Ambient Pressure-Dried Graphene–Composite Carbon Aerogel for Capacitive Deionization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of GCCAs
2.2. Material Characterizations
2.3. Electrochemical Tests and CDI Experiments
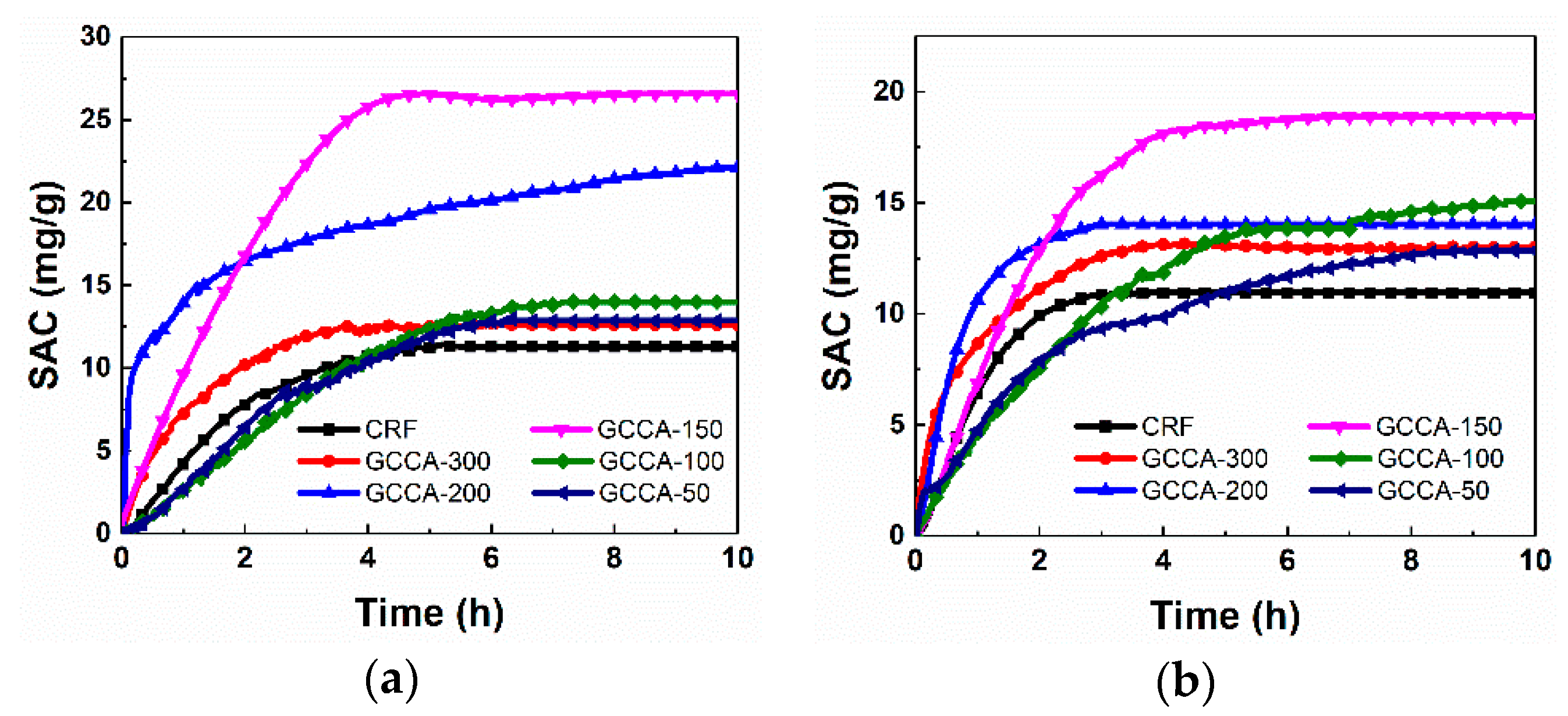
3. Results and Discussion
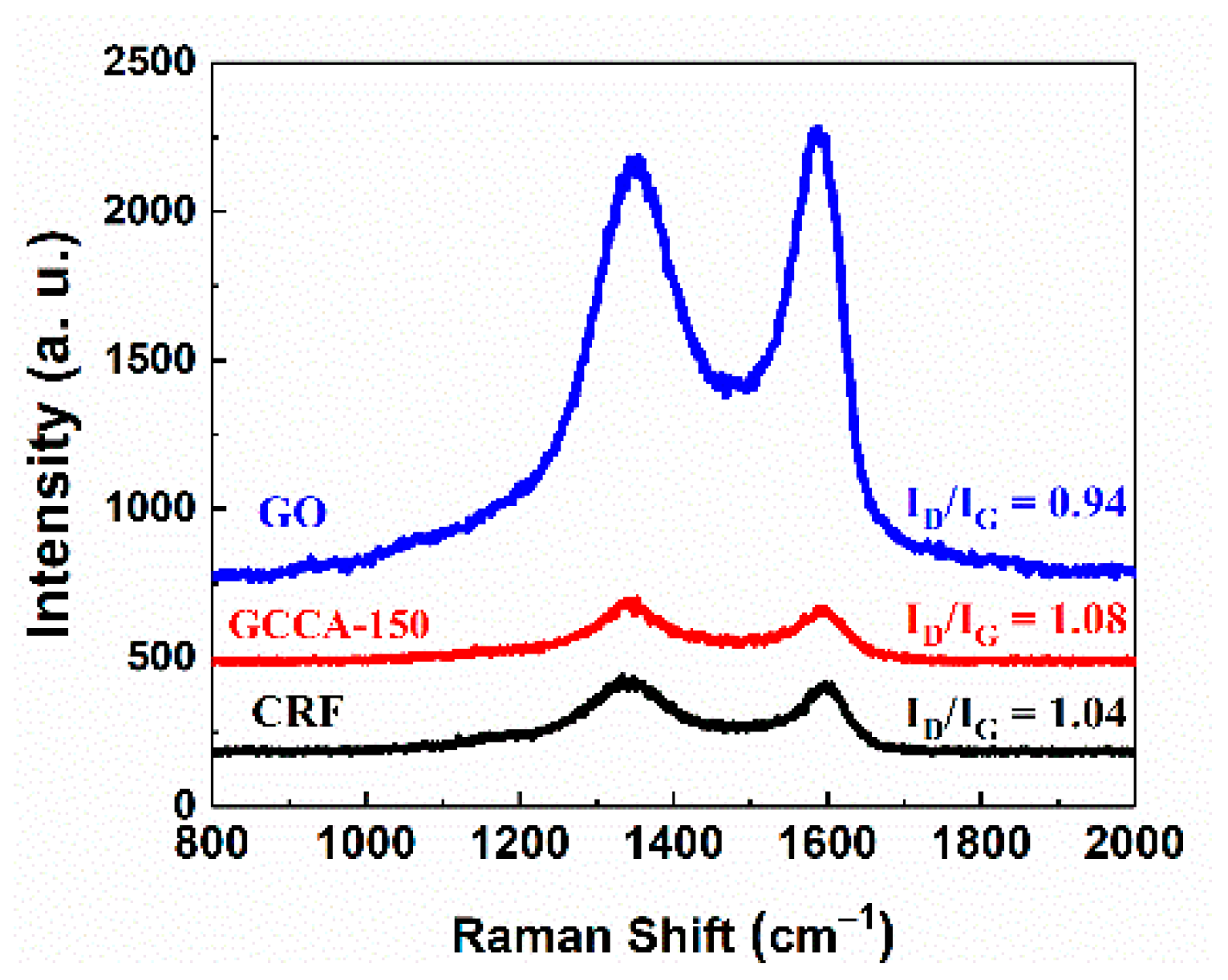
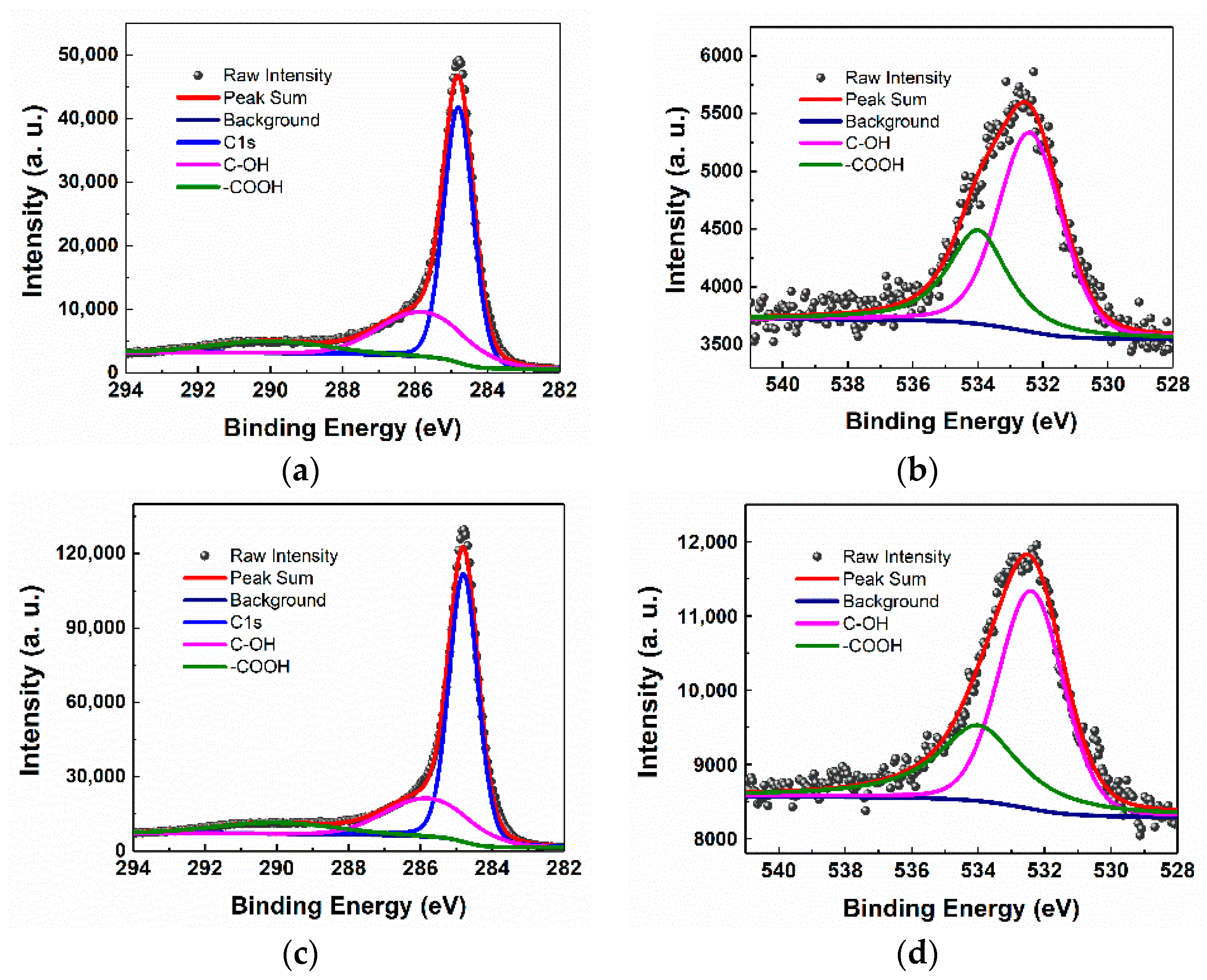
3.1. Physicochemical Characterization of GCCAs
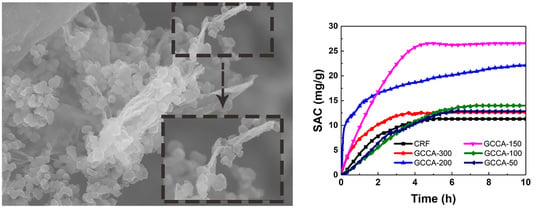
3.2. Surface Morphology and Pore Structure of GCCAs
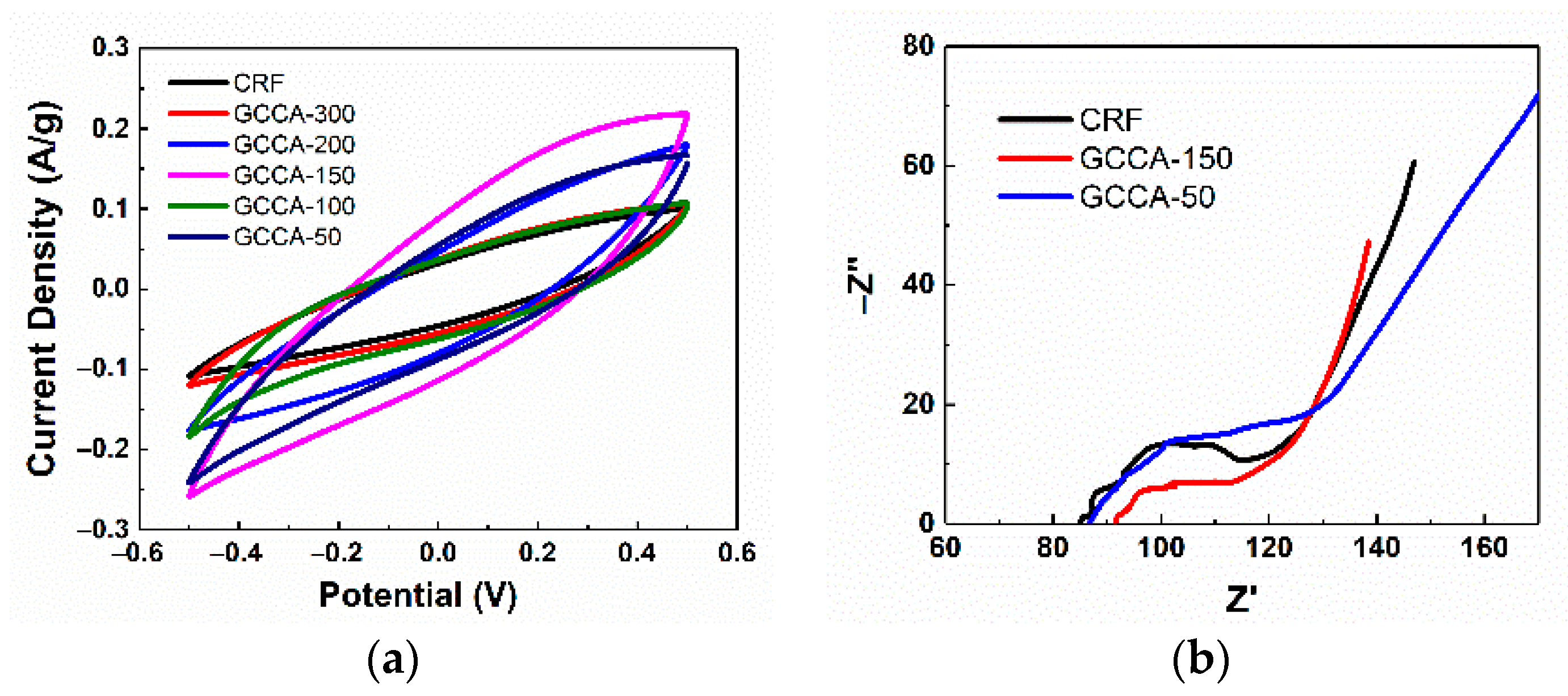
3.3. Electrochemical Characterization and Electrosorption Measurements
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Farmer, J.C.; Fix, D.V.; Mack, G.V.; Pekala, R.W.; Poco, J.F. The use of capacitive deionization with carbon aerogel electrodes to remove inorganic contaminants from water. Off. Sci. Tech. Inf. Tech. Rep. 1995, 15, 595–599. [Google Scholar]
- Porada, S.; Zhao, R.; van der Wal, A.; Presser, V.; Biesheuvel, P.M. Review on the science and technology of water desalination by capacitive deionization. Prog. Mater. Sci. 2013, 58, 1388–1442. [Google Scholar] [CrossRef] [Green Version]
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Mariñas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Humplik, T.; Lee, J.; O’Hern, S.C.; Fellman, B.A.; Baig, M.A.; Hassan, S.F.; Atieh, M.A.; Rahman, F.; Laoui, T.; Karnik, R. Nanostructured materials for water desalination. Nanotechnology 2011, 22, 292001. [Google Scholar] [CrossRef] [PubMed]
