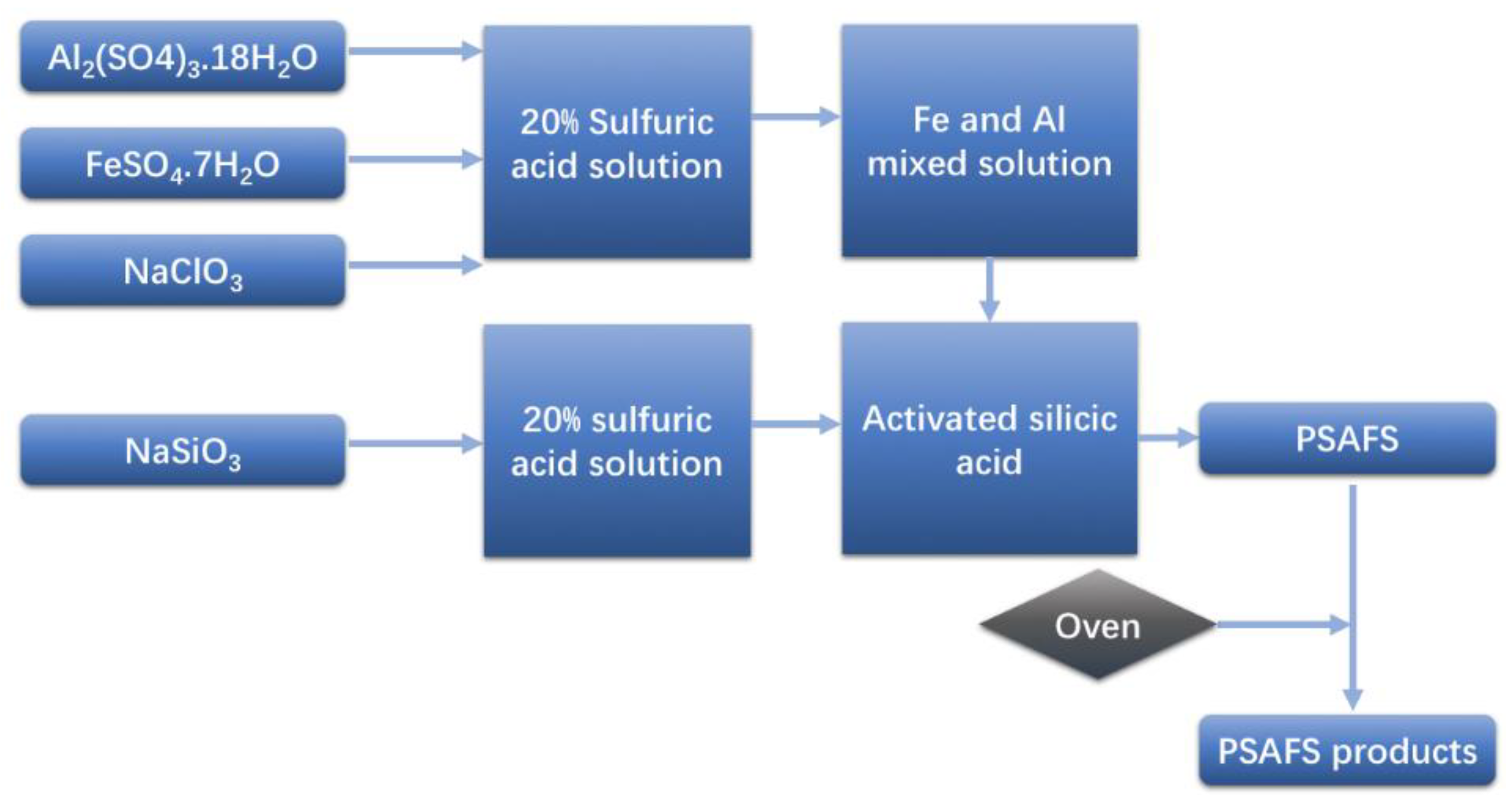
2.3. Preparation of PSAFS and Modified PSAFS
2.3.1. Choice of Modifier
2.3.2. PSAFS Preparation and Modification Process
2.4. Characterization
2.5. Coagulation and Coagulation-Flotation Tests
3. Results and Discussion
3.1. Selection of Modifiers
3.2. Factors Affecting Demulsification during Preparation of PSAFS
3.2.1. Sodium Silicate Concentration
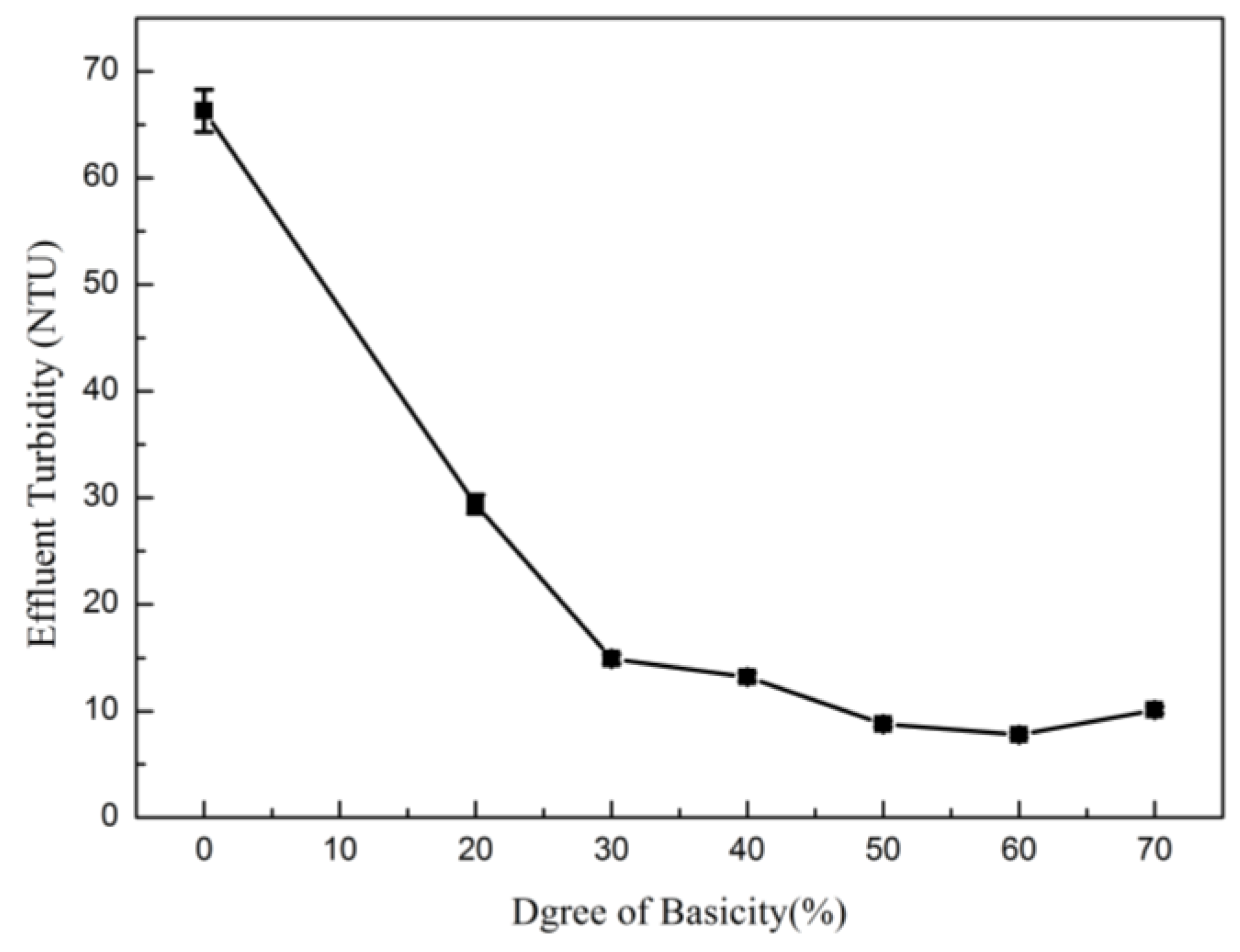
3.2.2. Degree of Basicity
3.2.3. n(Al+Fe):nSi and nAl:nFe
3.3. Modification Effects of PSAFS
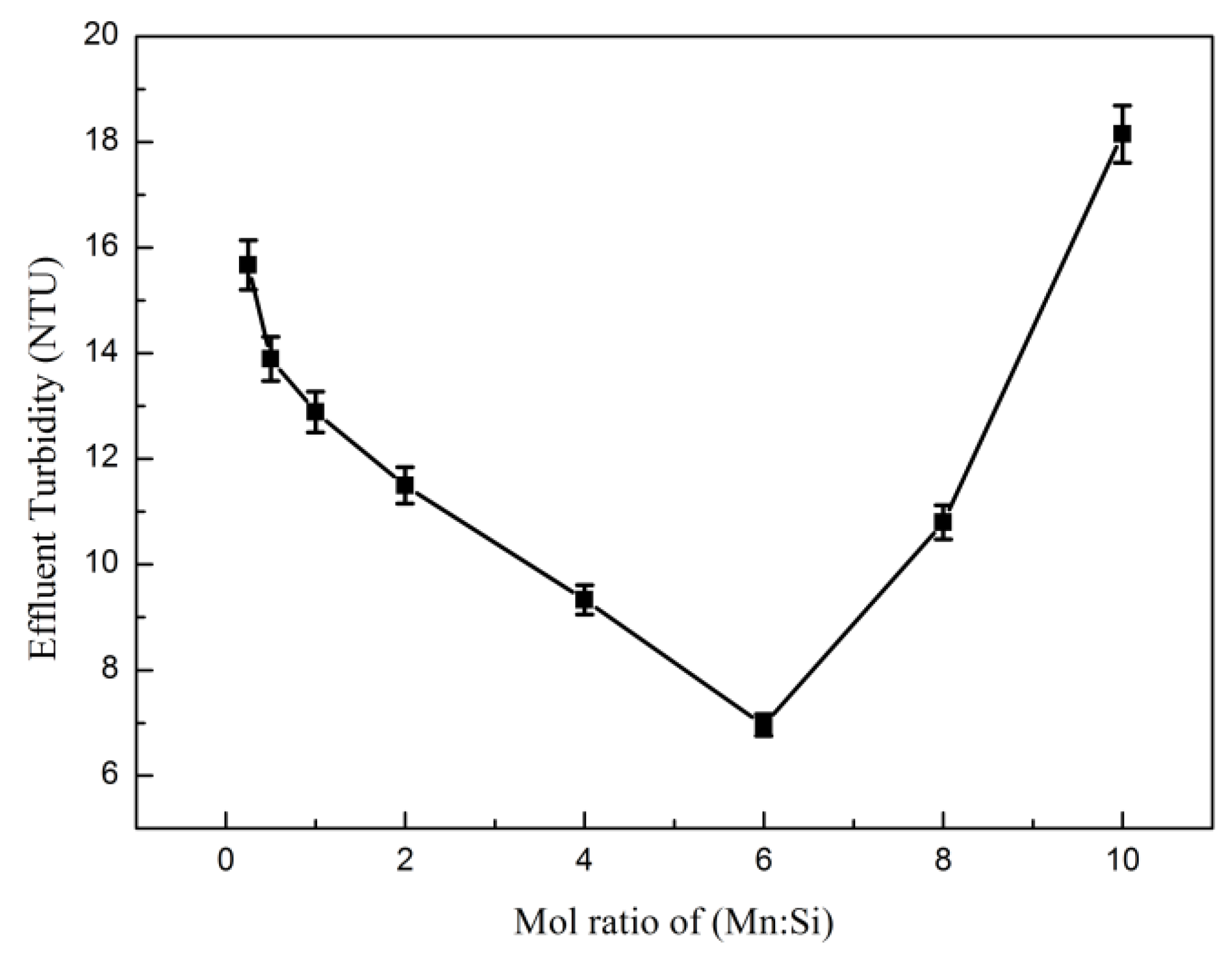
3.3.1. Manganese Salt
3.3.2. Magnesium Salt
3.3.3. Zinc Salt
3.4. Comparison of Modified Coagulants
3.5. Characterization Results Analysis
3.5.1. IR Spectroscopy Analysis Results
3.5.2. SEM Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Colla, V.; Branca, T.A.; Rosito, F.; Lucca, C.; Vivas, B.P.; Delmiro, V.M. Sustainable reverse osmosis application for wastewater treatment in the steel industry. J. Clean. Prod. 2016, 130, 103–115. [Google Scholar] [CrossRef]
- Razali, M.; Kim, J.F.; Attfield, M.; Budd, P.M.; Drioli, E.; Lee, Y.M.; Szekely, G. Sustainable wastewater treatment and recycling in membrane manufacturing. Green Chem. 2015, 17, 5196–5205. [Google Scholar] [CrossRef] [Green Version]
- Li, W.-W.; Yu, H.-Q.; He, Z. Towards sustainable wastewater treatment by using microbial fuel cells-centered technologies. Energy Environ. Sci. 2014, 7, 911–924. [Google Scholar] [CrossRef]
- Xue, Z.X.; Cao, Y.Z.; Liu, N.; Feng, L.; Jiang, L. Special wettable materials for oil/water separation. J. Mater. Chem. A 2014, 2, 2445–2460. [Google Scholar] [CrossRef]
