Mixed Ionic-Electronic Conducting Membranes (MIEC) for Their Application in Membrane Reactors: A Review
Abstract
:1. Introduction
2. Oxygen Transport through MIEC Membranes
- Bulk-to-surface mass transfer of gaseous oxygen (feed side to the membrane surface).
- Dissociation (surface exchange): The oxygen molecule is adsorbed on the membrane surface and dissociates catalytically in oxygen ions (O2−). On the high oxygen partial pressure side, this can be expressed using the Kröger–Vink notation [34] below.where refers to oxygen vacancies in the ceramic and to oxygen ions (O2−) occupying the oxygen lattice.
- Ionic transport (bulk diffusion): under a pressure gradient between the feed and permeate side, the oxygen ions diffuse through the ceramic crystal lattice (mainly oxygen vacancies, but also other defects). To maintain electrical neutrally, electrons are transported at the same time in the opposite direction.
- Association (surface exchange): The oxygen ions recombine to form oxygen molecules and desorb from the surface of the membrane. The reaction involved in this step can be represented by the following formula.
- Surface-to-bulk mass transfer of gaseous oxygen (permeate side): Gas transport in the permeate side alone or helped by a sweep gas (helium, CO2, etc.).
3. Materials and Methods for MIEC Oxygen Membranes
3.1. Single-Phase Ionic Transport Materials
3.1.1. Fluorites
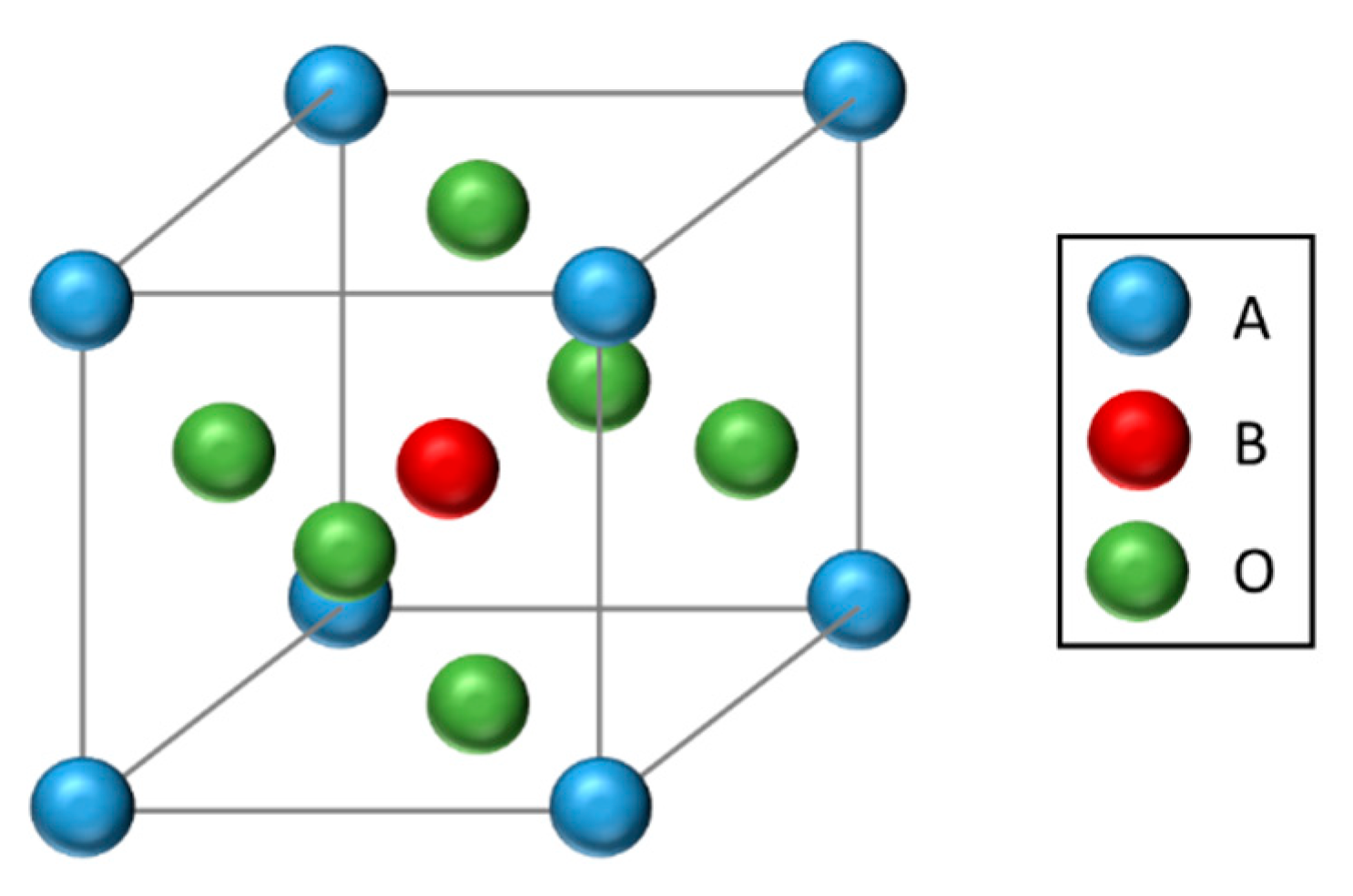
3.1.2. Perovskites
3.2. Dual-Phase Ionic-Electronic Transport Materials
3.2.1. Dual-Phase Based on Ceramic-Metallic Mixtures
3.2.2. Dual-Phase on Mixed Ceramics
4. Factors Affecting Permeation and Stability
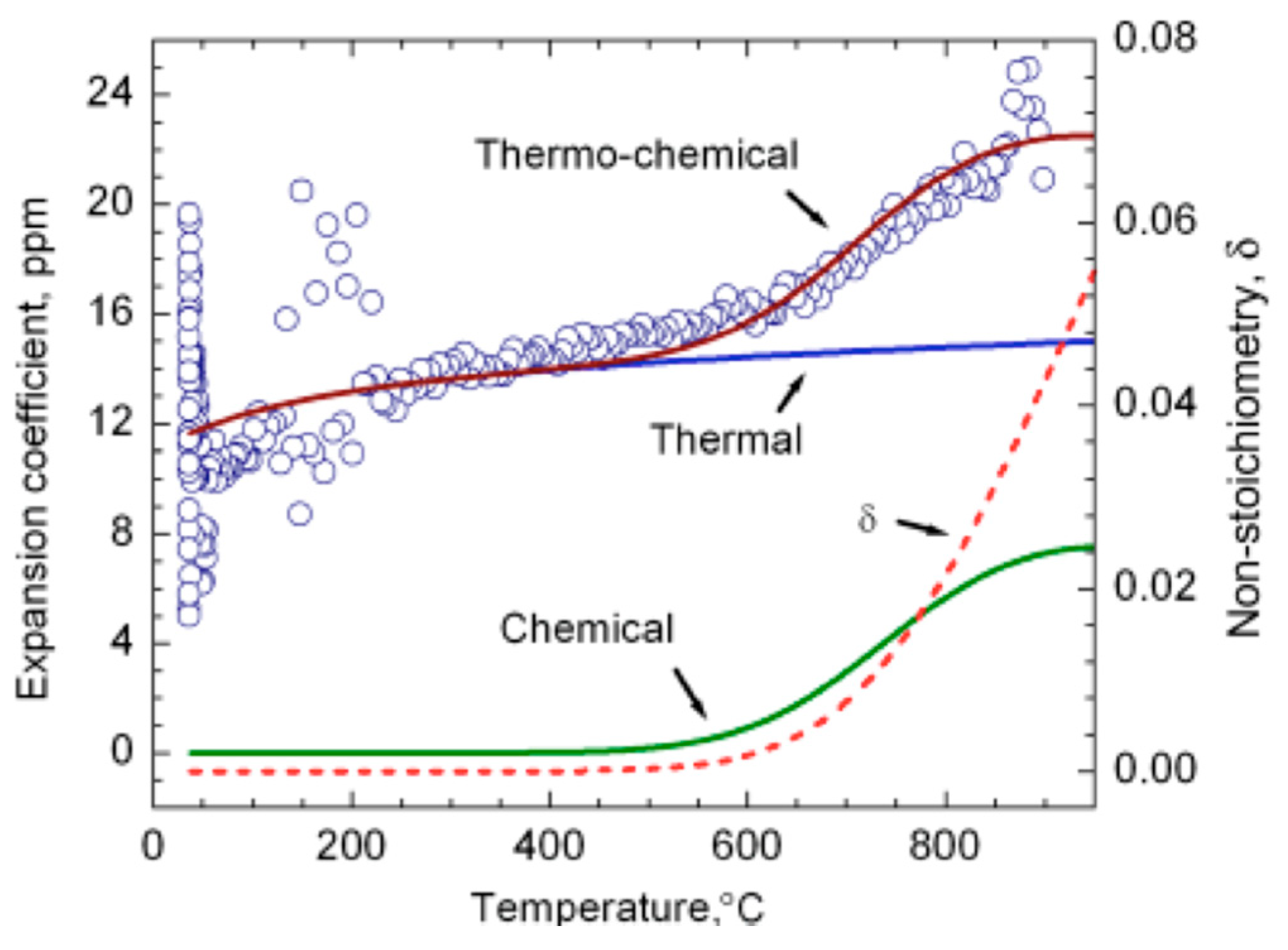
4.1. Chemical and Thermal Expansion
4.2. Phase Transformation
- Incorporation of zirconium in the B-site cation of perovskite compounds [53].
- Introduction of higher valence cations into the A site cation (i.e., La) [102] or (i.e., Ti, Cr, and Ga) [94,103,104] into the B site cation. The phase stability of SrFeO3 can be, thus, improved by introducing metal cations such as Ti, Ni, Mn, Cu, Cr, etc. Moderate amounts of Ti or Cr into the Co or Fe sub-lattice improves the structural stability, but might decrease the electron conductivity and oxygen permeability [50].
- Partial substitution of the A site or the B site cation with ions with larger radius. Phase stability could be improved by partial substitution of B-cations with bigger cations, like Nb [105] or Zr [106]. The main advantage of Zr doping is the reduction of the lower temperature limit of the perovskite phase stability range to below 800 °C. As an example, the SCFO cubic phase is stable under an oxygen content higher than 0.1 atm at high temperatures. However, the transformation of the perovskite structure to the brownmillerite phase occurs at an oxygen content below 0.1 atm, where the stoichiometry is around 2.5 (3-δ) at temperatures below 770 °C (when this stoichiometry increases) [107]. Similar results were observed for the phase stability of SrFeO3-δ [108]. In case of introducing Ba into SrCo0.8Fe0.2O3-δ by partial substitution of Sr, phase stability is obtained while the conductivity is not affected [109,110]. A single-phase BSFC membrane presented a cubic structure at temperatures over 900 °C for a range of oxygen partial pressures from 10−4 to 1 atm [92], whereas un-doped SCF changes from cubic perovskite to orthorhombic brownmillerite are below 677 °C. In this case, the cubic structure is stable at temperatures above 777 °C. Unger et al. [111] studied the effect of Yttrium doped BSCF for different Ytrium concentrations to analyze the partial transformation to Fe-depleted hexagonal phase during long term annealing in ambient air for 240 h for intermediate temperatures as well as cobalt precipitates and anomalies in the morphology. They concluded that the partial B site doped with 10% of Y extended the stability range of the cubic BSCF perovskite phase at lower temperatures. In addition, no secondary phase formation was observed at 800 ºC and, at lower temperatures, the degradation was significantly reduced.Fang et al. [105] investigated the performance and stability of niobium-substituted BSCF. They could demonstrate that the partial substitution of niobium for Co and Fe suppress the phase instability at intermediate temperatures (below 850 ºC). At 800–900 ºC with He as purge gas, the oxygen permeation flux only decreased 10% for the 10% mol Nb-subtituted BSCF compared to pure BSCF, but, over the long term, the test with CO2 in the purge gas, the Nb present was not enough to stabilize the cubic phase and consequently oxygen flux decreased dramatically.Ravkina et al. [112] extended the research done with Zr-doped BSCF on long-term experiments at an intermediate temperature range and they could conclude that a BSCF membrane with up to 3% (mol) Zr content at an intermediate temperature range (i.e., 773–1123 K) showed improved phase stability compared with pure BSCF. However, for a practical application, the Zr doped BSCF could not maintain a stable oxygen permeation flux and it decreased continuously, which concludes that BSCFZ materials are not an appropriate alternative for intermediate temperature oxygen transporting membranes. Not only the effect of the temperature in the phase transformation, but also the oxygen pressure need to be taken into account to consider the feasibility of perovskite membranes implementation. Ravkina et al. analyzed the phase separation of BSCF perovskite [113] at high and low temperature ranges. The influence of elevated oxygen pressure (from 1 to 50 bar) on the decomposition process of BSCF ceramic with a cubic structure was investigated from 300 to 1300 K. It could be found that, at high pressures, a mixture of cubic phase and a super structure (with double cell parameter) could be found in a single lamella decreasing oxygen permeation.
- The development of perovskite compounds without cobalt. Cobalt based perovskite type membranes present high oxygen permeability but the stability at intermediate temperatures or under reducing conditions is poor because the cobalt easily reduces and results in big changes in the unit cell dimension. Development of cobalt-free MIEC membranes could be another alternative to solve the long-term stability problems caused by the reaction with gas species like CO2, SO2, or water vapor [114].One of the most studied cobalt-free membranes is the BaFeO3-δ, but it shows low oxygen permeability because it crystalizes in the hexagonal structure, which permeates less than the cubic structure. The partial substitution in the A site with smaller cations like Sr, Ca, La, and Y can lead to the stabilization of the cubic structure. However, since the volume of the cubic unit cell is reduced, the oxygen flux also decreases. Yet, the partial substitution on the B site with low valence cations like Y, Cu, Ni, and Zr can increase the volume of the cubic unit cell and the oxygen vacancy concentration. Liang et al. [114] studied the influence of the partial substitution of La for Fe on the B-site of BaFe0.95Zr0.05O3-δ. Long-term tests suggest that BFLZ (x = 0.4) exhibits good oxygen permeability. Tan et al. [115] fabricated a cobalt-free La0.7Sr0.3FeO3-δ hollow fiber membrane and observed that the stability in He and CO2 atmosphere was higher than for the LSCF (La1-xSrxCo1-yFeyO3-δ) membrane. However, the LSF membrane still suffered from a reaction with H2 and CH4 and porous debris were formed, which resulted in membrane leaking or even a mechanical stability decrease.