- AlMarzooqi, F.A.; Al Ghaferi, A.A.; Saadat, I.; Hilal, N. Application of Capacitive Deionisation in water desalination: A review. Desalination 2014, 342, 3–15. [Google Scholar] [CrossRef]
- Suss, M.E.; Porada, S.; Sun, X.; Biesheuvel, P.M.; Yoon, J.; Presser, V. Water desalination via capacitive deionization: What is it and what can we expect from it? Energy Environ. Sci. 2015, 8, 2296–2319. [Google Scholar] [CrossRef]
- Sui, Z.; Meng, Q.; Zhang, X.; Ma, R.; Cao, B. Green synthesis of carbon nanotube–graphene hybrid aerogels and their use as versatile agents for water purification. J. Mater. Chem. 2012, 22, 8767. [Google Scholar] [CrossRef]
- Yan, C.; Kanaththage, Y.W.; Short, R.; Gibson, C.T.; Zou, L. Graphene/Polyaniline nanocomposite as electrode material for membrane capacitive deionization. Desalination 2014, 344, 274–279. [Google Scholar] [CrossRef]
- Pasta, M.; Wessells, C.D.; Cui, Y.; La Mantia, F. A desalination battery. Nano Lett. 2012, 12, 839–843. [Google Scholar] [CrossRef]
- Porada, S.; Weingarth, D.; Hamelers, H.V.M.; Bryjak, M.; Presser, V.; Biesheuvel, P.M. Carbon flow electrodes for continuous operation of capacitive deionization and capacitive mixing energy generation. J. Mater. Chem. A 2014, 2, 9313–9321. [Google Scholar] [CrossRef]
- Dermentzis, K.; Wessner, W. Continuous capacitive deionization with regenerative rotating film electrodes. Electrochem. Commun. 2018, 92, 5–8. [Google Scholar] [CrossRef]