- Hassler, B. Accidental Versus Operational Oil Spills from Shipping in the Baltic Sea: Risk Governance and Management Strategies. Ambio 2011, 40, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Long, H.; Tang, G.; Wan, J.; Li, H. The management in response to marine oil spill from ships in China: A systematic review. Mar. Pollut. Bull. 2015, 96, 7–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liubartseva, S.; De, D.M.; Oddo, P.; Coppini, G.; Pinardi, N.; Greggio, N. Oil spill hazard from dispersal of oil along shipping lanes in the Southern Adriatic and Northern Ionian Seas. Mar. Pollut. Bull. 2015, 90, 259–272. [Google Scholar] [CrossRef] [PubMed]
- King, G.M.; Kostka, J.E.; Hazen, T.C.; Sobecky, P.A. Microbial responses to the deepwater horizon oil spill: From coastal wetlands to the deep sea. Ann. Rev. Mar. Sci. 2015, 7, 377. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.W.; Zhang, Y.; Xia, Y. Fabrication of microbeads with a controllable hollow interior and porous wall using a capillary fluidic device. Adv. Funct. Mater. 2009, 19, 2943–2949. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Lin, C.-Y.; Jong, T.-C. Investigation of strategies to improve the recycling effectiveness of waste oil from fishing vessels. Mar. Policy 2007, 31, 415–420. [Google Scholar] [CrossRef]
- Tavakoli, M.T.; Amdahl, J.; Leira, B.J. Experimental investigation of oil leakage from damaged ships due to collision and grounding. Ocean Eng. 2011, 38, 1894–1907. [Google Scholar] [CrossRef]
- Teduka, M.; Nishioka, T. Ambient-temperature fusible filter: A preliminary experiment and a proposal for a filtration process. AIChE J. 2014, 60, 22–26. [Google Scholar] [CrossRef]