4.3. Cationic Diffusion and Creep
4.4. Gas Poisoning
4.4.1. CO2 and Steam Tolerance
4.4.2. Sulphur Resistance
4.5. Sintering Temperature
5. Oxygen Permeation Improvement
- Membrane thickness: Oxygen permeation through the membrane is related to the inverse of the thickness following the Wagner equation (Equation (4)). As thickness decreases, the bulk diffusion process become less relevant than the surface exchange.
- Ionic and electronic conduction capacity: Depends on selected materials and operating conditions (temperature, pressure, gases).
- When the limiting step is the surface exchange kinetics, the dissociation and association processes at both sides of the membrane need to be promoted. This could be improved by:
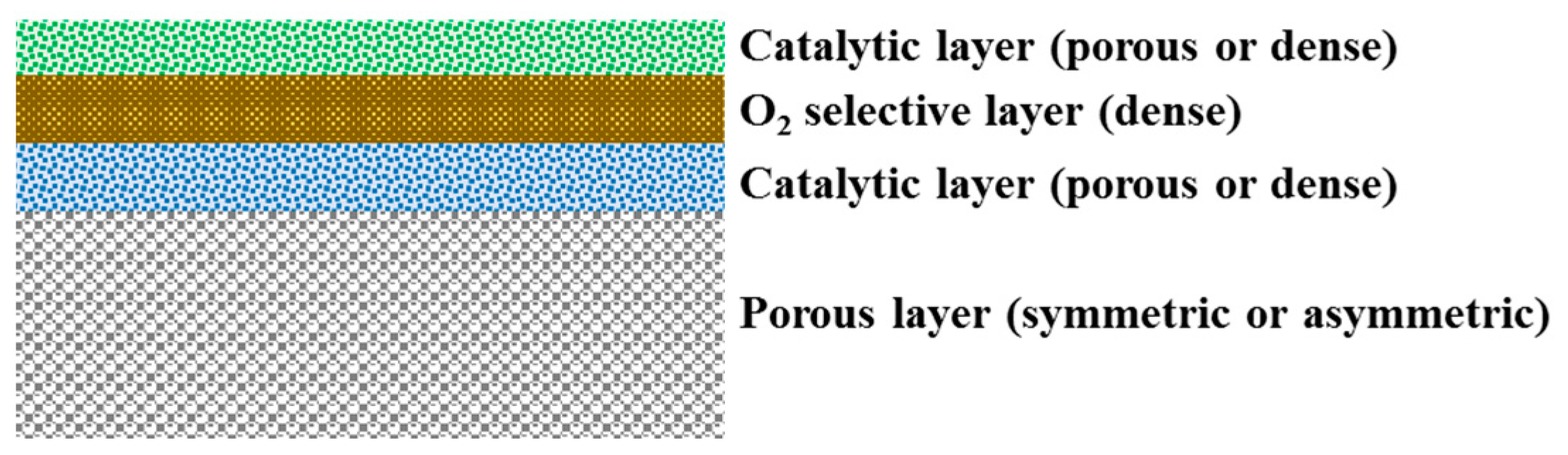
- Deposition of a very thin porous layer on top of a dense selective layer increasing the surface area for the dissociation and recombination of oxygen ions/molecules [151].
- Deposition of catalysts to improve oxygen splitting/recombination.
5.1. Surface Area Modification
5.2. Deposition of Catalyst
5.3. Thickness Reduction and Supported Thin Film Membranes
- The thermal and chemical expansion of the selective layer and the support must be as close as possible.
- No reactions should take place between the different materials at high temperatures.
- The dense selective layer should be free of defects, such as cracks and pinholes.
5.4. Application of Advanced Membrane Preparation Methods
6. Application of Oxygen Conducting Membranes in Membrane Reactors
6.1. OCM (Oxidative Coupling of Methane)
6.2. Partial Oxidation of Methane (POM)
6.3. Oxidative Dehydrogenation of Ethane
6.4. Other Applications
6.5. High Temperature Sealing
7. Conclusions and Future Trends
Acknowledgments
Conflicts of Interest
Acronyms
| BCFZ | BaCo1-x-yFeyZrxO3-δ |
| BLFZ | Ba1-xLaxFe1−yZryO3−δ |
| BSCF | Ba1-xSrxCo1-yFeyO3-δ |
| BSFM | Ba1-xSrxFe1-yMoyO3-δ |
| BYS | Bi2-x-yYxSmyO3-δ |
| CPO | Ce1-xPrxO2-δ |
| CTF | CaTi1-xFexO3-δ |
| CTO | Ce1-xTbxO2-δ |
| CGO or GDC | Ce1-xGdxO2-δ |
| LBCO | LaBaCo2O5+δ |
| LSC or LSCO | La1-xSrxCoO3-δ |
| LSCF | La1-xSrxCo1-yFeyO3-δ |
| LSCrF | La1-xSrxCr1-yFeyO3-δ |
| LSFN | La1-xSrxFe1−xNixO3−δ |
| LSFO or LSF | La1-xSrxFeO3-δ |
| LSFT | La1-xSrxFe1-yTayO3-δ |
| LSM | La1-xSrxMnO3-δ |
| LSTF | La1-xSrxTi1-yFeyO3-δ |
| NFO | NiFe2O4 |
| PNO | Pr2NiO4+δ |
| PNM | Pr2Ni1-xMoxO4+δ |
| PSFO | Pr1-xSrxFe2O3-δ |
| SCFO or SCF | SrCo1-xFexO3-δ |
| SDC | Ce1-xSmxO2-δ |
| SSAF | Sm1-xSrxAl1-yFeyO3-δ |
| SSF | Sm1-xSrxFeO3-δ |
| YCCC | Y1-xCaxCr1-yCoyO3 |
| YSZ | (ZrO2)1-x-(Y2O3)x |
Abbreviations
| d | Grain size |
| Diffusion coefficient of oxygen vacancies | |
| Ea | Activation energy |
| Faraday constant | |
| ΔHr | Enthalpy of reaction |
| Oxygen ion permeation | |
| Oxygen permeation through an MIEC membrane | |
| K | Pre-exponential factor |
| Reaction rate constant for the oxygen splitting step | |
| Reaction rate constant for the oxygen recombination step | |
| Membrane thickness | |
| Chemical potential gradient | |
| Oxygen ions occupying the lattice | |
| PO2 | Oxygen partial pressure |
| Oxygen partial pressure in the retentate | |
| Oxygen partial pressure in the permeate | |
| Transference number of electrons | |
| Transference number of oxygen ions | |
| Oxygen vacancies (Kröger-Vink notation) |
Greek Letters
| δ | Oxygen vacancies |
| Total electron and oxygen ions conductivity | |
| Creep rate | |
| σ | Stress |
References
- Bose, A.C. (Ed.) Inorganic Membranes for Energy and Enviromental Applications; Springer: New York, NY, USA, 2009. [Google Scholar]
- Baker, R.W. Future Directions of Membrane Gas Separation Technology. Ind. Eng. Chem. Res. 2002, 41, 1393–1411. [Google Scholar] [CrossRef]
- Diniz, C.; Leo, A.; Liu, S. Development of mixed conducting membranes for clean coal energy delivery. Int. J. Greenh. Gas Control 2009, 3, 357–367. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, W.; Chen, Y.; Chen, D.; Chen, J.; Liu, S.; Jin, W.; Shao, Z. Novel Approach for Developing Dual-Phase Ceramic Membranes for Oxygen Separation through Beneficial Phase Reaction. ACS Appl. Matetr. Interfaces 2015, 7, 22918–22926. [Google Scholar] [CrossRef] [PubMed]
- Bhide, B.C.; Stern, S.A. A new evaluation of membrane processes for the oxygen-enrichment of air. II. Effects of economic parameters and membrane properties. J. Membr. Sci. 1991, 62, 37–58. [Google Scholar] [CrossRef]
- Belaissaoui, B.; le Moullec, Y.; Hagi, H.; Favre, E. Energy efficiency of oxygen enriched air production technologies: Cryogeny vs membranes. Sep. Purif. Technol. 2014, 125, 142–150. [Google Scholar] [CrossRef]
- Sunarso, J.; Baumann, S.; Serra, J.M.; Meulenberg, W.A.; Liu, S.; Lin, Y.S.; da Costa, J.C.D. Mixed ionic–electronic conducting (MIEC) ceramic-based membranes for oxygen separation. J. Membr. Sci. 2008, 320, 13–41. [Google Scholar] [CrossRef]
- Bouwmeester, H.J.M. Dense ceramic membranes for methane conversion. Catal. Today 2003, 82, 141–150. [Google Scholar] [CrossRef]
- Gottzmann, C.F.; Prasad, R.; Robinson, E.T.; Schwartz, J.M. Syngas Production Utilizing an Oxygen Transport Membrane. Patent EP1390291A1, 25 February 2004. [Google Scholar]
- Christie, G.M.; Lane, J.A. Composite Oxygen Transport Membranes. U.S. Patent 20130156978A1, 20 June 2013. [Google Scholar]
- Bouwmeester, H.J.M.; Burggraaf, A.J. Dense ceramic membranes for oxygen separation. Fundam. Inorg. Membr. Sci. Technol. 1996, 4, 435–528. [Google Scholar] [CrossRef]
- Kharton, V.; Yaremchenko, A.; Kovalevsky, A.; Viskup, A.; Naumovich, E.; Kerko, P. Perovskite-type oxides for high-temperature oxygen separation membranes. J. Membr. Sci. 1999, 163, 307–317. [Google Scholar] [CrossRef]
- Akin, F.T.; Lin, Y.S. Selective oxidation of ethane to ethylene in a dense tubular membrane reactor. J. Membr. Sci. 2002, 209, 457–467. [Google Scholar] [CrossRef]
- Godini, H.R.; Trivedi, H.; de Villasante, A.G.; Görke, O.; Jašo, S.; Simon, U.; Berthold, A.; Witt, W.; Wozny, G. Design and demonstration of an experimental membrane reactor set-up for oxidative coupling of methane. Chem. Eng. Res. Des. 2013, 91, 2671–2681. [Google Scholar] [CrossRef]
- Godini, H.R.; Xiao, S.; Kim, M.; Holst, N.; Jašo, S.; Görke, O.; Steinbach, J.; Wozny, G. Experimental and model-based analysis of membrane reactor performance for methane oxidative coupling: Effect of radial heat and mass transfer. J. Ind. Eng. Chem. 2014, 20, 1993–2002. [Google Scholar] [CrossRef]
- Tong, J.; Yang, W.; Cai, R.; Zhu, B.; Lin, L. Novel and ideal zirconium-based dense membrane reactors for partial oxidation of methane to syngas. Catal. Lett. 2002, 78, 129–137. [Google Scholar] [CrossRef]
- Kim, J.; Hwang, G.; Lee, S.; Park, C.; Kim, J.; Kim, Y. Properties of oxygen permeation and partial oxidation of methane in La0.6Sr0.4CoO3−δ(LSC)-La0.7Sr0.3Ga0.6Fe0.4O3−δ (LSGF) membrane. J. Membr. Sci. 2005, 250, 11–16. [Google Scholar] [CrossRef]
- Takahashi, T.; Esaka, T.; Iwahara, H. Electrical conduction in the sintered oxides of the system Bi2O3-BaO. J. Solid State Chem. 1976, 16, 317–323. [Google Scholar] [CrossRef]
- Cales, B.; Baumard, J.F. Oxygen semipermeability and electronic conductivity in calcia-stabilized zirconia. J. Mater. Sci. 1982, 17, 3243–3248. [Google Scholar] [CrossRef]
- de Recherche, C.; Temperatures, H.; Orleans, C. Mixed Conduction and Defect Structure of ZrO-CeO-Y203 Solid Solutions. J. Electrochem. Soc. 1984, 131, 2407–2413. [Google Scholar]
- Yasutake, T.; Zhang, H.M.; Furukawa, S.; Yamazoe, N. Oxygen permeation through perovskite-type oxides. Chem. Lett. 1985, 14, 1743–1746. [Google Scholar]
- Boivin, J.C.; Mairesse, G. Recent Material Developments in Fast Oxide Ion Conductors. Chem. Mater. 1998, 10, 2870–2888. [Google Scholar] [CrossRef]
- Badwal, S.P.S.; Ciacchi, F.T. Ceramic Membrane Technologies for Oxygen Separation. Adv. Mater. 2001, 13, 993–996. [Google Scholar] [CrossRef]
- Yin, X.; Choong, C.; Hong, L. Crafting La0.2Sr0.8MnO3−δ membrane with dense surface from porous YSZ tube. J. Solid State Electrochem. 2006, 10, 643–650. [Google Scholar] [CrossRef]
- Meng, X.; Ding, W.; Jin, R.; Wang, H.; Gai, Y.; Ji, F.; Ge, Y. Two-step fabrication of BaCo0.7Fe0.2Nb0.1O3−δ asymmetric oxygen permeable membrane by dip coating. J. Membr. Sci. 2014, 450, 291–298. [Google Scholar] [CrossRef]
- Ramachandran, D.K.; Søgaard, M.; Clemens, F.; Gurauskis, J.; Kaiser, A. Fabrication and performance of a tubular ceria based oxygen transport membrane on a low cost MgO support. Sep. Purif. Technol. 2015, 147, 422–430. [Google Scholar] [CrossRef] [Green Version]
- Chen, A.J.B.C.S.; Kruidhof, H.; Bouwmeester, H.J.M.; Verweij, H. Thickness dependence of oxygen permeation through erbia-stabilized oxide-silver composites. Solid State Ion. 1997, 99, 215–219. [Google Scholar] [CrossRef]
- Teraoka, Y.; Fukuda, T.; Miura, N.; Yamazoe, N. Development of Oxygen Semipermeable Membrane Using Mixed Conductive Perovskite-Type Oxides (Part 2). J. Ceram. Soc. Jpn. 1989, 97, 533–538. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, B.J.; Rogan, R.C.; Richardson, J.W., Jr.; Ma, B.; Balachandran, U. Stability of the cubic perovskite SrFe0.8Co0.2O3−δ. Solid State Ion. 2002, 146, 313–321. [Google Scholar] [CrossRef]
- Sadykov, V.; Zarubina, V.; Pavlova, S.; Krieger, T.; Alikina, G.; Lukashevich, A.; Muzykantov, V.; Sadovskaya, E.; Mezentseva, N.; Zevak, E.; et al. Design of asymmetric multilayer membranes based on mixed ionic-electronic conducting composites supported on Ni-Al foam substrate. Catal. Today 2010, 156, 173–180. [Google Scholar] [CrossRef]
- Dong, X.; Jin, W.; Xu, N.; Li, K. Dense ceramic catalytic membranes and membrane reactors for energy and environmental applications. Chem. Commun. 2011, 47, 10886–10902. [Google Scholar] [CrossRef] [PubMed]
- Deibert, W.; Ivanova, M.E.; Baumann, S.; Guillon, O.; Meulenberg, W.A. Ion-conducting ceramic membrane reactors for high-temperature applications. J. Membr. Sci. 2017, 543, 79–97. [Google Scholar] [CrossRef]