- Zou, L.; Morris, G.; Qi, D. Using activated carbon electrode in electrosorptive deionisation of brackish water. Desalination 2008, 225, 329–340. [Google Scholar] [CrossRef]
- Wang, L.; Wang, M.; Huang, Z.H.; Cui, T.; Gui, X.; Kang, F.; Wang, K.; Wu, D. Capacitive deionization of NaCl solutions using carbon nanotube sponge electrodes. J. Mater. Chem. 2011, 21, 18295–18299. [Google Scholar] [CrossRef]
- Tsouris, C.; Mayes, R.; Kiggans, J.; Sharma, K.; Yiacoumi, S.; DePaoli, D.; Dai, S. Mesoporous carbon for capacitive deionization of saline water. Environ. Sci. Technol. 2011, 45, 10243–10249. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Sen Gupta, S.; Katiyar, S.; Raman, V.K.; Varigala, S.K.; Pradeep, T.; Sharma, A. Carbon aerogels through organo-inorganic co-assembly and their application in water desalination by capacitive deionization. Carbon 2016, 99, 375–383. [Google Scholar] [CrossRef]
- Quan, X.; Fu, Z.; Yuan, L.; Zhong, M.; Mi, R.; Yang, X.; Yi, Y.; Wang, C. Capacitive deionization of NaCl solutions with ambient pressure dried carbon aerogel microsphere electrodes. RSC Adv. 2017, 7, 35875–35882. [Google Scholar] [CrossRef] [Green Version]
- Thamilselvan, A.; Nesaraj, A.S.; Noel, M. Review on carbon-based electrode materials for application in capacitive deionization process. Inter. J. Environ. Sci. Technol. 2016, 13, 2961–2976. [Google Scholar] [CrossRef]
- Liu, Y.; Nie, C.; Liu, X.; Xu, X.; Sun, Z.; Pan, L. Review on carbon-based composite materials for capacitive deionization. RSC Adv. 2015, 5, 15205–15225. [Google Scholar] [CrossRef]