- Gu, Y.; Li, D. Electric charge on small silicone oil droplets dispersed in ionic surfactant solutions. Colloids Surf. A Physicochem. Eng. Asp. 1998, 139, 213–225. [Google Scholar] [CrossRef]
- Gupta, V.K.; Ali, I.; Saleh, T.A.; Nayak, A.; Agarwal, S. Chemical treatment technologies for waste-water recycling—An overview. RSC Adv. 2012, 2, 6380–6388. [Google Scholar] [CrossRef]
- Zhu, L.; Chen, M.; Dong, Y.; Tang, C.Y.; Huang, A.; Li, L. A low-cost mullite-titania composite ceramic hollow fiber microfiltration membrane for highly efficient separation of oil-in-water emulsion. Water Res. 2016, 90, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Huang, R.; Qi, W.; Su, R.; He, Z. Bioinspired peptide-coated superhydrophilic poly (vinylidene fluoride) membrane for oil/water emulsion separation. Langmuir 2018, 34, 6621–6627. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.D.; Xu, Y.; Jin, J.H.; Zhong, Z.P.; Liu, Y.; Luo, M.; Liu, Z.P. Pilot scale ex-situ bioremediation of heavily PAHs-contaminated soil by indigenous microorganisms and bioaugmentation by a PAHs-degrading and bioemulsifier-producing strain. J. Hazard. Mater. 2012, 233–234, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Dellagnezze, B.M.; Vasconcellos, S.P.; Angelim, A.L.; Melo, V.M.M.; Santisi, S.; Cappello, S.; Oliveira, V.M. Bioaugmentation strategy employing a microbial consortium immobilized in chitosan beads for oil degradation in mesocosm scale. Mar. Pollut. Bull. 2016, 107, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.E.; Morad, N.; Teng, T.T.; Poh, B.T. Development, characterization and the application of hybrid materials in coagulation/flocculation of wastewater: A review. Chem. Eng. J. 2012, 203, 370–386. [Google Scholar] [CrossRef]