- Liu, Y.; Tan, X.; Li, K. Membrane Processing Mixed Conducting Ceramics for Catalytic Membrane Processing. Catal. Rev. 2006, 48, 145–198. [Google Scholar] [CrossRef]
- Steele, B.C.H. Oxygen ion conductors and their technological applications. Mater. Sci. Eng. B 1992, 13, 79–87. [Google Scholar] [CrossRef]
- Xu, S.J.; Thomson, W.J. Oxygen permeation rates through ion-conducting perovskite membranes. Chem. Eng. Sci. 1999, 54, 3839–3850. [Google Scholar] [CrossRef]
- Wei, Y.; Yang, W.; Caro, J.; Wang, H. Dense ceramic oxygen permeable membranes and catalytic membrane reactors. Chem. Eng. J. 2013, 220, 185–203. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Q.; Faraji, S.; Slade, D.A.; Stagg-williams, S.M. A Review of Mixed Ionic and Electronic Conducting Ceramic Membranes as Oxygen Sources for High-Temperature Reactors. In Inorganic Polymeric and Composite Membranes: Structure, Function and Other Correlations, 1st ed.; Elsevier BV.: Amsterdam, The Netherlands, 2011; pp. 235–273. [Google Scholar] [CrossRef]
- Repasky, J.M.; Anderson, L.L.; Stein, E.E.; Armstrong, P.A.; Foster, E.P. ITM Oxygen technology: Scale-up toward clean energy applications. In Proceedings of the International Pittsburgh Coal Conference, Pittsburgh, PA, USA, 15–18 October 2012. [Google Scholar]
- Kharton, V.; Marques, F.; Atkinson, A. Transport properties of solid oxide electrolyte ceramics: A brief review. Solid State Ion. 2004, 174, 135–149. [Google Scholar] [CrossRef]
- Mogensen, M.; Lindegaard, T.; Hansen, U.R.; Mogensen, G. Physical Properties of Mixed Conductor Solid Oxide Fuel Cell Anodes of Doped CeO2. J. Electrochem. Soc. 1994, 141, 2122–2128. [Google Scholar] [CrossRef]
- Fagg, D.P.; Shaula, A.L.; Kharton, V.V.; Frade, J.R. High oxygen permeability in fluorite-type Ce0.8Pr0.2O2−δ via the use of sintering aids. J. Membr. Sci. 2007, 299, 1–7. [Google Scholar] [CrossRef]
- Massey, A.; Schwartz, S. The CRC Handbook of Solid State Electrochemistry; CRC: Boca Raton, FL, USA, 1996. [Google Scholar]
- Zhu, X.; Yang, W. Critical Factors Affecting Oxygen Permeation Through Dual-phase Membranes. In Inorganic Polymeric and Composite Membranes: Structure, Function and Other Correlations, 1st ed.; Elsevier BV.: Amsterdam, The Netherlands, 2011; pp. 275–293. [Google Scholar] [CrossRef]
- Fernández-garcía, M. Metal Oxide Nanoparticles. In Encyclopedia of Inorganic Chemistry; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar] [CrossRef]
- Srinivasan, R.; de Angelis, R.J.; Ice, G.; Davis, B.H. Identification of tetragonal and cubic structures of zirconia using synchrotron x-radiation source. J. Mater. Res. 1991, 6, 1287–1292. [Google Scholar] [CrossRef] [Green Version]
- Nawaz, M.; Gorgishvili, L.; Li, J.; Gorelik, T.; Kolb, U.; Nasdala, L.; Tremel, W. Facile synthesis and characterization of monocrystalline cubic. Solid State Sci. 2007, 9, 1105–1109. [Google Scholar] [CrossRef]
- Hoon, J.; Sook, G.; Yoo, C.; Haeng, J. Contribution of the surface exchange kinetics to the oxygen transport properties in Gd0.1Ce0.9O2−δ-La0.6Sr0.4Co0.2Fe0.8O3−δ dual-phase membrane. Solid State Ion. 2013, 253, 64–69. [Google Scholar] [CrossRef]
- Peña, M.A.; Fierro, J.L.G. Chemical Structures and Performance of Perovskite Oxides. Chem. Rev. 2001, 101, 1981–2017. [Google Scholar] [CrossRef] [PubMed]
- Cook, R.L.; Sammells, A.F. On the systematic selection of perovskite solid electrolytes for intermediate temperature fuel cells. Solid State Ion. 1991, 45, 311–321. [Google Scholar] [CrossRef]
- Kharton, A.V.K.; Viskup, A.; Yaremchenko, A.A.; Kerko, P.F.; Naumovich, E.N. Ionic transport in SrCo0.85Ti0.15O3−δCeramics at high oxygen pressures. Mater. Res. Bull. 2000, 34, 1921–1928. [Google Scholar] [CrossRef]
- Kharton, V.V.; Shaula, a.L.; Snijkers, F.M.M.; Cooymans, J.F.C.; Luyten, J.J.; Yaremchenko, A.A.; Valente, A.A.; Tsipis, E.V.; Frade, J.R.; Marques, F.M.B.; Rocha, J. Processing, stability and oxygen permeability of Sr(Fe, Al)O3-based ceramic membranes. J. Membr. Sci. 2005, 252, 215–225. [Google Scholar] [CrossRef]
- Zhu, X.; Cong, Y.; Yang, W. Oxygen permeability and structural stability of BaCe0.15Fe0.85O3−δ membranes. J. Membr. Sci. 2006, 283, 38–44. [Google Scholar] [CrossRef]
- Tong, J.; Yang, W.; Zhu, B.; Cai, R. Investigation of ideal zirconium-doped perovskite-type ceramic membrane materials for oxygen separation. J. Membr. Sci. 2002, 203, 175–189. [Google Scholar] [CrossRef] [Green Version]
- Cheng, H.; Yao, W.; Lu, X.; Zhou, Z.; Li, C.; Liu, J. Structural stability and oxygen permeability of BaCo0.7Fe0.2M0.1O3−δ (M = Ta, Nb, Zr) ceramic membranes for producing hydrogen from coke oven gas Hongwei. Fuel Process. Technol. 2015, 131, 36–44. [Google Scholar] [CrossRef]
- Liu, J.; Cheng, H.; Jiang, B.; Lu, X.; Ding, W. Effects of tantalum content on the structure stability and oxygen permeability of BaCo0.7Fe0.3−xTaxO3−δ ceramic membrane. Int. J. Hydrog. Energy 2013, 38, 11090–11096. [Google Scholar] [CrossRef]
- Yao, W.; Cheng, H.; Zhao, H.; Lu, X.; Zou, X.; Li, S.; Li, C. Synthesis, oxygen permeability, and structural stability of BaCo0.7Fe0.3-xZrxO3−δ ceramic membranes. J. Membr. Sci. 2016, 504, 251–262. [Google Scholar] [CrossRef]
- Ganji, E.; Towfighi, J.; Shirazi, L.; Nakhaei, A. Order–disorder transition and phase stability of BaxSr1−xCo0.8Fe0.2O3−δ oxides. J. Membr. Sci. 2011, 376, 78–82. [Google Scholar] [CrossRef]
- Babakhani, E.G.; Towfighi, J.; Shirazi, L.; Nakhaeipour, A.; Zamaniyan, A.; Shafiei, Z. Structure Stability and Oxygen Permeability of Perovskite-type Oxides of Ba0.5Sr0.5Co0.8Fe0.1R0.1O3−δ (R = Al, Mn, Fe, Ce, Cr, Ni, Co). J. Mater. Sci. Technol. 2012, 28, 177–183. [Google Scholar] [CrossRef]
- Niedrig, C.; Taufall, S.; Burriel, M.; Menesklou, W.; Wagner, S.F.; Baumann, S.; Ivers-Tiffée, E. Thermal stability of the cubic phase in Ba0.5Sr0.5Co0.8Fe0.2O3−δ (BSCF)1. Solid State Ion. 2011, 197, 25–31. [Google Scholar] [CrossRef]
- Stevenson, J.W.; Armstrong, I.; Carneim, R.D.; Pederson, L.I.; Weber, W.J. Electrochemical Properties of Mixed Conducting Perovskites La1−xMxCo1−yFeyO3−δ (M = Sr,Ba,Ca). J. Electrochem. Soc. 1996, 143, 2722–2729. [Google Scholar] [CrossRef]
- Li, S.; Jin, W.; Huang, P.; Xu, N.; Shi, J.; Hu, M.Z.; Payzant, E.A. Comparison of Oxygen Permeability and Stability of Perovskite Type La0.2A0.8Co0.2Fe0.8O3−δ (A = Sr,Ba,Ca) Membranes. Solid State Ion. 1999, 38, 2963–2972. [Google Scholar] [CrossRef]
- Li, X.; Kerstiens, T.; Markus, T. Oxygen permeability and phase stability of Ba0.5Sr0.5Co0.8Fe0.2O3−δ perovskite at at intermediate temperatures. J. Membr. Sci. 2013, 438, 83–89. [Google Scholar] [CrossRef]
- Baumann, S.; Serra, J.M.; Lobera, M.P.; Escolástico, S.; Schulze-küppers, F.; Meulenberg, W.A. Ultrahigh oxygen permeation flux through supported Ba0.5Sr0.5Co0.8Fe0.2O3−δ membranes. J. Membr. Sci. 2011, 377, 198–205. [Google Scholar] [CrossRef]
- Shubnikova, E.V.; Bragina, O.A.; Nemudry, A.P. Mixed conducting molybdenum doped BSCF materials. J. Ind. Eng. Chem. 2018, 59, 242–250. [Google Scholar] [CrossRef]
- Zhang, X.; Motuzas, J.; Liu, S.; da Costa, J.C.D. Zinc-doped BSCF perovskite membranes for oxygen separation. Sep. Purif. Technol. 2017, 189, 399–404. [Google Scholar] [CrossRef]
- Fan, C.-G.; Huang, X.-X.; Liu, W.; Chen, C.-S. Preparation and Oxygen Permeation for SrCo0.8Fe0.2O3−δ Tubular Asymmetric Membrane. J. Inorg. Mater. 2008, 23, 1221–1224. [Google Scholar] [CrossRef]
- Shao, X.; Wang, Z.; Xu, S.; Xie, K.; Hu, X.; Dong, D.; Parkinson, G.; Li, C. Microchannel structure of ceramic membranes for oxygen separation. J. Eur. Ceram. Soc. 2016, 36, 3193–3199. [Google Scholar] [CrossRef] [Green Version]
- Mazanec, T.J.; Cable, T.L.; Frye, J.G. Electrocatalytic cells for chemical reaction. Solid State Ion. 1992, 56, 111–118. [Google Scholar] [CrossRef]
- Chen, C.S.; Boukamp, B.A.; Bouwmeester, H.J.M.; Cao, G.Z.; Kruidhof, H.; Winnubst, A.J.A.; Burggraaf, A.J. Microstructural development, electrical properties and oxygen permeation of zirconia-palladium composites. Solis State Ion. 1995, 76, 23–28. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Lin, Y.S. Synthesis and oxygen permeation properties of ceramic-metal dual-phase membranes. J. Membr. Sci. 2000, 167, 123–133. [Google Scholar] [CrossRef]
- Kim, J.; Lin, Y.S. Synthesis and oxygen-permeation properties of thin YSZ/Pd composite membranes. AIChE J. 2000, 46, 1521–1529. [Google Scholar] [CrossRef]
- He, W.; Huang, H.; Gao, J.; Winnubst, L.; Chen, C. Phase-inversion tape casting and oxygen permeation properties of supported ceramic membranes. J. Membr. Sci. 2014, 452, 294–299. [Google Scholar] [CrossRef]
- Han, D.; Sunarso, J.; Tan, X.; Yan, Z.; Liu, L.; Liu, S. Optimizing Oxygen Transport Through La0.6Sr0.4Co0.2Fe0.8O3−δ Hollow Fiber by Microstructure Modification and Ag/Pt Catalyst Deposition. Energy Fuels 2012, 26, 4728–4734. [Google Scholar] [CrossRef]
- Schulze-Küppers, F.; Baumann, S.; Meulenberg, W.A.; Stöver, D.; Buchkremer, H.-P. Manufacturing and performance of advanced supported Ba0.5Sr0.5Co0.8Fe0.2O3−δ (BSCF) oxygen transport membranes. J. Membr. Sci. 2013, 433, 121–125. [Google Scholar] [CrossRef]
- Chena, C.S.; Kruidhof, H.; Verweij, H.; Burggraaf, A.J. Oxygen permeation through oxygen ion oxide-noble metal dual phase composites. Solid State Ion. 1996, 88, 569–572. [Google Scholar] [CrossRef]
- Wu, K.; Xie, S.; Jiang, G.S.; Liu, W.; Chen, C.S. Oxygen permeation through (Bi2O3)0.74(SrO)0.26-Ag (40%v/o) composite. J. Membr. Sci. 2001, 188, 189–193. [Google Scholar] [CrossRef]
- Capoen, E.; Steil, M.; Nowogrocki, G.; Malys, M.; Pirovano, C.; Lofberg, a.; Bordesrichard, E.; Boivin, J.; Mairesse, G.; Vannier, R. Oxygen permeation in bismuth-based materials. Part I: Sintering and oxygen permeation fluxes. Solid State Ion. 2006, 177, 483–488. [Google Scholar] [CrossRef] [Green Version]
- Balager, M.; Garcia-Fayos, J.; Solis, C.; Serra, J.M. Fast Oxygen Separation Through SO2- and CO2-Stable Dual-Phase Membrane Membrane Based on NiFe2O4–Ce0.8Tb0.2O2−δ. Chem. Mater. 2013, 25, 4986–4993. [Google Scholar] [CrossRef]
