Purification of Amygdalin from the Concentrated Debitterizing-Water of Apricot Kernelsusing XDA-1 Resin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples and Preparation
2.2. Chemicals
2.3. Pre-Treatment of the Macroporous Resins
2.4. Experiments of Static Adsorption/De-Adsorption of Macroporous Resin
2.5. Statics Adsorption Kinetics
2.6. Static Adsorption Isotherm Procedure
2.7. Effect of Ethanol Concentration on the De-Adsorption of Amygdalin for the XDA-1 Resin
2.8. Effect of Time on the De-Adsorption Capacity of XAD-1 for the Amygdalin
2.9. Purification of Amygdalin in DWC Solutions by the XAD-1 Resin
2.9.1. Dynamic Adsorption of XDA-1 Resin on Amygdalin
2.9.2. Dynamic De-Adsorption of XDA-1 Resin on Amygdalin
2.9.3. Determination of the Recovery Rate and Relative Content of Amygdalin
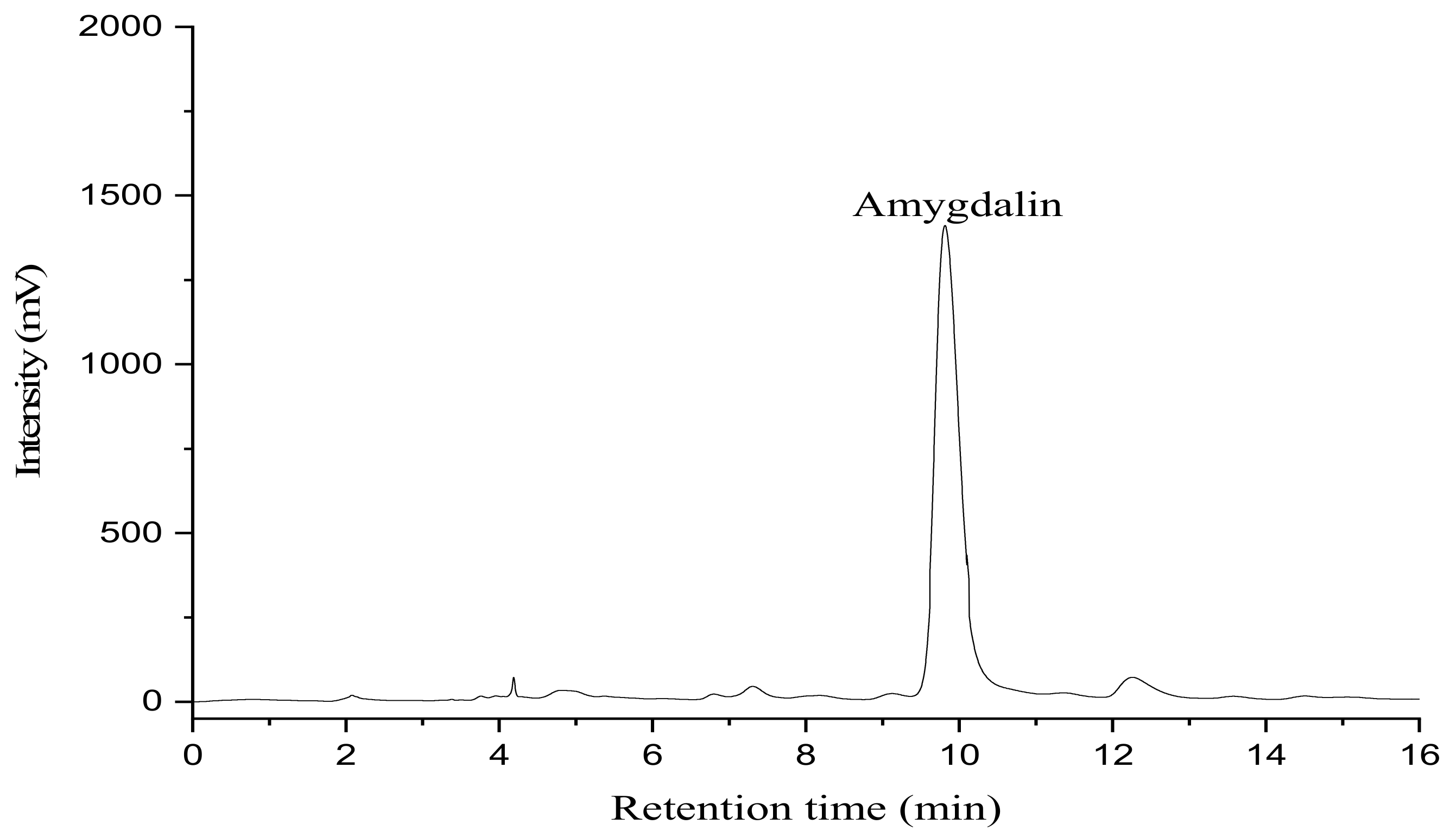
2.10. Determination of Amygdalin by HPLC
3. Results and Discussion
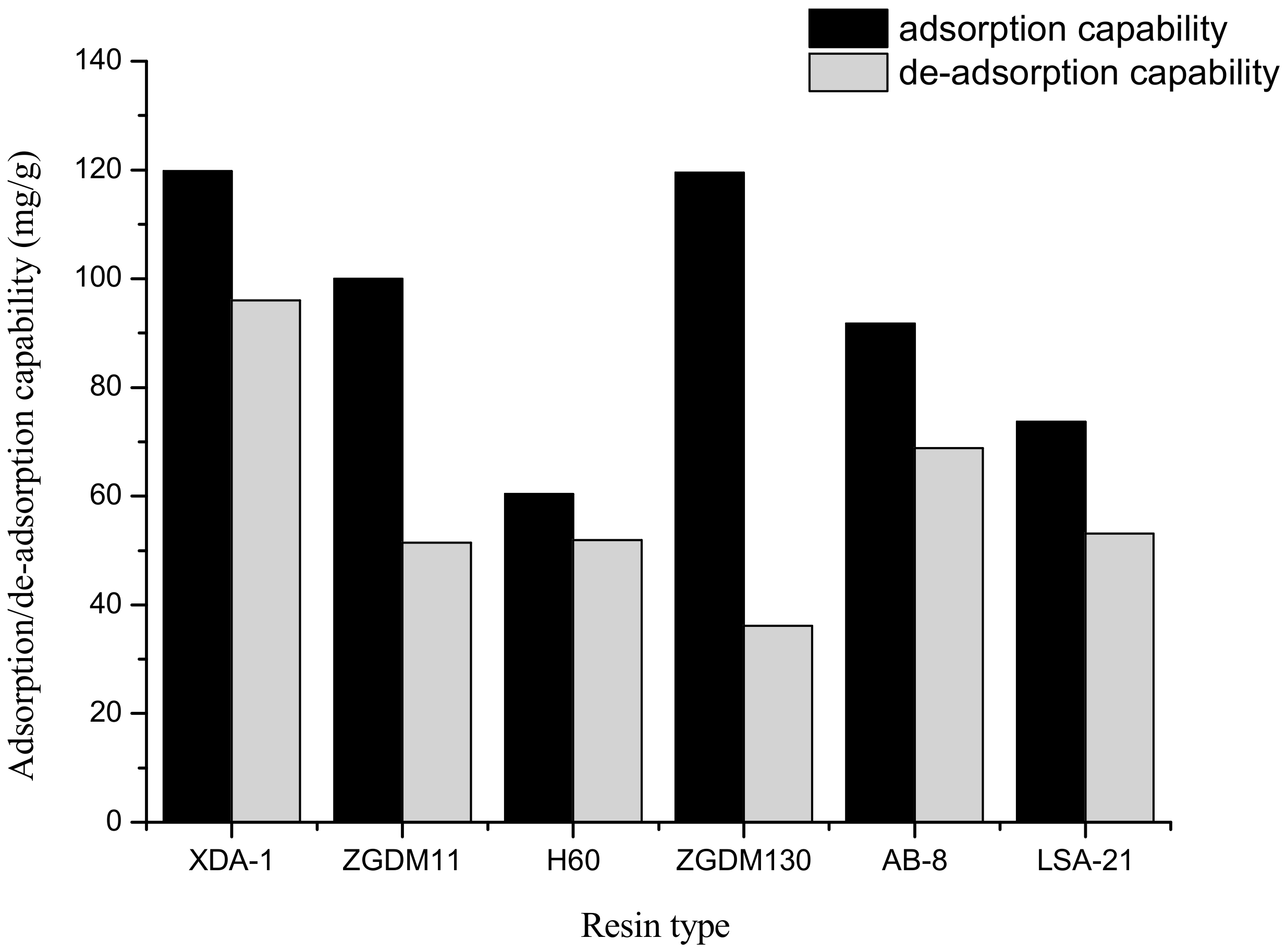
3.1. Selection of Resins Suitable for Adsorption/De-Adsorption of Amygdalin
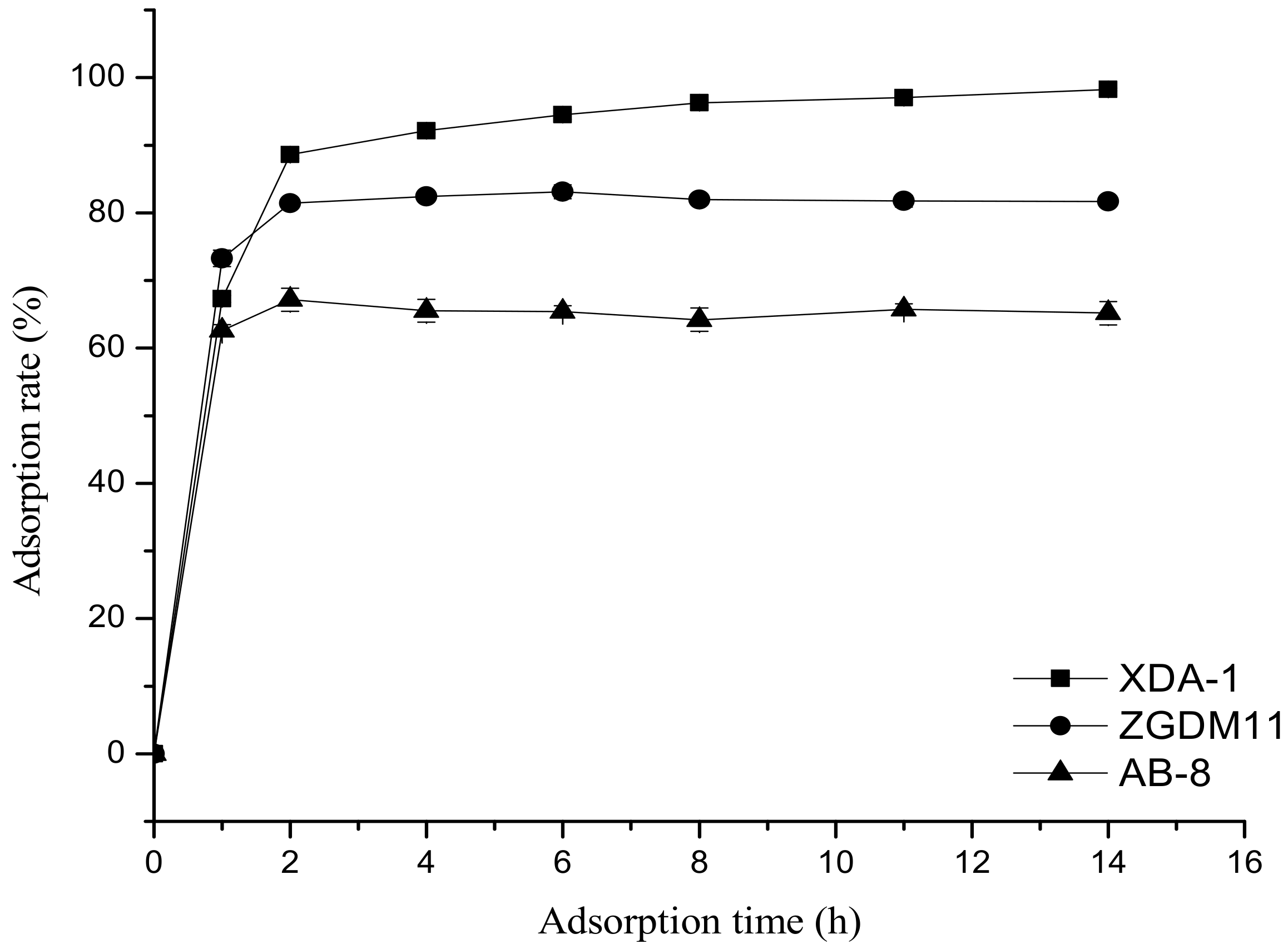