- Elimelech, M.; Gregory, J.; Jia, X. Particle Deposition and Aggregation: Measurement, Modelling and Simulation; Butterworth-Heinemann: Oxford, UK, 2013; ISBN 1483161374. [Google Scholar]
- Matilainen, A.; Vepsäläinen, M.; Sillanpää, M. Natural organic matter removal by coagulation during drinking water treatment: A review. Adv. Colloid Interface Sci. 2010, 159, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci Total Environ. 2014, 473, 619–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutiérrez, G.; Lobo, A.; Benito, J.M.; Coca, J.; Pazos, C. Treatment of a waste oil-in-water emulsion from a copper-rolling process by ultrafiltration and vacuum evaporation. J. Hazard. Mater. 2011, 185, 1569–1574. [Google Scholar] [CrossRef] [PubMed]
- Daud, Z.; Awang, H.; Nasir, N.; Ridzuan, M.B.; Ahmad, Z. Suspended solid, color, COD and oil and grease removal from biodiesel wastewater by coagulation and flocculation processes. Procedia Soc. Behav. Sci. 2015, 195, 2407–2411. [Google Scholar] [CrossRef]
- Zhao, J.-R.; Xie, H.; Shi, C.-M.; Chen, Z. Study on Treatment of Landfill Leachate by Combined Coagulation-Electrolytic Method. Environ. Sci. Technol. 2010, 33, 188–191. [Google Scholar]
- Wei, Y.; Ding, A.; Dong, L.; Tang, Y.; Yu, F.; Dong, X. Characterisation and coagulation performance of an inorganic coagulant-poly-magnesium-silicate-chloride in treatment of simulated dyeing wastewater. Colloids Surf. A Physicochem. Eng. Asp. 2015, 470, 137–141. [Google Scholar] [CrossRef]