- Shaula, A.L.; Kharton, V.V.; Marques, F.M.B.; Kovalevsky, A.V.; Viskup, A.P.; Naumovich, E.N. Oxygen permeability of mixed-conducting composite membranes: Effects of phase interaction. J. Solid State Electrochem. 2005, 10, 28–40. [Google Scholar] [CrossRef]
- Lide, D.R. (Ed.) CRC Handbook of Chemistry and Physics; CRC: Boca Raton, FL, USA, 2005. [Google Scholar]
- Luo, H.; Jiang, H.; Klande, T.; Cao, Z.; Liang, F.; Wang, H.; Caro, J. Novel Cobalt-Free, Noble Metal-Free Oxygen-Permeable 40Pr0.6Sr0.4FeO3−δ-60Ce0.9Pr0.1O2−δ Dual-phase Membrane. Cem. Mater. 2012, 24, 2148–2154. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, W.; Zhu, Z.; Liu, T.; Liu, W. A novel cobalt-free, CO2-stable, and reduction-tolerant dual-phase oxygen-permeable membrane. Appl. Mater. Interfaces 2013, 5, 11038–11043. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Zhao, H.; Xu, N.; Li, Y.; Lu, X.; Ding, W.; Li, F. Synthesis and oxygen permeation properties of a Ce0.8Sm0.2O2−δ-LaBaCo2O5+δ dual-phase composite membrane. J. Membr. Sci. 2011, 370, 158–165. [Google Scholar] [CrossRef]
- Kharton, V.V.; Kovalensky, A.V.; Viskup, A.P.; Figueiredo, F.M.; Yaremchenko, A.A.; Naumovich, E.N. Oxygen permeability and Faradaic efficiency of Ce0.8Gd0.2O2−d-La0.7Sr0.3MnO3−d composite. J. Am. Chem. Soc. 2001, 21, 1763–1767. [Google Scholar]
- Shao, X.; Dong, D.; Parkinson, G.; Li, C.-Z. Improvement of oxygen permeation through microchanneled ceramic membranes. J. Membr. Sci. 2014, 454, 444–450. [Google Scholar] [CrossRef]
- Xue, J.; Liao, Q.; Wei, Y.; Li, Z.; Wang, H. A CO2-tolerance oxygen permeable 60Ce0.9Gd0.1O2−δ-40Ba0.5Sr0.5Co0.8Fe0.2O3−δ dual phase membrane. J. Membr. Sci. 2013, 443, 124–130. [Google Scholar] [CrossRef]
- Joong, K.; Marina, O.A. Highly stable dual-phase Y0.8Ca0.2Cr0.8Co0.2O3-Sm0.2Ce0.8O1.9 ceramiccomposite membrane for oxygen separation. J. Membr. Sci. 2016, 499, 301–306. [Google Scholar] [CrossRef]
- Adler, S.B. Chemical Expansivity of Electrochemical Ceramics. J. Am. Ceram. Soc. 2001, 19, 2117–2119. [Google Scholar] [CrossRef]
- Marrocchelli, D.; Bishop, S.R.; Tuller, H.L.; Yildiz, B. Understanding Chemical Expansion in Non-Stoichiometric Oxides: Ceria and Zirconia Case Studies. Adv. Funct. Mater. 2012, 22, 1958–1965. [Google Scholar] [CrossRef]
- Omar, S.; Nino, J.C. Consistency in the chemical expansion of fluorites: A thermal revision of the doped ceria. Acta Mater. 2013, 61, 5406–5413. [Google Scholar] [CrossRef]
- Bishop, S.R.; Duncan, K.L.; Wachsman, E.D. Surface and bulk oxygen non-stoichiometry and bulk chemical expansion in gadolinium-doped cerium oxide. Acta Mater. 2009, 57, 3596–3605. [Google Scholar] [CrossRef]
- Yaremchenko, A.A.; Mikhalev, S.M.; Kravchenko, E.S.; Frade, J.R. Thermochemical expansion of mixed-conducting (Ba,Sr)Co0.8Fe0.2O3−δ ceramics. J. Eur. Ceram. Soc. 2014, 34, 703–715. [Google Scholar] [CrossRef]
- Mcintosh, S.; Vente, J.F.; Haije, W.G.; Blank, D.H.A.; Bouwmeester, H.J.M. Phase stability and oxygen non-stoichiometry of SrCo0.8Fe0.2O3−δ measured by in situ neutron diffraction. Solis State Ion. 2006, 177, 833–842. [Google Scholar] [CrossRef]
- Kharton, V.V.; Viskup, A.P.; Kovalevsky, A.V.; Jurado, J.R.; Naumovich, E.N.; Vecher, A.A.; Frade, J.R. Oxygen ionic conductivity of Ti-containing strontium ferrite. Solis State Ion. 2000, 133, 57–65. [Google Scholar] [CrossRef]
- Wang, H.; Tablet, C.; Feldhoff, A.; Caro, J. Investigation of phase structure, sintering, and permeability of perovskite-type Ba0.5Sr0.5Co0.8Fe0.2O3−δ membranes. J. Membr. Sci. 2005, 262, 20–26. [Google Scholar] [CrossRef]
- Choi, M.-B.; Jeon, S.-Y.; Im, H.-N.; Wachsman, E.D.; Song, S.-J. Oxygen Exchange Kinetics and Ionic Conductivity from Chemical Expansion Relaxation of Mixed Conducting Ba0.5Sr0.5Co0.8Fe0.2O3−δ. J. Electrochem. Soc. 2012, 159, 23–28. [Google Scholar] [CrossRef]
- Bishop, S.R.; Duncan, K.L.; Wachsman, E.D. Thermo-Chemical Expansion in Strontium-Doped Lanthanum Cobalt Iron Oxide. J. Am. Ceram. Soc. 2010, 93, 4115–4121. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, S.; Meng, B.; Ding, J.; Tan, X. Preparation and the superior oxygen permeability of a new CO2-resistant Ruddlesden–Popper composite oxide Pr2Ni0.9Mo0.1O4+δ. J. Alloy. Compd. 2018, 742, 966–976. [Google Scholar] [CrossRef]
- Švarcová, S.; Wiik, K.; Tolchard, J.; Bouwmeester, H.J.M.; Grande, T. Structural instability of cubic perovskite BaxSr1−xCo1−yFeyO3−δ. Solid State Ion. 2008, 178, 1787–1791. [Google Scholar] [CrossRef]
- Pei, S.; Kleefisch, M.S.; Kobylinski, T.P.; Faber, J.; Udovich, C.A.; Zhang-McCoy, V.; Dabrowski, B.; Balachandran, U.; Mieville, R.L.; Poeppel, R.B. Failure mechanisms of ceramic membrane reactors in partial oxidation of methane to synthesis gas. Catal. Lett. 1995, 30, 201–212. [Google Scholar] [CrossRef]
- Balachandran, U.; Dusek, J.T.; Maiya, P.S.; Ma, B.; Mieville, R.L.; Kleefisch, M.S.; Udovich, C.A. Ceramic membrane reactor for converting methane to syngas. Catal. Today 1997, 36, 265–272. [Google Scholar] [CrossRef]
- Prado, F.; Grunbaum, N.; Caneiro, A.; Manthiram, A. Effect of La3+doping on the perovskite-to-brownmillerite transformation in Sr1−xLaxCo0.8Fe0.2O3−δ(0 ≤ x ≤ 0.4). Solid State Ion. 2004, 167, 147–154. [Google Scholar] [CrossRef]
- Yang, W.; Wang, H.; Zhu, X.; Lin, L. Development and application of oxygen permeable membrane in selective oxidation of light alkanes. Top. Catal. 2005, 35, 155–167. [Google Scholar] [CrossRef]
- Arikawa, H.; Yamada, T.; Ishihara, T.; Nishiguchi, H.; Takita, Y. Mixed Electronic-Oxide Ionic Conductivity and Oxygen Permeating Property in Ni Doped LaGaO3 Perovskite Oxide. Chem. Lett. 1999, 135, 1257–1258. [Google Scholar] [CrossRef]
- Fang, S.M.; Yoo, C.; Bouwmeester, H.J.M. Performance and stability of niobium-substituted Ba0.5Sr0.5Co0.8Fe0.2O3−δ membranes. Solid State Ion. 2011, 195, 1–6. [Google Scholar] [CrossRef]
- Meng, X.; Yang, N.; Meng, B.; Tan, X.; Ma, Z.-F.; Liu, S. Zirconium stabilized Ba0.5Sr0.5(Co0.8−xZrx)Fe0.2O3−α perovskite hollow fibre membranes for oxygen separation. Ceram. Int. 2011, 37, 2701–2709. [Google Scholar] [CrossRef]
- Qiu, L.; Lee, T.H.; Liu, L.; Yang, Y.L.; Jacobson, A.J. Oxygen permeation studies of SrCo0.8Fe0.2O3−δ. Solid State Ion. 1995, 76, 321–329. [Google Scholar] [CrossRef]
- Takeda, Y.; Kanno, K.; Takada, T.; Takano, M.; Nakayama, N. Phase Relation in the Oxygen Nonstoichiometric System, SrFeOx (2.5 < x < 3.0). J. Solid State Chem. 1986, 249, 237–249. [Google Scholar]
- Shao, Z.; Yang, W.; Cong, Y.; Dong, H.; Tong, J.; Xiong, G. Investigation of the permeation behavior and stability of a Ba0.5Sr0.5Co0.8Fe0.2O(3−δ) oxygen membrane. J. Membr. Sci. 2000, 172, 177–188. [Google Scholar] [CrossRef]
- Shao, Z.; Xiong, G.; Dong, H.; Yang, W.; Lin, L. Synthesis, oxygen permeation study and membrane performance of a Ba0.5Sr0.5Co0.8Fe0.2O3−δ oxygen-permeable dense ceramic reactor for partial oxidation of methane to syngas. Sep. Purif. Technol. 2001, 25, 97–116. [Google Scholar] [CrossRef]
- Unger, L.S.; Ruhl, R.; Meffert, M.; Niedrig, C.; Menesklou, W.; Wagner, S.F.; Gerthsen, D.; Bouwmeester, H.J.M.; Ivers-Tiffée, E. Yttrium doping of Ba0.5Sr0.5Co0.8Fe0.2O3−δ part II: Influence on oxygen transport and phase stability. J. Eur. Ceram. Soc. 2018, 38, 2388–2395. [Google Scholar] [CrossRef]
- Ravkina, O.; Klande, T.; Feldhoff, A. Investigation of Zr-doped BSCF perovskite membrane for oxygen separation in the intermediate temperature range. J. Solid State Chem. 2013, 201, 101–106. [Google Scholar] [CrossRef]
- Ravkina, O.; Yaremchenko, A.; Feldhoff, A. Phase separation in BSCF perovskite under elevated oxygen pressures ranging from 1 to 50 bar. J. Membr. Sci. 2016, 520, 76–88. [Google Scholar] [CrossRef] [Green Version]
- Liang, F.; Partovi, K.; Jiang, H.; Luo, H.; Caro, J. B-site La-doped BaFe0.95−xLaxZr0.05O3−δ perovskite-type membranes for oxygen separation. J. Mater. Chem. A 2013, 1, 746–751. [Google Scholar] [CrossRef]
- Tan, X.; Shi, L.; Hao, G.; Meng, B.; Liu, S. La0.7Sr0.3FeO3−α perovskite hollow fiber membranes for oxygen permeation and methane conversion. Sep. Purif. Technol. 2012, 96, 89–97. [Google Scholar] [CrossRef]
- Lein, H.L.; Wiik, K.; Grande, T. Kinetic demixing and decomposition of oxygen permeable membranes. Solid State Ion. 2006, 177, 1587–1590. [Google Scholar] [CrossRef]
- Zhu, X.; Yang, W. Mixed Conducting Ceramic Membranes, Green Chemistry and Sustainable Technology; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar] [CrossRef]
- Kharton, V.V. Handbook of Solid Fuel State Electrochemistry: Fundamentals, Methodologies, Applications; Wiley-VCH: Wernheim, Germany, 2009. [Google Scholar]
- Chi, X.; Zhang, J.; Wen, Z.; Liu, Y. Modified Pechini Synthesis of Proton-Conducting Ba(Ce,Ti)O3 and Comparative Studies of the Effects of Acceptors on its Structure, Stability, Sinterability, and Conductivity. J. Am. Ceram. Soc. 2014, 1109, 1103–1109. [Google Scholar] [CrossRef]
- Zhu, Z.; Sun, W.; Yan, L.; Liu, W.; Liu, W. Synthesis and hydrogen permeation of Ni-Ba(Zr0.1Ce0.7Y0.2)O3−δ metal e ceramic asymmetric membranes. Int. J. Hydrog. Energy 2011, 36, 6337–6342. [Google Scholar] [CrossRef]
- Kathiraser, Y.; Wang, Z.; Yang, N.-T.; Zahid, S.; Kawi, S. Oxygen permeation and stability study of La0.6Sr0.4Co0.8Ga0.2O3−δ (LSCG) hollow fiber membrane with exposure to CO2, CH4 and He. J. Membr. Sci. 2013, 427, 240–249. [Google Scholar] [CrossRef]
- Jaiswal, S.K.; Kumar, J. A novel series of Ba0.5Sr0.5Al0.2−xMgxFe0.8O3−ξ (x ≤ 0.2) membranes for oxygen permeation application. J. Eur. Ceram. Soc. 2014, 34, 381–390. [Google Scholar] [CrossRef]
- Cheng, H.; Luo, L.; Yao, W.; Lu, X.; Zou, X.; Zhou, Z. Novel cobalt-free CO2-tolerant dual-phase membranes of Ce0.8Sm0.2O2−δ-Ba0.95La0.05Fe1−xZrxO3−δ for oxygen separation. J. Membr. Sci. 2015, 492, 220–229. [Google Scholar] [CrossRef]
- Arnold, M.; Wang, H.; Feldhoff, A. Influence of CO2 on the oxygen permeation performance and the microstructure of perovskite-type (Ba0.5Sr0.5)(Co0.8Fe0.2)O3−δ membranes. J. Membr. Sci. 2007, 293, 44–52. [Google Scholar] [CrossRef]