- Jung, H.-H.; Hwang, S.-W.; Hyun, S.-H.; Lee, K.-H.; Kim, G.-T. Capacitive deionization characteristics of nanostructured carbon aerogel electrodes synthesized via ambient drying. Desalination 2007, 216, 377–385. [Google Scholar] [CrossRef]
- Zafra, M.C.; Lavela, P.; Rasines, G.; Macías, C.; Tirado, J.L.; Ania, C.O. A novel method for metal oxide deposition on carbon aerogels with potential application in capacitive deionization of saline water. Electrochim. Acta 2014, 135, 208–216. [Google Scholar] [CrossRef] [Green Version]
- Macías, C.; Lavela, P.; Rasines, G.; Zafra, M.C.; Tirado, J.L.; Ania, C.O. On the correlation between the porous structure and the electrochemical response of powdered and monolithic carbon aerogels as electrodes for capacitive deionization. J. Solid State Chem. 2016, 242, 21–28. [Google Scholar] [CrossRef]
- Du, A.; Zhou, B.; Zhang, Z.; Shen, J. A Special Material or a New State of Matter: A Review and Reconsideration of the Aerogel. Materials 2013, 6, 941–968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, J.; Hou, J.; Guo, Y.; Xue, H.; Wu, G.; Zhou, B. Microstructure Control of RF and Carbon Aerogels Prepared by Sol-Gel Process. J. Sol-Gel Sci. Technol. 2005, 36, 131–136. [Google Scholar] [CrossRef]
- Liu, N.P.; Shen, J.; Guan, D.Y.; Liu, D.; Zhou, X.W.; Li, Y.J. Effect of Carbon Aerogel Activation on Electrode Lithium Insertion Performance. Acta Phys.-Chim. Sin. 2013, 29, 966–972. [Google Scholar]