- Yi, Q.; Long, L. Synthesis of PSAFS using Bayer red mud and its flocculation effect evaluation. Environ. Sci. Technol. 2012, 35, 134–138. [Google Scholar]







| Composition (g/1000 g) | Freshwater | Fuel oil A | Fuel oil B | SDBS | Fe3O4 |
| 947.8 | 25.0 | 25.0 | 0.5 | 1.7 | |
| Characteristics | Oil concentration (mg·L−1) | Turbidity (NTU) | pH | Droplets diameter (μm) | COD (mg·L−1) |
| 3000~5000 | 7000~10,000 | 6.5~6.8 | 10~15 | 7800~17,000 |
| nFe:nAl | |||||||
|---|---|---|---|---|---|---|---|
| 4:1 | 2:1 | 1:1 | 1:2 | 1:4 | 1:6 | ||
| Untreated | 7000 | ||||||
| n(Fe+Al):nSi | 20:1 | 74.0 | 27.8 | 12.7 | 14.3 | 33.3 | 46.2 |
| 15:1 | 85.0 | 33.5 | 11.8 | 13.5 | 14.2 | 27.9 | |
| 10:1 | 85.7 | 26.8 | 9.69 | 11.3 | 12.16 | 21.4 | |
| 5:1 | 108.1 | 89.8 | 76.5 | 55.8 | 55.6 | 87.6 | |
| 1:1 | 100.8 | 57.2 | 118 | 125.6 | 125.3 | 143.6 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, Z.; Zhang, L.; Zhang, S.; Sun, Y.; Shah, K.J. Treatment of Oil-Contaminated Water by Modified Polysilicate Aluminum Ferric Sulfate. Processes 2018, 6, 95. https://doi.org/10.3390/pr6070095
You Z, Zhang L, Zhang S, Sun Y, Shah KJ. Treatment of Oil-Contaminated Water by Modified Polysilicate Aluminum Ferric Sulfate. Processes. 2018; 6(7):95. https://doi.org/10.3390/pr6070095
Chicago/Turabian StyleYou, Zhaoyang, Li Zhang, Shujuan Zhang, Yongjun Sun, and Kinjal J. Shah. 2018. "Treatment of Oil-Contaminated Water by Modified Polysilicate Aluminum Ferric Sulfate" Processes 6, no. 7: 95. https://doi.org/10.3390/pr6070095