- Efimov, K.; Klande, T.; Juditzki, N.; Feldhoff, A. Ca-containing CO2-tolerant perovskite materials for oxygen separation. J. Membr. Sci. 2012, 389, 205–215. [Google Scholar] [CrossRef]
- Li, K.; Zhao, H.; Lu, Y.; Ma, Y.; Du, Z.; Zhang, Z. High CO2 tolerance oxygen permeation membranes BaFe0.95−xCa0.05TixO3−δ. J. Membr. Sci. 2018, 550, 302–312. [Google Scholar] [CrossRef]
- Waindich, A.; Möbius, A.; Müller, M. Corrosion of Ba1−xSrxCo1−yFeyO3−δ and La0.3Ba0.7Co0.2Fe0.8O3−δ materials for oxygen separating membranes under Oxycoal conditions. J. Membr. Sci. 2009, 337, 182–187. [Google Scholar] [CrossRef]
- Zeng, Q.; Zuo, Y.B.; Fan, C.G.; Chen, C.S. CO2-tolerant oxygen separation membranes targeting CO2 capture application. J. Membr. Sci. 2009, 335, 140–144. [Google Scholar] [CrossRef]
- Yi, J.; Schroeder, M.; Weirich, T.; Mayer, J. Behavior of Ba(Co,Fe,Nb)O3−δ Perovskite in CO2-Containing Atmospheres: Degradation Mechanism and Materials Design. Chem. Mater. 2010, 6246–6253. [Google Scholar] [CrossRef]
- Wang, Z.; Kathiraser, Y.; Kawi, S. High performance oxygen permeable membranes with Nb-doped BaBi0.05Co0.95O3−δ perovskite oxides. J. Membr. Sci. 2013, 431, 180–186. [Google Scholar] [CrossRef]
- Chen, W.; Chen, C.; Winnubst, L. Ta-doped SrCo0.8Fe0.2O3−δ membranes: Phase stability and oxygen permeation in CO2 atmosphere. Solid State Ion. 2011, 196, 30–33. [Google Scholar] [CrossRef]
- Yi, J.; Weirich, T.E.; Schroeder, M. CO2 corrosion and recovery of perovskite-type BaCo1-x-yFexNbyO3−δ membranes. J. Membr. Sci. 2013, 437, 49–56. [Google Scholar] [CrossRef]
- Popov, M.P.; Bychkov, S.F.; Nemudry, A.P. Modification of mixed conducting Ba0.5Sr0.5Co0.8Fe0.2O3–δ by partial substitution of cobalt with tungsten. Russ. J. Electrochem. 2016, 52, 648–654. [Google Scholar] [CrossRef]
- Kharton, V.V.; Viskup, A.P.; Naumovich, E.N.; Lapchuk, N.M. Mixed electronic and ionic conductivity of LaCo(M)O3 (M = Ga, Cr, Fe or Ni). I. Oxygen transport in perovskites LaCoO3-LaGaO3. Solid State Ion. 1997, 104, 67–78. [Google Scholar] [CrossRef]
- Carolan, M.F.; Dyer, P.N.; LaBar, J.M., Sr.; Thorogood, R.M. Process for Restoring Permeance of an Oxygen-Permeable on Transport Membrane Utilized to Recover Oxygen Froman Oxygen-Contaning Gaseous mixture. Patent Application CA002104821A, August 1993. [Google Scholar]
- Yi, J.; Feng, S.; Zuo, Y.; Liu, W.; Chen, C. Oxygen permeability and stability of Sr0.95Co0.8Fe0.2O3−δ in a CO2-and H2O-containing atmosphere. Chem. Mater. 2005, 17, 5856–5861. [Google Scholar] [CrossRef]
- Wang, H.; Kölsch, P.; Schiestel, T.; Tablet, C.; Werth, S.; Caro, J. Production of high-purity oxygen by perovskite hollow fiber membranes swept with steam. J. Membr. Sci. 2006, 284, 5–8. [Google Scholar] [CrossRef]
- Cheng, S.; Søgaard, M.; Han, L.; Zhang, W.; Chen, M.; Kaiser, A.; Hendriksen, P.V. A novel CO2- and SO2-tolerant dual phase composite membrane for oxygen separation. Chem. Commun. 2015, 51, 7140–7143. [Google Scholar] [CrossRef] [PubMed]
- Khatib, S.J.; Yun, S.; Oyama, S.T. Sulfur resistant Pd and Pd alloy membranes by phosphidation. J. Membr. Sci. 2014, 455, 283–293. [Google Scholar] [CrossRef]
- Gao, J.; Li, L.; Yin, Z.; Zhang, J.; Lu, S.; Tan, X. Poisoning effect of SO2 on the oxygen permeation behavior of La0.6Sr0.4Co0.2Fe0.8O3−δ perovskite hollow fiber membranes. J. Membr. Sci. 2014, 455, 341–348. [Google Scholar] [CrossRef]
- Alqaheem, Y.; Thurs, A.; Zhang, G.; Metcalfe, I.S. The impact of sulfur contamination on the performance of La0.6Sr0.4Co0.2Fe0.8O3−δ oxygen transport membranes. Solid State Ion. 2014, 262, 262–265. [Google Scholar] [CrossRef]
- Wei, Y.; Ravkina, O.; Klande, T.; Wang, H.; Feldhoff, A. Effect of CO2 and SO2 on oxygen permeation and microstructure of (Pr0.9La0.1)2(Ni0.74Cu0.21Ga0.05)O4+δ membranes. J. Membr. Sci. 2013, 429, 147–154. [Google Scholar] [CrossRef]
- Wei, Y.; Liao, Q.; Xue, J.; Li, Z.; Wang, H. Influence of SO2 on the phase structure, oxygen permeation and microstructure of K2NiF4-type hollow fiber membranes. Chem. Eng. J. 2013, 217, 34–40. [Google Scholar] [CrossRef]
- Garcia-Fayos, J.; Balaguer, M.; Baumann, S.; Serra, J.M. Dual-phase membrane based on LaCo0.2Ni0.4Fe0.4O3−x-Ce0.8Gd0.2O2−x composition for oxygen permeation under CO2/SO2-rich gas environments. J. Membr. Sci. 2018, 548, 117–124. [Google Scholar] [CrossRef]
- Wu, C.; Zhou, J.; Tang, X.; Luo, W.; Zhang, Y.; Ding, W.; Sun, C. The migration behavior of sulfur impurity contained in the dual-phase membrane of Ce0.9Gd0.1O2−δ-SrCo0.8Fe0.1Nb0.1O3−δunder CO2atmosphere. J. Membr. Sci. 2016, 511, 162–169. [Google Scholar] [CrossRef]
- Diethelm, S.; Van, J.; Sfeir, J.; Buffat, P. Correlation between oxygen transport properties and microstructure in La0.5Sr0.5FeO3−δ. J. Eur. Ceram. Soc. 2005, 25, 2191–2196. [Google Scholar] [CrossRef]
- Martynczuk, J.; Arnold, M.; Feldhoff, A. Influence of grain size on the oxygen permeation performance of perovskite-type (Ba0.5Sr0.5)(Fe0.8Zn0.2)O3−δ membranes. J. Membr. Sci. 2008, 322, 375–382. [Google Scholar] [CrossRef]
- Li, Q.; Zhu, X.; He, Y.; Cong, Y.; Yang, W. Effects of sintering temperature on properties of dual-phase oxygen permeable membranes. J. Membr. Sci. 2011, 367, 134–140. [Google Scholar] [CrossRef]
- Wagner, C. Diffusion and high temperature oxidation of metals. At. Mov. 1951, 194–199, 153–173. [Google Scholar]
- Phair, J.W.; Badwal, S.P.S.Ã. Materials for separation membranes in hydrogen and oxygen production and future power generation. Sci. Technol. Adv. Mater. 2006, 7, 792–805. [Google Scholar] [CrossRef] [Green Version]
- Pinacci, P.; Louradour, E.; Wimbert, L.; Gindrat, M.; Jarligo, M.O.; Vassen, R.; Comite, a.; Serra, J.M.; Jewulski, J.; Mancuso, L.; Chiesa, P.; et al. Dense Membranes for Oxygen and Hydrogen Separation (DEMOYS): Project Overview and First Results. Energy Procedia 2013, 37, 1030–1038. [Google Scholar] [CrossRef] [Green Version]
- Buchler, O.; Serra, J.; Meulenberg, W.; Sebold, D.; Buchkremer, H. Preparation and properties of thin La1−xSrxCo1−yFeyO3−δ perovskitic membranes supported on tailored ceramic substrates. Solid State Ion. 2007, 178, 91–99. [Google Scholar] [CrossRef]
- Bouchara, A.; Grosso, D.; Durand, D.; Sanchez, C. Controlled formation of highly ordered cubic and hexagonal mesoporous nanocrystalline yttria–zirconia and ceria–zirconia thin films exhibiting high thermal stability. Angew. Chem. 2003, 42, 347–351. [Google Scholar]
- Corma, A.; Atienzar, P.; García, H.; Chane-Ching, J.-Y. Hierarchically mesostructured doped CeO2 with potential for solar-cell use. Nat. Mater. 2004, 3, 394–397. [Google Scholar] [CrossRef] [PubMed]
- Serra, J.M.; Uhlenbruck, S.; Meulenberg, W.A.; Buchkremer, H.P.; Sto, D. Nano-structuring of solid oxide fuel cells cathodes. Top. Catal. 2006, 40, 123–131. [Google Scholar] [CrossRef]
- Gurauskis, J.; Lohne, Ø.F.; Wiik, K. La0.2Sr0.8Fe0.8Ta0.2O3−δ based thin film membranes with surface modification for oxygen production. Solid State Ion. 2012, 225, 703–706. [Google Scholar] [CrossRef]
- Cheng, S.; Huang, H.; Ovtar, S.; Simonsen, S.B.; Chen, M.; Zhang, W.; Søgaard, M.; Kaiser, A.; Hendriksen, P.V.; Chen, C. High-Performance Microchanneled Asymmetric Gd0.1Ce0.9O1.95−δ-La0.6Sr0.4FeO3−δ-Based Membranes for Oxygen Separation. Appl. Mater. Interfaces 2016, 3, 4548–4560. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.P.; Park, J.H.; Magnole, E.; Lee, Y. Significant improvement of the oxygen permeation flux of tubular Ba0.5Sr0.5Co0.8Fe0.2O3−δ membranes covered by a thin La0.6Sr0.4Ti0.3Fe0.7O3−δ layer. Mater. Lett. 2011, 65, 2168–2170. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, H.; Li, Q.; Cong, Y.; Yang, W. Unsteady-state permeation and surface exchange of dual-phase membranes. Solid State Ion. 2011, 185, 27–31. [Google Scholar] [CrossRef] [Green Version]
- Hayamizu, Y.; Kato, M.; Takamura, H. Effects of surface modification on the oxygen permeation of Ba0.5Sr0.5Co0.8Fe0.2O3−δ membrane. J. Membr. Sci. 2014, 462, 147–152. [Google Scholar] [CrossRef]
- Serra, J.M.; Meulenberg, W.A. Thin-Film Proton BaZr0.85Y0.15O3 Conducting Electrolytes: Toward an Intermediate-Temperature Solid Oxide Fuel Cell Alternative. J. Am. Ceram. Soc. 2007, 90, 2082–2089. [Google Scholar] [CrossRef]
- Jacobs, M.; Fontaine, M.; Bredesen, R.; Michielsen, B.; Middelkoop, V.; Larring, Y. Surface activation of asymmetric CaTi1−xFexO3−δ tubular membranes forfor oxygen separation. J. Membr. Sci. 2015, 477, 58–64. [Google Scholar] [CrossRef]
- Liu, T.; Chen, Y.; Fang, S.; Lei, L.; Wang, Y.; Ren, C.; Chen, F. A dual-phase bilayer oxygen permeable membrane with hierarchically porous structure fabricated by freeze-drying tape-casting method. J. Membr. Sci. 2016, 520, 354–363. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Zhang, C.; Zhao, L.; Meng, B.; Liu, J.; Liu, S. Enhanced Oxygen Permeation Behavior of Ba0.5Sr0.5Co0.8Fe0.2O3−δ Membranes in a CO2-Containing Atmosphere with a Sm0.2Ce0.8O1.9 Functional Functional Shell. Energy Fuels 2016, 30, 1829–1834. [Google Scholar] [CrossRef]
- Kasai, M.; Miyazawa, A.; Suzuki, T.M. Controlled Heating of Palladium Dispersed Porous Alumina Tube and Continuous Oxidation of Ethylene Using Frequency-Variable Single-Mode Microwave Reactor. Ind. Eng. Chem. Res. 2014, 53, 1073–1078. [Google Scholar] [CrossRef]
- Hu, X.; Chen, W.; Huang, Y. Fabrication of Pd/ceramic membranes for hydrogen separation based on low-cost macroporous ceramics with pencil coating. Int. J. Hydrog. Energy 2010, 35, 7803–7808. [Google Scholar] [CrossRef]
- Callister, W.D.; Wiley, J. Thermal Properties. In Materials Science and Engineering; John Wiley and Sons: Hoboken, NJ, USA, 2007; pp. 713–729. [Google Scholar]
- Ovtar, S.; Gurauskis, J.; Haugen, A.B.; Chatzichristodoulou, C.; Kaiser, A.; Hendriksen, P.V. Oxygen transport properties of tubular Ce0.9Gd0.1O1.95-La0.6Sr0.4FeO3−d composite asymmetric oxygen permeation membranes supported on magnesium oxide. J. Membr. Sci. 2017, 523, 576–587. [Google Scholar] [CrossRef]
- Yin, X.; Hong, L.; Liu, Z. Development of oxygen transport membrane La0.2Sr0.8CoO3−δ/Ce0.8Gd0.2O2−δ on the tubular CeO2 support. Appl. Catal. A Gen. 2006, 300, 75–84. [Google Scholar] [CrossRef]
