High-Temperature Permittivity and Microwave Pretreatment Characteristics of Nickel-Containing Sludge from Battery Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Experimental Devices
2.2.1. Permittivity Measurement System
2.2.2. Microwave Oven for the Pretreatment Experiments
2.3. Data Analysis and Mathematical Modeling
3. Results and Discussion
3.1. TG-DSC of the NCS
3.2. Dielectric Properties of the NCS
3.3. Microwave Heating Curves of the NCS
3.4. Changes in Physical Properties of the NCS After Pretreatment
3.5. Effect of Microwave Pretreatment on Nickel Leaching Rate
3.6. Intensification Mechanism of the Microwave Heating Process
4. Conclusions
- The dielectric properties of the NCS were measured at different temperatures and moisture contents using the microwave cavity perturbation method at 2.45 GHz. The values of the dielectric constant and relative loss factor of the NCS increase with increasing temperature. The tanδ and DP are also determined using Equations (2) and (3). At temperatures below 200 °C, the tanδ value reaches a stable level of 0.013 and then rapidly increases when the temperature is greater than 300 °C, reaching a maximum value of 0.055. The optimum thickness for uniform heat varies from 86 to 17 cm from room temperature to 600 °C. The dielectric properties of NCS significantly increase with the moisture content. When the moisture content of the materials is ~4%, the value of the loss angle tangent (tanδ) of the NCS is far higher than that of the temperature.
- Within 22 min, the dried material temperature reaches more than 600 °C in a microwave field. The heating curve can be divided into two parts. The sample temperature slowly increases at a heating rate of 16.2 °C/min below 200 °C and rapidly increases at a heating rate of 42.1 °C/min from 200 °C to 600 °C. The comparison of the heating characteristics of the materials with different moisture contents shows that the heating rate of the material is 36.4% and 11.1% higher than that of the dried materials when the initial moisture content is 4% or 8%, respectively.
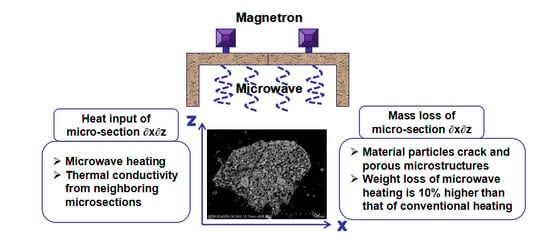
- The microwave pretreatment process for the NCS is feasible and the dried sample can be reduced by 21% without changing the major phases. The weight loss of microwave heating is 10% higher, the particle size of the roasted products is smaller and while the weight of NCS is reduced by more than 20%, the particle size is significantly reduced and the leaching time reduce 20 min than that of conventional heating. The microwave roasting mechanism was evaluated using modern analysis methods such as chemical analysis, XRD, and SEM.
Author Contributions
Funding
Conflicts of Interest
References
- Yan, X.; Li, Q.; Chai, L.; Wang, Q. Formation of abiological granular sludge—A facile and bioinspired proposal for improving sludge settling performance during heavy metal wastewater treatment. Chemosphere 2014, 113, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.H.; Shih, K.; Hu, C.Y.; Lo, S.L.; Li, N.H.; Cheng, Y.T. The effects of salinity and temperature on phase transformation of copper-laden sludge. J. Hazard. Mater. 2013, 244–245, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Ma, Y.; Xie, F. Impacts of ultrasound on selective leaching recovery of heavy metals from metal-containing waste sludge. J. Mater. Cycles Waste 2013, 15, 530–538. [Google Scholar] [CrossRef]
- Silva, J.E.; Paiva, A.P.; Soares, D.; Labrincha, A.; Castro, F. Solvent extraction applied to the recovery of heavy metals from galvanic sludge. J. Hazard. Mater. 2005, 120, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.J.; Chai, L.Y.; Min, X.B.; Tang, C.J.; Zhang, H.J.; Ke, Y.; Xie, X.D. Hydrothermal sulfidation and floatation treatment of heavy-metal-containing sludge for recovery and stabilization. J. Hazard. Mater. 2012, 217–218, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Shih, K.; White, T.; Leckie, J.O. Nickel stabilization efficiency of aluminate and ferrite spinels and their leaching behavior. Environ. Sci. Technol. 2006, 40, 5520–5526. [Google Scholar] [CrossRef] [PubMed]