- Macías, C.; Rasines, G.; Lavela, P.; Zafra, M.C.; Tirado, J.L.; Ania, C.O. Mn-Containing N-Doped Monolithic Carbon Aerogels with Enhanced Macroporosity as Electrodes for Capacitive Deionization. ACS Sustain. Chem. Eng. 2016, 4, 2487–2494. [Google Scholar] [Green Version]
- Bai, Y.; Huang, Z.-H.; Yu, X.-L.; Kang, F. Graphene oxide-embedded porous carbon nanofiber webs by electrospinning for capacitive deionization. Colloid Surf. A 2014, 444, 153–158. [Google Scholar] [CrossRef] [Green Version]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Guo, K.; Song, H.; Chen, X.; Du, X.; Zhong, L. Graphene oxide as an anti-shrinkage additive for resorcinol-formaldehyde composite aerogels. Phys. Chem. Chem. Phys. 2014, 16, 11603–11608. [Google Scholar] [CrossRef]
- Sun, W.; Du, A.; Gao, G.; Shen, J.; Wu, G. Graphene-templated carbon aerogels combining with ultra-high electrical conductivity and ultra-low thermal conductivity. Microporous Mesoporous Mater. 2017, 253, 71–79. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2009, 39, 228–240. [Google Scholar] [CrossRef]
- Wang, X.; Lu, L.L.; Yu, Z.L.; Xu, X.W.; Zheng, Y.R.; Yu, S.H. Scalable template synthesis of resorcinol-formaldehyde/graphene oxide composite aerogels with tunable densities and mechanical properties. Angew. Chem. Int. Ed. 2015, 54, 2397–2401. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.-H.; Zhang, X.-F.; Yi, S.-Q.; Liu, H.-B.; Chen, Y.