- Fan, E.S.C.; Kesler, O. Deposition of Lanthanum Strontium Cobalt Ferrite (LSCF) Using Suspension Plasma Spraying for Oxygen Transport Membrane Applications. J. Therm. Spray Technol. 2015, 24, 1081–1092. [Google Scholar] [CrossRef]
- Niu, S.; Zhou, K.; Xu, L.; Deng, C.; Liu, M.; Mao, J. A comparative study of La0.6Sr0.4Co0.2Fe0.8O3−δ oxygen transport membranes deposited on porous metal supports prepared by supersonic air-gas plasma spraying (SAPS) and low pressure plasma spraying-physical vapor deposition (PS-PVD). Surf. Coat. Technol. 2016, 307, 963–970. [Google Scholar] [CrossRef]
- Cao, Z.; Zhu, X.; Li, W.; Xu, B.; Yang, L.; Yang, W. Asymmetric dual-phase membranes prepared via tape-casting and co-lamination for oxygen permeation. Mater. Lett. 2015, 147, 88–91. [Google Scholar] [CrossRef]
- Escribano, J.A.; García-fayos, J.; Serra, J.M. Shaping of 3YSZ porous substrates for oxygen separation membranes. J. Eur. Ceram. Soc. 2017, 37, 5223–5231. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, R.; He, Z.; Gao, J.; Chen, C. Phase inversion tape casting and oxygen permeation properties of composite membrane. Solid State Ion. 2016, 288, 342–346. [Google Scholar] [CrossRef]
- Lemes, P.; Motuzas, J.; Machado, R.A.F.; Hotza, D.; Diniz, J.C. Influence of porous structures on O2 flux of BSCF asymmetric membranes. Sep. Purif. Technol. 2017, 175, 164–169. [Google Scholar] [CrossRef]
- Li, Q.; Zhu, X.; Yang, W. Single-step fabrication of asymmetric dual-phase composite membranes for oxygen separation. J. Membr. Sci. 2008, 325, 11–15. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, G.; Dong, X.; Jiang, W.; Jin, W.; Xu, N. Fabrication of asymmetric tubular mixed-conducting dense membranes by a combined spin-spraying and co-sintering process. J. Membr. Sci. 2012, 415–416, 313–319. [Google Scholar] [CrossRef]
- Fontaine, M.; Smith, J.B.; Larring, Y.; Bredesen, R. On the preparation of asymmetric CaTi0.9Fe0.1O3−δ membranes by tape-casting and co-sintering process. J. Membr. Sci. 2009, 326, 310–315. [Google Scholar] [CrossRef]
- Yang, C.; Xu, Q.; Liu, C.; Liu, J.; Chen, C.; Liu, W. Bi1.5Y0.3Sm0.2O3-La0.8Sr0.2MnO3−δ dual-phase composite hollow fiber membrane for oxygen separation. Mater. Lett. 2011, 65, 3365–3367. [Google Scholar] [CrossRef]
- Chang, X.; Zhang, C.; Jin, W.; Xu, N. Match of thermal performances between the membrane and the support for supported dense mixed-conducting membranes. J. Membr. Sci. 2006, 285, 232–238. [Google Scholar] [CrossRef]
- Jin, W.; Li, S.; Huang, P.; Xu, N.; Shi, J. Preparation of an asymmetric perovskite-type membrane and its oxygen permeability. J. Membr. Sci. 2001, 185, 237–243. [Google Scholar] [CrossRef]
- Fang, W.; Gao, J.; Chen, C. La0.8Sr0.2Cr0.5Fe0.5O3−d (LSCF)-Zr0.8Y0.2O2−d (YSZ) based multilayer membrane for CO2 decomposition. Ceram. Int. 2013, 39, 7269–7272. [Google Scholar] [CrossRef]
- Fang, W.; Zhang, Y.; Gao, J.; Chen, C. Oxygen permeability of asymmetric membrane of functional La0.8Sr0.2Cr0.5Fe0.5O3−δ(LSCrF)-Zr0.8Y0.2O-δ(YSZ) supported on porousl porous YSZ. Ceram. Int. 2014, 40, 799–803. [Google Scholar] [CrossRef]
- Yin, X.; Hong, L.; Liu, Z.L. Oxygen permeation through the LSCO-80/CeO2 asymmetric tubular membrane reactor. J. Membr. Sci. 2006, 268, 2–12. [Google Scholar] [CrossRef]
- Geffroy, P.M.; Bassat, J.M.; Vivet, A.; Fourcade, S.; Chartier, T.; del Gallo, P.; Richet, N. Oxygen semi-permeation, oxygen diffusion and surface exchange coefficient of La(1−x)SrxFe(1−y)GayO3−δ perovskite membranes. J. Membr. Sci. 2010, 354, 6–13. [Google Scholar] [CrossRef]
- Trunec, M.; Cihlar, J.; Diethelm, S.; van Herle, J. Tubular La0.7Ca0.3Fe0.85Co0.15O3−δ perovskite membranes, Part I: Preparation and properties. J. Am. Ceram. Soc. 2006, 89, 949–954. [Google Scholar] [CrossRef]
- Zhu, X.; Sun, S.; Cong, Y.; Yang, W. Operation of perovskite membrane under vacuum and elevated pressures for high-purity oxygen production. J. Membr. Sci. 2009, 345, 47–52. [Google Scholar] [CrossRef]
- Salehi, M.; Pfaff, E.M.; Junior, R.M.; Bergmann, C.P.; Diethelm, S.; Neururer, C.; Graule, T.; Grobéty, B.; Clemens, F.J. Ba0.5Sr0.5Co0.8Fe0.2O3−δ (BSCF) feedstock development and optimization for thermoplastic forming of thin planar and tubular oxygen separation membranes. J. Membr. Sci. 2013, 443, 237–245. [Google Scholar] [CrossRef]
- Wu, Z.; Othman, N.H.; Zhang, G.; Liu, Z.; Jin, W.; Li, K. Effects of fabrication processes on oxygen permeation of Nb2O5-doped SrCo0.8Fe0.2O3−δ micro-tubular membranes. J. Membr. Sci. 2013, 442, 1–7. [Google Scholar] [CrossRef]
- Bi, X.; Meng, X.; Liu, P.; Yang, N.; Zhu, Z.; Ran, R.; Liu, S. A novel CO2-resistant ceramic dual-phase hollow fiber membrane for oxygen separation. J. Membr. Sci. 2017, 522, 91–99. [Google Scholar] [CrossRef]
- Tan, X.; Liu, Y.; Li, K. Preparation of LSCF Ceramic Hollow-Fiber Membranes for Oxygen Production by a Phase-Inversion/Sintering Technique. Ind. Eng. Chem. Res. 2005, 44, 61–66. [Google Scholar] [CrossRef]
- Liu, H.; Tan, X.; Pang, Z.; da Costa, J.C.D.; Lu, G.Q.; Liu, S. Novel dual structured mixed conducting ceramic hollow fibre membranes. Sep. Purif. Technol. 2008, 63, 243–247. [Google Scholar] [CrossRef]
- Huang, H.; Cheng, S.; Gao, J.; Chen, C.; Yi, J. Phase-inversion tape-casting preparation and significant performance enhancement of Ce0.9Gd0.1O1.95-La0.6Sr0.4Co0.2Fe0.8O3−δ dual-phase asymmetric membrane for oxygen separation. Mater. Lett. 2014, 137, 245–248. [Google Scholar] [CrossRef]
- Shao, X.; Dong, D.; Parkinson, G.; Li, C.Z. Thin ceramic membrane with dendritic microchanneled sub structure and high oxygen permeation rate. J. Membr. Sci. 2017, 541, 653–660. [Google Scholar] [CrossRef]
- Liu, T.; Zhao, W.; Wang, Y. Robust Freeze-Cast Bilayer Dual-Phase Oxygen Transport Membrane Targeting Chemical Reactor Application. ACS Appl. Nano Mater. 2018, 1, 3774–3778. [Google Scholar] [CrossRef]
- Stansch, Z.; Mleczko, L.; Baerns, M. Comprehensive kinetics of oxidative coupling of methane over the La2O3/CaO catalyst. Ind. Eng. Chem. Res. 1997, 36, 2568–2579. [Google Scholar] [CrossRef]
- Cruellas, A.; Melchiori, T.; Gallucci, F.; Annaland, M.v. Advanced reactor concepts for oxidative coupling of methane. Catal. Rev. 2018, 59, 234–294. [Google Scholar] [CrossRef]
- Tan, X.; Pang, Z.; Gu, Z.; Liu, S. Catalytic perovskite hollow fibre membrane reactors for methane oxidative coupling. J. Membr. Sci. 2007, 302, 109–114. [Google Scholar] [CrossRef]
- Lu, Y.; Dixon, A.G.; Moser, W.R.; Hua, Y.; Balachandran, U. Oxygen-permeable dense membrane reactor for the oxidative coupling of methane. J. Membr. Sci. 2000, 170, 27–34. [Google Scholar] [CrossRef]
- Czuprat, O.; Schiestel, T.; Voss, H. Oxidative Coupling of Methane in a BCFZ Perovskite Hollow Fiber Membrane Reactor. Ind. Eng. Chem. Res. 2010, 49, 10230–10236. [Google Scholar] [CrossRef]
- Wang, H.; Cong, Y.; Yang, W. Oxidative coupling of methane in Ba0.5Sr0.5Co0.8Fe0.2O3−δ tubular membrane reactors. Catal. Today 2005, 104, 160–167. [Google Scholar] [CrossRef]
- Olivier, L.; Haag, S.; Mirodatos, C.; van Veen, A.C. Oxidative coupling of methane using catalyst modified dense perovskite membrane reactors. Catal. Today 2009, 142, 34–41. [Google Scholar] [CrossRef]
- Akin, F.T.; Lin, Y.S. Controlled Oxidative Coupling of Methane by Ionic Conducting Ceramic Membrane. Catal. Lett. 2002, 78, 239–242. [Google Scholar] [CrossRef]
- Bhatia, S.; Thien, C.Y.; Mohamed, A.R. Oxidative coupling of methane (OCM) in a catalytic membrane reactor and comparison of its performance with other catalytic reactors. Chem. Eng. J. 2009, 148, 525–532. [Google Scholar] [CrossRef]
- Othman, N.H.; Wu, Z.; Li, K. An oxygen permeable membrane microreactor with an in-situ deposited Bi1.5Y0.3Sm0.2O3−δ catalyst for oxidative coupling of methane. J. Membr. Sci. 2015, 488, 182–193. [Google Scholar] [CrossRef]
- Santamaria, J.; Menéndez, M.; Pena, J.A.; Barahona, J.I. Methane oxidative coupling in fixed bed catalytic reactors with a distributed oxygen feed. A simulation study. Catal. Today 1992, 13, 353–360. [Google Scholar] [CrossRef]
- Coronas, J.; Menéndez, M.; Santamaria, J. Methane oxidative coupling using porous ceramic membrane reactors–II. Reaction studies. Chem. Eng. Sci. 1994, 49, 2015–2025. [Google Scholar] [CrossRef]
- Tiemersma, T.P.; Chaudhari, A.S.; Gallucci, F.; Kuipers, J.A.M.; Annaland, M.V. Integrated autothermal oxidative coupling and steam reforming of methane. Part 2: Development of a packed bed membrane reactor with a dual function catalyst. Chem. Eng. Sci. 2012, 82, 232–245. [Google Scholar] [CrossRef]
- Haag, S.; van Veen, A.C.; Mirodatos, C. Influence of oxygen supply rates on performances of catalytic membrane reactors. Catal. Today 2007, 127, 157–164. [Google Scholar] [CrossRef]
- York, A.P.E.; Xiao, T.; Green, M.L.H. Brief Overview of the Partial Oxidation of Methane to Synthesis Gas. Top. Catal. 2003, 22, 345–358. [Google Scholar] [CrossRef]
- Babakhani, E.G.; Towfighi, J.; Taheri, Z.; Pour, A.N.; Zekordi, M.; Taheri, A. Partial oxidation of methane in Ba0.5Sr0.5Co0.8Fe0.1Ni0.1O3−δ ceramic membrane reactor. J. Nat. Gas Chem. 2012, 21, 519–525. [Google Scholar] [CrossRef]
- Song, S.; Zhang, P.; Zhang, X.; Han, M. Partial oxidation of methane reaction in Ba0.9Co0.7Fe0.2Nb0.1O3−δ oxygen permeation membrane with three-layer structure. Int. J. Hydrog. Energy 2015, 40, 10894–10901. [Google Scholar] [CrossRef]
- Gong, Z.; Hong, L. Integration of air separation and partial oxidation of methane in the La0.4Ba0.6Fe0.8Zn0.2O3−δ membrane reactor. J. Membr. Sci. 2011, 380, 81–86. [Google Scholar] [CrossRef]
- Song, S.; Zhang, P.; Han, M.; Singhal, S.C. Oxygen permeation and partial oxidation of methane reaction in Ba0.9Co0.7Fe0.2Nb0.1O3−δ oxygen permeation membrane. J. Membr. Sci. 2012, 415, 654–662. [Google Scholar] [CrossRef]
- Kathiraser, Y.; Kawi, S. La0.6Sr0.4Co0.8Ga0.2O3−δ (LSCG) hollow fiber membrane reactor: Partial oxidation of methane at medium temperature. AIChE J. 2013, 59, 3874–3885. [Google Scholar] [CrossRef]