- Li, C.T.; Lee, W.J.; Huang, K.L.; Fu, S.F.; Lait, Y.C. Vitrification of chromium electroplating sludge. Environ. Sci. Technol. 2007, 41, 2950–2956. [Google Scholar] [CrossRef]
- Yi, Z.; Wang, Z.K.; Xia, X.; Chen, Y.Q.; Qi, T. Recovery of heavy metals from electroplating sludge and stainless steel pickle waste liquid by ammonia leaching method. J. Environ. Sci. China 1999, 11, 381–384. [Google Scholar]
- Silva, J.E.; Soares, D.; Paiva, A.P.; Labrincha, J.A.; Castro, F. Leaching behaviour of a galvanic sludge in sulphuric acid and ammoniacal media. J. Hazard. Mater. 2005, 121, 195–202. [Google Scholar] [CrossRef]
- Dang, X.E.; Lan, X.Z.; Dong, Y. Study on Recovering of Value Metals from Acid Leaching Solution of Roasted Electroplating Sludge. Adv. Mater. Res. 2012, 524–527, 1951–1955. [Google Scholar] [CrossRef]
- Jones, D.A.; Lelyveld, T.P.; Mavrofidis, S.D.; Kingman, S.W.; Miles, N.J. Microwave heating applications in environmental engineering—A review. Resour. Conserv. Recycl. 2002, 34, 75–90. [Google Scholar] [CrossRef]
- Kingman, S.W.; Rowson, N.A. Microwave treatment of minerals-a review. Miner. Eng. 1998, 11, 1081–1087. [Google Scholar] [CrossRef]
- Roy, R.; Agrawal, D.; Cheng, J.; Gedevanishvili, S. Full sintering of powdered-metal bodies in a microwave field. Nature 1999, 399, 304–304. [Google Scholar] [CrossRef]
- Liao, C.; Sun, S.; Chen, Q. Study on metal recovery and innoxious diposal of eleetroplating sludge using ineineration. Shanghai Environ. Sci. 2002, 21, 491–500. (In Chinese) [Google Scholar]
- Menéndez, J.A.; Domínguez, A.; Inguanzo, M.; Pis, J.J. Microwave-induced drying, pyrolysis and gasification (MWDPG) of sewage sludge: Vitrification of the solid residue. J. Anal. Appl. Pyrolysis 2005, 74, 406–412. [Google Scholar] [CrossRef]
- Chen, C.L.; Lo, S.L.; Kuan, W.H.; Hsieh, C.H. Stabilization of Cu in acid-extracted industrial sludge using a microwave process. J. Hazard. Mater. 2005, 123, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.H.; Lo, S.L.; Chiueh, P.T.; Kuan, W.H.; Chen, C.L. Microwave enhanced stabilization of heavy metal sludge. J. Hazard. Mater. 2007, 139, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.; Wong, W.; Liao, P.; Lo, K. Sewage sludge nutrient solubilization using a single-stage microwave treatment. Environ. Lett. 2007, 42, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Dawson, E.A.; Parkes, G.M.B.; Barnes, P.A.; Bond, G.; Mao, R. The generation of microwave-induced plasma in granular active carbons under fluidised bed conditions. Carbon 2008, 46, 220–228. [Google Scholar] [CrossRef]
- Zlotorzynski, A. The application of microwave radiation to analytical and environmental chemistry. Crit. Rev. Anal. Chem. 1995, 25, 43–76. [Google Scholar] [CrossRef]
- Araneta, J.C.; Brodwin, M.E.; Kriegsmann, G.A. High-temperature microwave characterization of dielectric rods. IEEE Trans. Microw. Theory 1984, 32, 1328–1335. [Google Scholar] [CrossRef]
- Lin, G.; Liu, C.; Zhang, L.; Hu, T.; Peng, J.; Li, J.; Wang, X. High temperature dielectric properties of spent adsorbent with zinc sulfate by cavity perturbation technique. J. Hazard. Mater. 2017, 330, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Liu, B.; Liu, P.; Peng, J.; Zhang, L. Dielectric properties and oxidation roasting of molybdenite concentrate by using microwave energy at 2.45 GHz frequency. Metall. Mater. Trans. B 2017, 48, 3047–3057. [Google Scholar]
- Catalá-Civera, J.M.; Canós, A.J.; Plaza-González, P.; Gutiérrez, J.D.; García-Baños, B.; Peñaranda-Foix, F.L. Dynamic Measurement of Dielectric Properties of Materials at High Temperature During Microwave Heating in a Dual Mode Cylindrical Cavity. IEEE Trans. Microw. Theory 2015, 63, 2905–2914. [Google Scholar] [CrossRef]