-X.; Chen, H.; Yang, L.; He, Y.-D. Preparation of high specific surface area composite carbon cryogels from self-assembly of graphene oxide and resorcinol monomers for supercapacitors. J. Solid State Electrochem. 2016, 20, 1793–1802. [Google Scholar] [CrossRef]
- He, S.; Chen, W. High performance supercapacitors based on three-dimensional ultralight flexible manganese oxide nanosheets/carbon foam composites. J. Power Sources 2014, 262, 391–400. [Google Scholar] [CrossRef]
- Rasines, G.; Lavela, P.; Macías, C.; Zafra, M.C.; Tirado, J.L.; Parra, J.B.; Ania, C.O. N-doped monolithic carbon aerogel electrodes with optimized features for the electrosorption of ions. Carbon 2015, 83, 262–274. [Google Scholar] [CrossRef] [Green Version]
- Li, S.M.; Yang, S.Y.; Wang, Y.S.; Lien, C.H.; Tien, H.W.; Hsiao, S.T.; Liao, W.H.; Tsai, H.P.; Chang, C.L.; Ma, C.C.M. Controllable synthesis of nitrogen-doped graphene and its effect on the simultaneous electrochemical determination of ascorbic acid, dopamine, and uric acid. Carbon 2013, 59, 418–429. [Google Scholar] [CrossRef]
- Terzyk, A.P. The influence of activated carbon surface chemical composition on the adsorption of acetaminophen (paracetamol) in vitro: Part II. TG, FTIR, and XPS analysis of carbons and the temperature dependence of adsorption kinetics at the neutral pH. Colloid Surf. A 2001, 177, 23–45. [Google Scholar] [CrossRef]