- Meng, X.; Bi, X.; Meng, B.; Yang, N.; Tan, X.; Liu, L.; Liu, S. H2/CH4/CO2-tolerant properties of SrCo0.8Fe0.1Ga0.1O3−δ hollow fiber membrane reactors for methane partial oxidation to syngas. Fuel Process. Technol. 2017, 161, 265–272. [Google Scholar] [CrossRef]
- Wang, Z.; Ashok, J.; Pu, Z.; Kawi, S. Low temperature partial oxidation of methane via BaBi0.05Co0.8Nb0.15O3−δ-Ni phyllosilicate catalytic hollow fiber membrane reactor. Chem. Eng. J. 2017, 315, 315–323. [Google Scholar] [CrossRef]
- Liao, Q.; Chen, Y.; Wei, Y.; Zhou, L.; Wang, H. Performance of U-shaped BaCo0.7Fe0.2Ta0.1O3−δ hollow-fiber membranes reactor with high oxygen permeation for methane conversion. Chem. Eng. J. 2014, 237, 146–152. [Google Scholar] [CrossRef]
- Wei, Y.; Liao, Q.; Li, Z.; Wang, H.; Feldhoff, A.; Caro, J. Partial oxidation of methane in hollow-fiber membrane reactors based on alkaline-earth metal-free CO2-tolerant oxide. AIChE J. 2014, 60, 3587–3595. [Google Scholar] [CrossRef]
- Zhu, J.; Guo, S.; Liu, G.; Liu, Z.; Zhang, Z.; Jin, W. A robust mixed-conducting multichannel hollow fiber membrane reactor. AIChE J. 2015, 61, 2592–2599. [Google Scholar] [CrossRef]
- Yuan, R.; He, Z.; Zhang, Y.; Wang, W.; Chen, C.; Wu, H.; Zhan, Z. Partial Oxidation of Methane to Syngas in a Packed Bed Catalyst Membrane Reactor. AIChE J. 2016, 62, 2170–2176. [Google Scholar] [CrossRef]
- Ruiz-Trejo, E.; Boldrin, P.; Medley-Hallam, J.L.; Darr, J.; Atkinson, A.; Brandon, N.P. Partial oxidation of methane using silver/gadolinia-doped ceria composite membranes. Chem. Eng. Sci. 2015, 127, 269–275. [Google Scholar] [CrossRef] [Green Version]
- Jiang, W.; Zhang, G.; Liu, Z.; Zhang, K.; Jin, W. A novel porous-dense dual-layer composite membrane reactor with long-term stability. AIChE J. 2013, 59, 4355–4363. [Google Scholar] [CrossRef]
- Balachandran, U.; Dusek, J.T.; Mieville, R.L.; Poeppel, R.B.; Kleefisch, M.S.; Pei, S.; Kobylinski, T.P.; Udovich, C.A.; Bose, A.C. Dense ceramic membranes for partial oxidation of methane to syngas. Appl. Catal. A Gen. 1995, 133, 19–29. [Google Scholar] [CrossRef]
- Caro, J.; Schiestel, T.; Werth, S.; Wang, H.; Kleinert, A.; Kölsch, P. Perovskite hollow fibre membranes in the partial oxidation of methane to synthesis gas in a membrane reactor. Desalination 2006, 199, 415–417. [Google Scholar] [CrossRef]
- Wei, Y.Y.; Huang, L.; Tang, J.; Zhou, L.Y.; Li, Z.; Wang, H.H. Syngas production in a novel perovskite membrane reactor with co-feed of CO2. Chin. Chem. Lett. 2011, 22, 1492–1496. [Google Scholar] [CrossRef]
- Jansen, L. Aristotle’s theory of dispositions from the principle of movement to the unmoved mover, Debating Dispositions: Issues in Metaphysics. Epistemol. Philos. Mind 2009, 202, 24–46. [Google Scholar] [CrossRef]
- Wang, H.; Cong, Y.; Yang, W. Continuous Oxygen Ion Transfer Medium as a Catalyst for High Selective Oxidative Dehydrogenation of Ethane. Catal. Lett. 2002, 84, 101–106. [Google Scholar] [CrossRef]
- Wang, H.; Tablet, C.; Schiestel, T.; Caro, J. Hollow fiber membrane reactors for the oxidative activation of ethane. Catal. Today 2006, 118, 98–103. [Google Scholar] [CrossRef]
- Rebeilleau-Dassonneville, M.; Rosini, S.; van Veen, A.C.; Farrusseng, D.; Mirodatos, C. Oxidative activation of ethane on catalytic modified dense ionic oxygen conducting membranes. Catal. Today 2005, 104, 131–137. [Google Scholar] [CrossRef]
- Lobera, M.P.; Escolástico, S.; Garcia-Fayos, J.; Serra, J.M. Ethylene Production by ODHE in Catalytically Modified Ba0.5Sr0.5Co0.8Fe0.2O3−δ Membrane Reactors. ChemSusChem 2012, 5, 1587–1596. [Google Scholar] [CrossRef] [PubMed]
- Lobera, M.P.; Escolástico, S.; Serra, J.M. High Ethylene Production through Oxidative Dehydrogenation of Ethane Membrane Reactors Based on Fast Oxygen-Ion Conductors. ChemCatChem 2011, 3, 1503–1508. [Google Scholar] [CrossRef]
- Wang, H.; Cong, Y.; Zhu, X.; Yang, W. Oxidative dehydrogenation of propane in a dense tubular membrane reactor. React. Kinet. Catal. Lett. 2003, 79, 351–356. [Google Scholar] [CrossRef]
- Yan, R.; Liu, W.; Song, C. Oxidative Dehydrogenation of Alkanes using Oxygen-Permeable Membrane Reactor. Chin. J. Chem. Phys. 2014, 27, 690–696. [Google Scholar] [CrossRef]
- Balachandran, U.; Lee, T.H.; Wang, S.; Dorris, S.E. Use of mixed conducting membranes to produce hydrogen by water dissociation. Int. J. Hydrog. Energy 2004, 29, 291–296. [Google Scholar] [CrossRef]
- Li, W.; Cao, Z.; Zhu, X.; Yang, W. High-rate hydrogen separation using an MIEC oxygen permeable membrane reactor. AIChE J. 2017, 63, 1278–1286. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, H.; Werth, S.; Schiestel, T.; Caro, J. Simultaneous Production of Hydrogen and Synthesis Gas by Combining Water Splitting with Partial Oxidation of Methane in a Hollow-Fiber Membrane Reactor. Angew. Chem. Int. Ed. 2008, 47, 9341–9344. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Steinbach, F.; Cao, Z.; Zhu, X.; Feldhoff, A. A Highly Efficient Sandwich-Like Symmetrical Dual-Phase Oxygen-Transporting Membrane Reactor for Hydrogen Production by Water Splitting. Angew. Chem. Int. Ed. 2016, 55, 8648–8651. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Jiang, H.; Luo, H.; Baumann, S.; Meulenberg, W.A.; Assmann, J.; Mleczko, L.; Liu, Y.; Caro, J. Natural Gas to Fuels and Chemicals: Improved Methane Aromatization in an Oxygen-Permeable Membrane Reactor. Angew. Chem. Int. Ed. 2013, 52, 13794–13797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- di Felice, L.; Middelkoop, V.; Anzoletti, V.; Snijkers, F.; Annaland, M.v.; Gallucci, F. New high temperature sealing technique and permeability data for hollow fiber BSCF perovskite membranes. Chem. Eng. Process. Process Intensif. 2016, 107, 206–219. [Google Scholar] [CrossRef] [Green Version]
- Qi, X.; Akin, F.T.; Lin, Y.S. Ceramic–glass composite high temperature seals for dense ionic-conducting ceramic membranes. J. Membr. Sci. 2001, 193, 185–193. [Google Scholar] [CrossRef]
- Vivet, A.; Geffroy, P.M.; Coudert, V.; Fouletier, J.; Richet, N.; Chartier, T. Influence of glass and gold sealants materials on oxygen permeation performances in La0.8Sr0.2Fe0.7Ga0.3O3−δ perovskite membranes. J. Membr. Sci. 2011, 366, 132–138. [Google Scholar] [CrossRef]
- Chen, Y.; Qian, B.; Hao, Y.; Liu, S.; Tade, M.O.; Shao, Z. Influence of sealing materials on the oxygen permeation fluxes of some typical oxygen ion conducting ceramic membranes. J. Membr. Sci. 2014, 470, 102–111. [Google Scholar] [CrossRef]
- Chen, H.; Li, L.; Kemps, R.; Michielsen, B.; Jacobs, M.; Snijkers, F.; Middelkoop, V. Reactive air brazing for sealing mixed ionic electronic conducting hollow fibre membranes. Acta Mater. 2015, 88, 74–82. [Google Scholar] [CrossRef]
- Deng, X.; Duquette, J.; Petric, A. Silver–Glass Composite for High Temperature Sealing. Int. J. Appl. Ceram. Technol. 2007, 4, 145–151. [Google Scholar] [CrossRef]
- Kiebach, R.; Engelbrecht, K.; Kwok, K.; Molin, S.; Søgaard, M.; Niehoff, P.; Schulze-Küppers, F.; Kriegel, R.; Kluge, J.; Hendriksen, P.V. Joining of ceramic Ba0.5Sr0.5Co0.8Fe0.2O3 membranes for oxygen production to high temperature alloys. J. Membr. Sci. 2016, 506, 11–21. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, T.; Zhang, J.; Wu, C.; Lu, X.; Ding, W. Induction brazing BaCo0.7Fe0.2Nb0.1O3−δ membrane tubes to steel supports with Ag-based filler in air. J. Membr. Sci. 2017, 533, 19–27. [Google Scholar] [CrossRef]
- Godini, H.R.; Gili, A.; Görke, O.; Simon, U.; Hou, K.; Wozny, G. Performance Analysis of a Porous Packed Bed Membrane Reactor for Oxidative Coupling of Methane: Structural and Operational Characteristics. Energy Fuels. 2014, 28, 877–890. [Google Scholar] [CrossRef]












| Membrane | Temperature (°C) | Thickness (µm) | Oxygen Flux (mol cm−2 s−1) | (atm) | (atm) | Feed Gas | Sweep Gas | Ea (kJ/mol) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| (YSZ)0.7-(Pd)0.3 | 1100 | 2000 | 10−10 | 0.209 | 1.4·10−3 | Air | He | 170 | [69] |
| (YSZ)0.6-(Pd)0.4 | 1100 | 2000 | 4.3 ;× 10−8 | 0.209 | 0.014 | Air | He | 82.6 | [69] |
| (YSZ)0.6-(Pd)0.4 | 800 | 1720 | 1.6 ;× 10−8 | 0.209 | 0.026 | Air | He | - | [75] |
| (YSZ)0.5-(Pd)0.5 | 1100 | 800 | 1.56 ;× 10−6 | 0.209 | - | Air | CH4a | - | [68] |
| (YSZ)0.5-(Pt)0.5 | 1100 | 800 | 1.34 ;× 10−6 | 0.209 | - | Air | CH4a | - | [68] |
| (YSZ)0.6-(In90Pr10)0.4 | 1100 | 800 | 8.18 ;× 10−7 | 0.209 | - | Air | CH4a | - | [68] |
| (YSZ)0.5-(In90Pr10)0.5 | 1100 | 800 | 1.71 ;× 10−6 | 0.209 | - | Air | CH4a | - | [68] |
| (YSZ)0.5-(In90Pr10)0.5 | 1100 | 300 | 4.09 ×·10−6 | 0.209 | - | Air | CH4a | - | [68] |
| (YSZ)0.5-(In95Pr2.5Zr2.5)0.5 | 1100 | 300 | 5.80 ;× 10−6 | 0.209 | - | Air | CH4a | - | [68] |
| [(Bi2O3)(Er2O3)]0.6-Ag0.4 | 800 | 1600 | 1.19 ;× 10−7 | 0.209 | 0.026 | Air | He | - | [75] |
| YSZ-Pd-YSZ | 1050 | 10 | 2.0 ;× 10−8 | 6 × 10−3 | 1 ;× 10−5 | Air | - | 193 | [71] |
| YSZ-Pd-YSZ | 1050 | 10 | 4.8 ;× 10−8 | 6× 10−3 | 1 ;× 10−5 | O2 | - | 193 | [71] |
| [(Bi2O3)0.74(SrO)0.26]0.6-Ag0.4 | 680 | 1000 | 5 ;× 10−8 | 0.209 | 0.0024 | Air | He | 185 | [76] |
| [(Bi2O3)0.75(Er2O3)0.25]0.6-Ag0.4 | 852 | 230 | 3.08 ;× 10−7 | 0.209 | 0.046 | Air | He | 48.9 | [27] |
| [(Bi2O3)0.75(Er2O3)0.25]0.6-Ag0.4 | 680 | 129 | 1.79 ;× 10−7 | 1 | 2 ;× 10−6 | O2 | Ar | - | [77] |
| [(Bi2O3)0.75(CaO)0.25]0.6-Ag0.4 | 680 | 75 | 2.95 ;× 10−8 | 1 | 2 ;× 10−6 | O2 | Ar | - | [77] |
| (Bi1.5Y0.3Sm0.2O3)0.6-Ag0.4 | 850 | 1300 | 5.80 ;× 10−7 | 0.21 | 0.009 | O2/N2 | He | 87.30 | [70] |
| (NiFe2O4)0.4(Ce0.8Tb0.2O2-δ)0.6 [(Ce0.8Ga0.2O2-δ)(La0.8Sr0.2MnO2-δ)] +Pd | 1000 | 680b | 1.26 ;× 10−7 | 0.21 | - | Air | Ar | - | [78] |
| (NiFe2O4)0.4(Ce0.8Tb0.2O2-δ)0.6 [(Ce0.8Ga0.2O2-δ)(La0.8Sr0.2MnO2-δ)] +Pd | 1000 | 680b | 1.49 ;× 10−7 | 0.21 | - | Air | CO2 | - | [78] |
| Element | Z | Tm (°C) | TEC × 106 (°C−1) | Cp at 25 °C (J g−1 K−1) | Electrical Resistivity ×10−8 (Ω·m) | Price (€ kg−1) | ||
|---|---|---|---|---|---|---|---|---|
| 700 °C | 800 °C | 900 °C | ||||||
| Zr | 40 | 1855 | 5.7 | 0.278 | 104.2 | 114.9 | 123.1 | - |
| Pd | 46 | 1555 | 11.8 | 0.246 | 24.2 | 27.1 | 29.4 | 40000 |