- Li, E.; Nie, Z.P.; Guo, G.; Zhang, Q.; Li, Z.; He, F. Broadband measurements of dielectric properties of low-loss materials at high temperatures using circular cavity method. Prog. Electromagn. Res. 2009, 92, 103–120. [Google Scholar] [CrossRef]
- Huang, K.M.; Cao, X.J.; Liu, C.; Liu, C.J.; Xu, X.B. Measurement/computation of effective permittivity of dilute solution in saponification reaction. IEEE Trans. Microw. Theory 2003, 51, 2106–2111. [Google Scholar] [CrossRef]
- Sun, H.R.; Huang, K.M. A novel microwave cancellation circuit for measuring nonlinear dielectric changes of polar solution under microwave fields. J. Phys. D Appl. Phys. 2015, 48, 475–502. [Google Scholar] [CrossRef]
- Huang, K.; Zhu, H.; Wu, L. Temperature cycle measurement for effective permittivity of biodiesel reaction. Bioresour. Technol. 2013, 131, 541–544. [Google Scholar] [CrossRef]
- Yang, X.; Huang, K. Study on the key problems of interaction between microwave and chemical reaction. Front. Electr. Electr. Eng. China SPCU 2007, 2, 473–480. [Google Scholar] [CrossRef]
- Sheen, J. Study of microwave dielectric properties measurements by various resonance techniques. Measurement 2005, 37, 123–130. [Google Scholar] [CrossRef]
- Henmi, H.; Mori, M.; Hirayama, T.; Mizutani, N.; Kato, M. Influence of the self-generated and controlled atmosphere on the thermal decomposition of basic nickel carbonate, NiCO3·2Ni(OH)2·4H2O. Thermochim. Acta 1986, 104, 101–109. [Google Scholar] [CrossRef]
- Cronan, C.L.; Micale, F.J.; Topić, M.; Leidheiser, H., Jr.; Zettlemoyer, A.C. Surface properties of Ni(OH)2, and NiO. II. Mechanism for the thermal decomposition of Ni(OH)2, and other metal hydroxides. J. Colloid Interface Sci. 2012, 55, 546–557. [Google Scholar] [CrossRef]
- Haque, K.E. Microwave energy for mineral treatment processes—A brief review. Int. J. Miner. Process. 1999, 57, 1–24. [Google Scholar] [CrossRef]
- Pickles, C.A. Microwave heating behaviour of nickeliferous limonitic laterite ores. Miner. Eng. 2004, 17, 775–784. [Google Scholar] [CrossRef]
- Chiteme, C.; Mulaba-Bafubiandi, A.F. An investigation on electrical properties of microwave treated natural ilmenite (FeTiO3). J. Mater. Sci. 2006, 41, 2365–2372. [Google Scholar] [CrossRef]
- Laybourn, A.; Katrib, J.; Palade, P.A.; Easun, T.L.; Champness, N.R.; Schroder, M.; Kingman, S.W. Understanding the electromagnetic interaction of metal organic framework reactants in aqueous solution at microwave frequencies. Phys. Chem. Chem. Phys. 2016, 18, 5419–5431. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, B.K.; De, S.; Meikap, B.C. Improvement of grinding characteristics of Indian coal by microwave pre-treatment. Fuel Process. Technol. 2011, 92, 1920–1928. [Google Scholar] [CrossRef]
- Kumar, P.; Sahoo, B.K.; De, S.; Kar, D.D.; Chakraborty, S.; Meikap, B.C. Iron ore grindability improvement by microwave pre-treatment. J. Ind. Eng. Chem. 2010, 16, 805–812. [Google Scholar] [CrossRef]













| Composition | O | Ca | Mg | C | Ni | Fe | Si | Cd |
|---|---|---|---|---|---|---|---|---|
| Content (%) | 53.42 | 14.09 | 8.67 | 8.30 | 8.02 | 4.64 | 1.57 | 0.71 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Z.; Guo, P.; Su, G.; Zhai, D.; Cheng, F.; Li, F. High-Temperature Permittivity and Microwave Pretreatment Characteristics of Nickel-Containing Sludge from Battery Production. Processes 2019, 7, 257. https://doi.org/10.3390/pr7050257
Guo Z, Guo P, Su G, Zhai D, Cheng F, Li F. High-Temperature Permittivity and Microwave Pretreatment Characteristics of Nickel-Containing Sludge from Battery Production. Processes. 2019; 7(5):257. https://doi.org/10.3390/pr7050257
Chicago/Turabian StyleGuo, Zhanyong, Ping Guo, Guang Su, Demei Zhai, Fang Cheng, and Fachuang Li. 2019. "High-Temperature Permittivity and Microwave Pretreatment Characteristics of Nickel-Containing Sludge from Battery Production" Processes 7, no. 5: 257. https://doi.org/10.3390/pr7050257