- Sun, W.; Gao, G.; Zhang, K.; Liu, Y.; Wu, G. Self-assembled 3D N-CNFs/V2O5 aerogels with core/shell nanostructures through vacancies control and seeds growth as an outstanding supercapacitor electrode material. Carbon 2018, 132, 667–677. [Google Scholar] [CrossRef]
- Liu, Y.; Guan, D.; Gao, G.; Liang, X.; Sun, W.; Zhang, K.; Bi, W.; Wu, G. Enhanced electrochemical performance of electrospun V2O5 nanotubes as cathodes for lithium ion batteries. J. Alloys Compd. 2017, 726, 922–929. [Google Scholar] [CrossRef]


| Samples | SBET 1 (m2/g) | Smic (m2/g) | Vtot (cc/g) | Vmic (cc/g) | Vmic/Vtot (%) |
|---|---|---|---|---|---|
| CRF | 901.9 | 276.0 | 1.847 | 0.221 | 12.0 |
| GCCA-300 | 820.9 | 166.8 | 2.023 | 0.119 | 5.9 |
| GCCA-200 | 554.8 | 143.9 | 1.794 | 0.086 | 4.7 |
| GCCA-150 | 546.2 | 108.5 | 1.746 | 0.046 | 2.6 |
| GCCA-100 | 475.5 | 80.3 | 0.742 | 0.034 | 4.6 |
| GCCA-50 | 93.6 | 35.3 | 0.177 | 0.016 | 9.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Wang, X.; Wang, H.; Wu, X.; Shen, J. Ambient Pressure-Dried Graphene–Composite Carbon Aerogel for Capacitive Deionization. Processes 2019, 7, 29. https://doi.org/10.3390/pr7010029
Zhang C, Wang X, Wang H, Wu X, Shen J. Ambient Pressure-Dried Graphene–Composite Carbon Aerogel for Capacitive Deionization. Processes. 2019; 7(1):29. https://doi.org/10.3390/pr7010029
Chicago/Turabian StyleZhang, Chen, Xiaodong Wang, Hongqiang Wang, Xueling Wu, and Jun Shen. 2019. "Ambient Pressure-Dried Graphene–Composite Carbon Aerogel for Capacitive Deionization" Processes 7, no. 1: 29. https://doi.org/10.3390/pr7010029