| Ag | 47 | 962 | 18.9 | 0.235 | 4.21 | 4.91 | 5.64 | 470 |
| In | 49 | 156.6 | 32.1 | 0.233 | - | - | - | - |
| Pr | 59 | 931 | 6.7 | 0.193 | - | - | - | - |
| Pt | 78 | 1768 | 8.8 | 0.133 | 25.4 | 28.7 | 32 | 25500 |
| Au | 79 | 1064 | 14.2 | 0.129 | 5.82 | 6.81 | 7.86 | 37000 |
| Membrane | Temperature (°C) | Thickness (µm) | Oxygen Flux (mol cm−2 s−1) | (atm) | (atm) | Feed Gas | Sweep Gas | Ea (kJ mol−1) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| (LSM)(YSZ) two-step sequential tape casting | 900 | 150 | 3.31 ;× 10−8 | 0.21 | 0.002 | Air | He | 145.3 | [72] |
| (LSM)(YSZ) phase-inversion tape-casting | 900 | 150 | 1.90 ;× 10−7 | 0.21 | 0.002 | Air | He | 142.5 | [72] |
| (PSFO)0.4(CPO)0.6 | 950 | 600 | 1.34 ;× 10−7 | - | - | 20% O2 80% N2 | CO2 | - | [81] |
| (PSFO)0.4(CPO)0.6 | 950 | 600 | 1.93 ;× 10−7 | - | - | 20% O2 80% N2 | He | - | [81] |
| (SDCδ)0.7(LSFO)0.3 | 950 | 1100 | 1.59 ;× 10−7 | - | - | Air | He | 115 | [82] |
| (SDCδ)0.7(LSFO)0.3 | 950 | 1100 | 1.59 ;× 10−7 | - | - | Air | CO2 | 115 | [82] |
| (SDCδ)0.7(LSFO)0.3 | 950 | 1100 | 8.92 ;× 10−7 | - | - | Air | CO2 | 96.2 | [82] |
| (SDCδ)0.7(LBCO)0.3 | 950 | 600 | 4.59 ;× 10−7 | - | - | 21% O2 79% N2 | He | 84.8 | [83] |
| (LSM)0.5(CGO)0.5 | 807 | 1000 | 1.8 ;× 10−8 | 18 | 1 | O2 | - | - | [84] |
| (LSCF)0.7(CGO)0.3 | 950 | 800 | 1.56 ;× 10−6 | 0.209 | - | Air | Ar | 95 | [85] |
| [(LSCF)0.7(CGO)0.3]+Pt | 950 | 700 | 2.83 ;× 10−6 | 0.209 | - | Air | Ar | 71 | [85] |
| (BSCF)0.4(CGO)0.6 | 875 | 500 | 8.04 ;× 10−7 | - | - | Air | He | - | [86] |
| (BSCF)0.4(CGO)0.6 | 950 | 500 | 1.33 ;× 10−6 | - | - | Air | He | - | [86] |
| (BSCF)0.4(CGO)0.6 | 875 | 500 | 1.93 ;× 10−7 | - | - | Air | a | 46.75 | [86] |
| (BSCF)0.4(CGO)0.6 | 950 | 500 | 6.32 ;× 10−7 | - | - | Air | a | 46.75 | [86] |
| (YCCC) + (SDC) | 950 | 1300 | 2.1 ;× 10−6 | - | - | Air | b | 82-90 | [87] |
| Membrane | p(O2) atm | Temperature Range (°C) | × 106 (K−1) | Reference |
|---|---|---|---|---|
| SrCo0.8Fe0.2O3-δ | 1.00 1.00 | RT–430 430–1000 | 18.5 31.1 | [92] |
| 1.00 | 600–900 | 31.1 | [93] | |
| 0.21 0.21 | RT–500 500–1000 | 17.8 30.3 | [92] | |
| 0.21 0.21 | 27–427 427–827 | 18.8 ± 0.3 29.4 ± 0.8 | [94] | |
| 0.21 | Room–1000 | 17.9 | [95] | |
| 10−4 | RT–540 540–1000 | 12.3 19.7 | [92] | |
| Ba0.5Sr0.5Co0.8Fe0.2O3-δ | 1.00 | RT–440 440–1000 | 12.2 24.5 | [92] |
| 0.21 | RT–440 440–1000 | 13.6 24.1 | [92] | |
| 0.21 | RT–500 700–1000 | 13.6 24.8 a | [96] | |
| 0.21 | Room–1000 | 11.5 | [95] | |
| 10−4 | RT–1000 | 18.2 | [92] | |
| (ZrO2)0.92-(Y2O3)0.08 (YSZ) | 0.21 | Room–1000 | 10.7 | [92] |
| (ZrO2)0.85-(Y2O3)0.15 (YSZ) | 0.21 | 50–1000 | 10.8 | [40] |
| CeO2 | 0.21 | 50–1000 | 12.3 | [40] |
| Ce0.9Gd0.1O2-δ | 0.21 | Room–1000 | 12.4 | [92] |
| Ce0.8Gd0.2O2-δ | 0.21 | 50–1000 | 12.5 | [40] |
| Ce0.6Gd0.4O2-δ | 0.21 | 50–1000 | 12.1 | [40] |
| Ce0.5Er0.5O2-δ | 0.21 | 50–1000 | 11.4 | [40] |
| Ce0.9Ca0.1O2-δ | 0.21 | 50–1000 | 12.8 | [40] |
| Ce0.8Ca0.2O2-δ | 0.21 | 50–1000 | 13.6 | [40] |
| Material (Membrane/support) | Temperature (°C) | Thickness (µm) | Oxygen Flux (mol cm−2 s−1) | p’O2 (atm) | p’’O2 (atm) | Feed Gas | Sweep Gas | Reference |
|---|---|---|---|---|---|---|---|---|
| BSCF/BSCF | 1000 | 70 | 4.62 ;× 10−5 | 1 | - | O2 | Ar | [63] |
| CGO-LSF/MgO | 850 | 10 | 1.12 ;× 10−5 | 0.21 | - | Air | H2 | [168] |
| BSCF/BSCF | 1000 | 70 | 8.32 ;× 10−6 | 0.21 | - | Air | Ar | [63] |
| CGO-LSF/CGO-LSF | 900 | 100 | 7.51 ;× 10−6 | 0.209 | 9.87 ;× 10−3 | Air | CO | [169] |
| BCFN/BCFN | 900 | 20 | 3.35 ;× 10−6 | 0.21 | - | Air | He | [25] |
| CGO/MgO | 900 | 31 | 2.97 ;× 10−6 | 0.209 | 2.96 ;× 10−3 | Air | He | [26] |
| SDC-SSAF/SDC-SSAF | 950 | 40 | 2.90 ;× 10−6 | 0.209 | 4.93 ;× 10−3 | Air | He | [172] |
| BSCF/BSCF | 1000 | 20 | 2.01 ;× 10−6 | 0.209 | - | Air | Ar | [74] |
| BSCF/BSCF | 850 | 40 | 1.95 ;× 10−6 | 0.21 | - | Air | Ar | [175] |
| YSZ-LSCF-SCO/YSZ-LSCF | 900 | 30 | 1.64 ;× 10−6 | 0.21 | - | Air | CO | [174] |
| CGO-LSF/MgO | 850 | 10 | 1.56 ;× 10−6 | 0.21 | - | Air | N2 | [142] |
| CDS-SSF/CDS-SSF | 950 | 160 | 8.06 ;× 10−7 | 0.209 | 0.0316 | Air | He | [176] |
| SCFZ/SCFZ | 800 | 20 | 7.41 ;× 10−7 | 0.209 | 2.96 ;× 10−3 | Air | He | [177] |
| CGO-NFO/YSZ | 850 | 20 | 3.41 ;× 10−7 | - | - | Air | Ar | [173] |
| CGO/CeO2 | 900 | 10-20 | 3.35× 10−7 | 0.21 | - | Air | - | [169] |
| CTF/CTF | 1000 | 30 | 3.35 ;× 10−7 | 0.209 | - | Air | Ar | [178] |
| BYS-LSM/ BYS-LSM | 850 | 290 | 2.56 ;× 10−7 | 1.05 | 1.05 | Air | He | [179] |
| SCFZ-MgO/SCFZ-MgO | 900 | 200 | 2.00 ;× 10−7 | 0.209 | 9.87 × 10−4 | Air | He | [180] |
| LSM-YSZ/LSM-YSZ/ | 900 | 150 | 1.90 ;× 10−7 | 0.209 | 0.002 | Air | He | [72] |
| LSCF/LSCF | 800 | 200 | 1.45 ;× 10−7 | 0.21 | 1·10−3 | Air | He | [181] |
| LSCF-YSZ/YSZ | 900 | 60 | 1.12 ;× 10−7 | - | - | CO2 | H2 | [182] |
| LSCF-YSZ/YSZ | 900 | 120 | 4.46 ;× 10−8 | 0.209 | - | Air | CO | [183] |
| LSCO/CeO2 | 900 | 10 | 1.17 ;× 10−8 | 0.209 | - | Air | He | [184] |
| Membrane | Temperature (°C) | Oxygen Flux (mol cm−2 s−1) | Dilution (%) | Catalyst | CH4 Conversion | C2+ Selectivity | C2+ Yield | Reference |
|---|---|---|---|---|---|---|---|---|
| La0.6Sr0.4Co0.2Fe0.8O3-δ | 975 | - | 67.5 (He) | SrTi0.9Li0.1O3 | 0.3 | 0.7 | 0.21 | [198] |
| BaCe0.8Gd0.2O3-δ | 780 | - | 96 (He) | BaCe0.8Gd0.2O3 | 0.26 | 0.62 | 0.16 | [199] |
| BaCoxFeyZrzO3-δ | 800 | - | 75 (He) | MnNa2WO4/SiO2 | 0.35 | 0.5 | 0.17 | [200] |
| Ba0.5Sr0.5Co0.8Fe0.2O3-δ | 850 | 1.12 ;× 10−6 | 80 (He) | La-Sr/CaO | 0.22 | 0.67 | 0.15 | [201] |
| Ba0.5Sr0.5Co0.8Fe0.2O3-δ | 1000 | 4.09 ;× 10−6 | 47 (He) | Pt/MgO | 0.05 | 0.5 | 0.03 | [202] |
| Ba0.5Sr0.5Co0.8Fe0.2O3-δ | 900 | 2.60 ;× 10−6 | 66 (He) | LaSr/CaO | 0.25 | 0.7 | 0.18 | [202] |
| Ba0.5Sr0.5Co0.8Fe0.2O3-δ | 950 | 1.49 ;× 10−6 | 89 (He) | Sr/La2O3 | 0.25 | 0.37 | 0.09 | [202] |
| Bi1.5Y0.3Sm0.2O3-δ | 900 | 4.00 ;× 10−8 | 98 (He) | Bi1.5Y0.3Sm0.2O3-δ | 0.648 | 0.54 | 0.35 | [203] |
| Ba0.5Ce0.4Gd0.1Co0.8Fe0.2O3−δ | 850 | 1.04 ;× 10−6 | 50 (He) | Na-W-Mn/SiO2 | 0.516 | 0.67 | 0.35 | [204] |
| La0.6Sr0.4Co0.2Fe0.8O3-δ | 900 | 6.50 ;× 10−6 | 25 (Ar) | Bi1.5Y0.3Sm0.2O3-δ | 0.49 | 0.79 | 0.39 | [205] |
| Membrane | Temperature (°C) | Oxygen Flux (mol cm−2 s−1) | Shape | Catalyst | Operating Hours | CH4 Convulution | CO selectivity | Reference |
|---|---|---|---|---|---|---|---|---|
| Ba0.5Sr0.5Co0.8Fe0.2O3−δ | 875 | 8.56 ;× 10−6 | planar | LiLaNiOx/γ-Al2O3 | 500 | 0.97 | 0.96 | [110] |
| Ba0.5Sr0.5Co0.8Fe0.1Ni0.1O3−δ | 850 | 8.93 ;× 10−6 | planar | Ni | 120 | 0.98 | 0.97 | [211] |
| Ba0.9Co0.7Fe0.2Nb0.1O3-δ | 875 | 1.19 ;× 10−5 | planar | Ni | 100 | 0.97 | 0.75 | [212] |
| La0.4Ba0.6Fe1−xZnxO3−δ | 900 | 2.83 ;× 10−6 | planar | Ni | 500 | 0.99 | 0.97 | [213] |
| Ba0.9Co0.7Fe0.2Nb0.1O3-δ | 875 | 5.28 ;× 10−6 | planar | NiO/MgO | 400 | 0.93 | 0.95 | [214] |
| La0.6Sr0.4Co0.8Ga0.2O3-δ | 750 | 1.86 ;× 10−6 | hollow fiber | Ni/LaAlO3-Al2O3 | - | 0.97 | 0.91 | [215] |
| SrCo0.8Fe0.1Ga0.1O3-δ | 800 | 3.08 ;× 10−6 | hollow fiber | Ni/Al2O3 | 220 | 1 | 0.33 | [216] |
| BaBi0.05Co0.8Nb0.15O3-δ | 730 | 1.12 ;× 10−5 | hollow fiber | Ni phyllosilicate | <5 | 0.8 | 0.85 | [217] |
| BaCo0.7Fe0.2Ta0.1O3-δ | 875 | 1.49 ;× 10−5 | hollow fiber | Ni | 83 | 0.96 | 0.99 | [218] |
| (Pr0.9La0.1)2(Ni0.74Cu0.21Ga0.05)O4+δ | 900 | 7.81 ;× 10−6 | hollow fiber | Ni | 140 | 0.97 | 0.99 | [219] |
| SrFe0.8Nb0.2O3-δ | 900 | 1.43 ;× 10−5 | hollow fiber | Ni/Al2O3 | 120 | 0.95 | 0.99 | [220] |
| YSZ-La0.8Sr0.2Cr0.5Fe0.5O3-δ | 800 | 1.30 ;× 10−6 | planar | Ni/Al2O3 | - | 0.9 | 0.95 | [221] |
| Ag-Ce0.9Gd0.1O2-x | 700 | 1.34 ;× 10−7 | planar | Ni | - | 0.21 | 0.9 | [222] |
| (0.5 wt.% Nb2O5-doped SrCo0.8Fe0.2O3-δ) (Ba0.3Sr0.7Fe0.9Mo0.1O3-δ) | 900 | 1.38 ;× 10−5 | planar | Ni/Al2O3 | 1500 | 0.99 | 0.94 | [223] |
| Membrane | Temperature (°C) | Oxygen Flux (mol cm−2 s−1) | Shape | C2H6 Conv. | C2H4 Selectivity | C2H4 Yield | References |
|---|---|---|---|---|---|---|---|
| Ba0.5Sr0.5Co0.8Fe0.2O3-δ | 800 | 1.28 ;× 10−6 | planar | 0.84 | 0.8 | 0.67 | [228] |
| BaCoxFeyZrzO3-d | 800 | 8.56 ;× 10−7 | hollow fiber | 0.90 | 0.64 | 0.4 | [229] |
| Bi1.5Y0.3Sm0.2O3-δ | 875 | 6.40 ;× 10−7 | hollow fiber | 0.70 | 0.8 | 0.56 | [13] |
| Ba0.5Sr0.5Co0.8Fe0.2O3-δ | 775 | 1.15 ;× 10−6 | planar | 0.85 | 0.89 | 0.76 | [230] |
| Ba0.5Sr0.5Co0.8Fe0.2O3-δ | 775 | 7.44 ;× 10−7 | planar | 0.83 | 0.89 | 0.74 | [230] |
| La0.5Ce0.1Sr0.4Co0.5Fe0.5 O3-δ | 850 | - | planar | 0.86 | 0.91 | 0.78 | [231] |
| Sm0.6Sr0.4Co0.5Fe0.5O3-δ | 850 | - | planar | 0.85 | 0.91 | 0.77 | [231] |
| Nd0.6Sr0.4Co0.5Fe0.5O3-δ | 850 | - | planar | 0.86 | 0.89 | 0.77 | [231] |
| Ba0.6Sr0.4Fe O3-δ | 850 | - | planar | 0.86 | 0.91 | 0.78 | [231] |
| Ba0.5Sr0.5Co0.8Fe0.2O3-δ a | 850 | - | planar | 0.89 | 0.9 | 0.8 | [231] |
| Ba0.5Sr0.5Co0.8Fe0.2O3-δ | 850 | - | - | - | - | 0.73 | [232] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arratibel Plazaola, A.; Cruellas Labella, A.; Liu, Y.; Badiola Porras, N.; Pacheco Tanaka, D.A.; Sint Annaland, M.V.; Gallucci, F. Mixed Ionic-Electronic Conducting Membranes (MIEC) for Their Application in Membrane Reactors: A Review. Processes 2019, 7, 128. https://doi.org/10.3390/pr7030128
Arratibel Plazaola A, Cruellas Labella A, Liu Y, Badiola Porras N, Pacheco Tanaka DA, Sint Annaland MV, Gallucci F. Mixed Ionic-Electronic Conducting Membranes (MIEC) for Their Application in Membrane Reactors: A Review. Processes. 2019; 7(3):128. https://doi.org/10.3390/pr7030128
Chicago/Turabian StyleArratibel Plazaola, Alba, Aitor Cruellas Labella, Yuliang Liu, Nerea Badiola Porras, David Alfredo Pacheco Tanaka, Martin Van Sint Annaland, and Fausto Gallucci. 2019. "Mixed Ionic-Electronic Conducting Membranes (MIEC) for Their Application in Membrane Reactors: A Review" Processes 7, no. 3: 128. https://doi.org/10.3390/pr7030128
APA StyleArratibel Plazaola, A., Cruellas Labella, A., Liu, Y., Badiola Porras, N., Pacheco Tanaka, D. A., Sint Annaland, M. V., & Gallucci, F. (2019). Mixed Ionic-Electronic Conducting Membranes (MIEC) for Their Application in Membrane Reactors: A Review. Processes, 7(3), 128. https://doi.org/10.3390/pr7030128