3.2. Comparison of Static Adsorption Kinetics for Three Resins on the Amygdalin
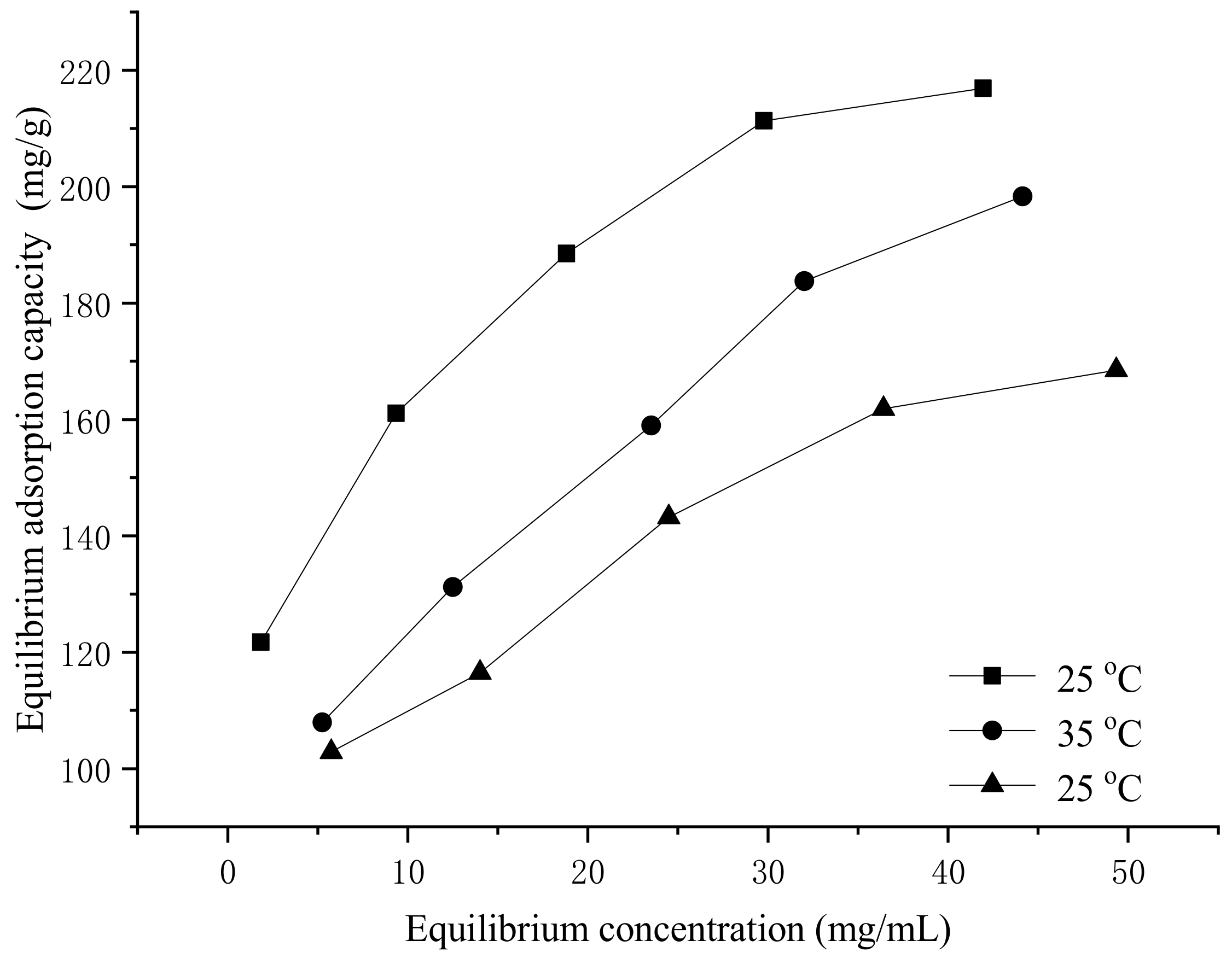
3.3. Static Adsorption Isotherm of XDA-1 Resin on the Amygdalin
3.4. Thermodynamics Adsorption of Amygdalin by the XDA-1 Resin
3.5. Effect of Ethanol Concentration on the De-Adsorption of Amygdalin for the XDA-1 Resin
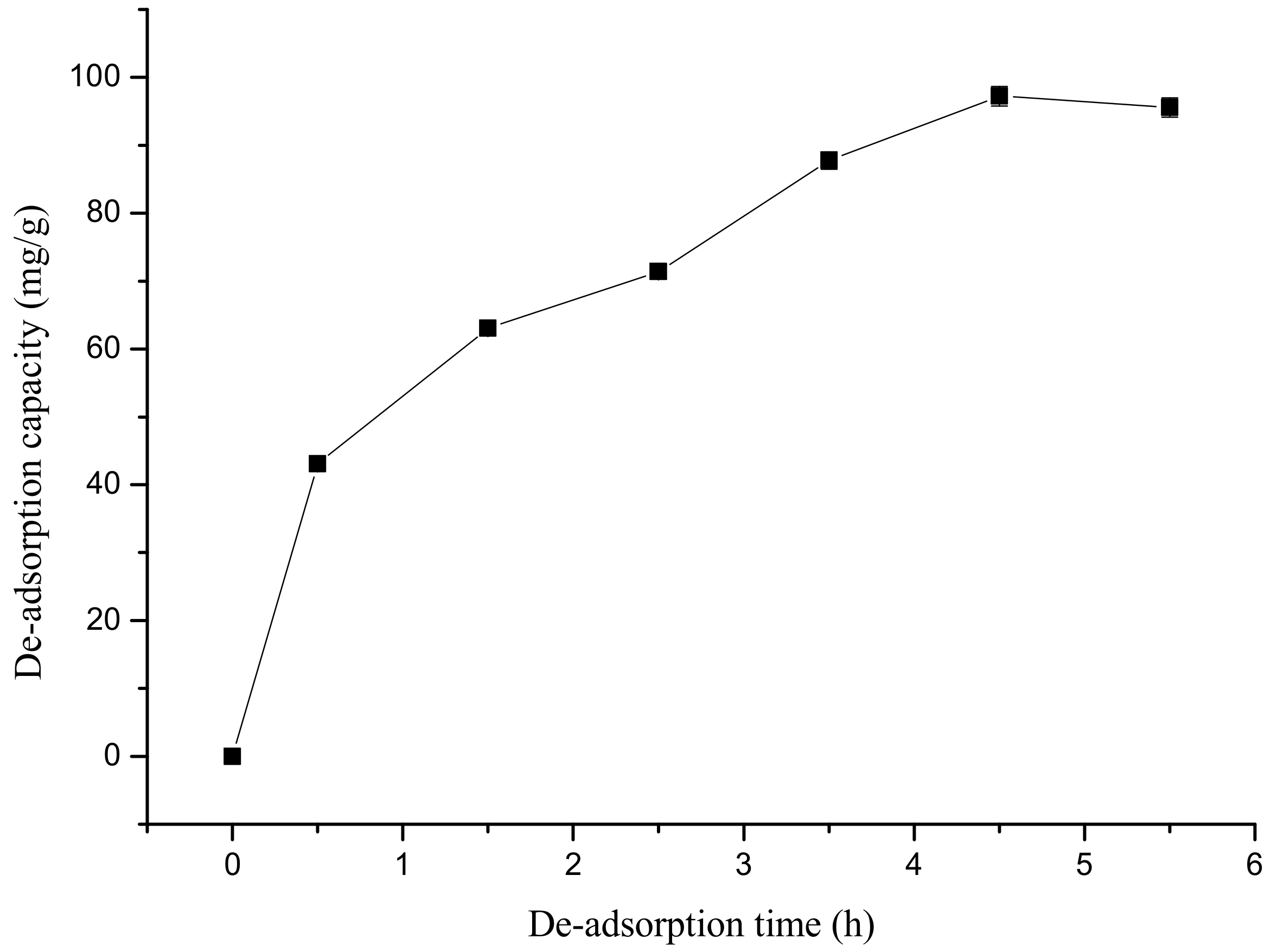
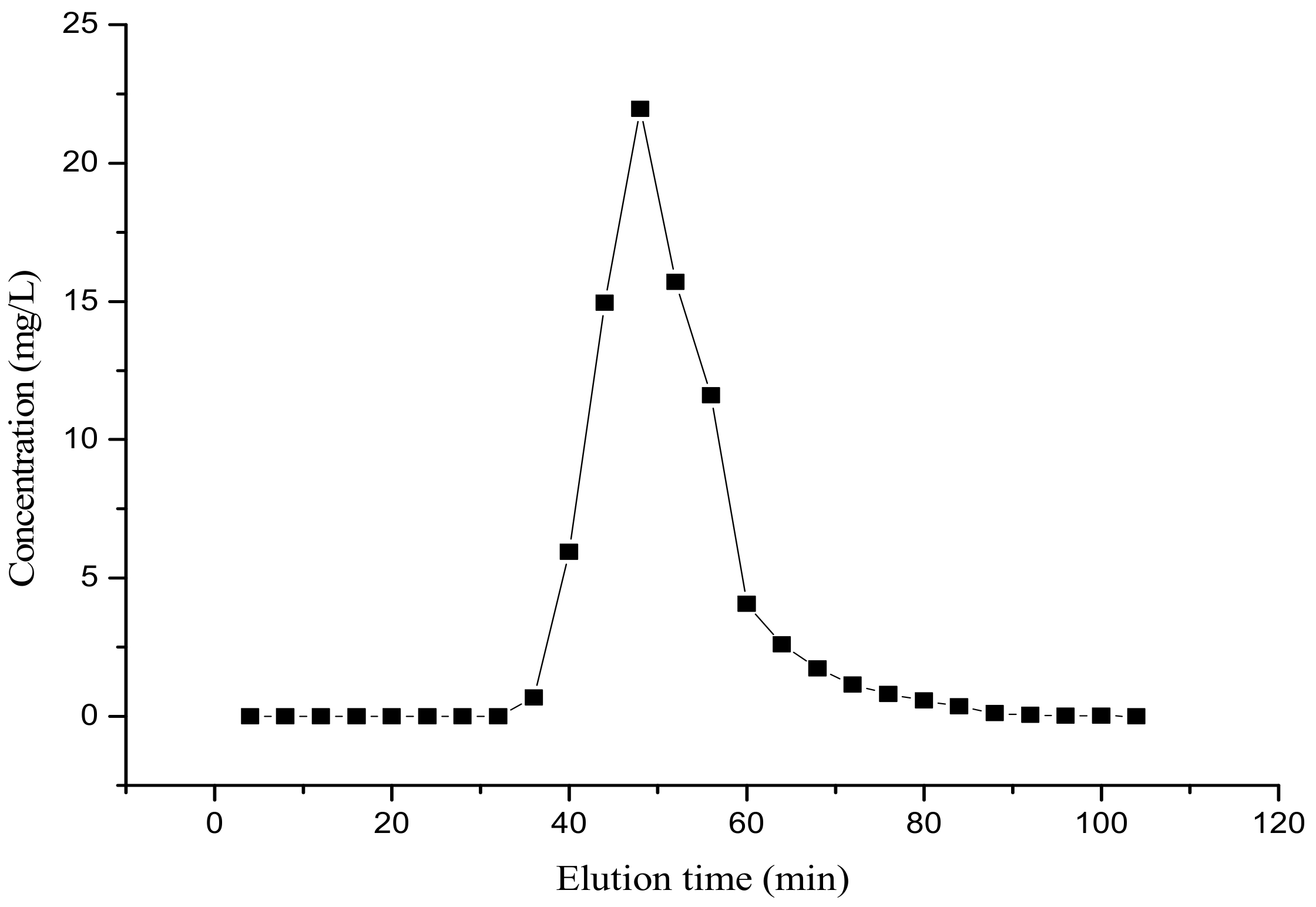
3.6. Static Elution Curve of Amygdalin from the XDA-1 Resin
3.7. Dynamic Adsorption and De-Adsorption Experiments
3.7.1. Effect of Sample Concentration on the Adsorption of Amygdalin for the XDA-1 Resin
3.7.2. Dynamic De-Adsorption Curve of Amygdalin with XDA-1 Resin
3.8. Effect of Purification of XDA-1 Resin on the Amygdalin
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Erdogan-Orhan, I.; Kartal, M. Insights into research on phytochemistry and biological activities of Prunus armeniaca L. (apricot). Food Res. Int. 2011, 44, 1238–1243. [Google Scholar] [CrossRef]
- Górnaś, P.; Siger, A.; Segliņa, D. Physicochemical characteristics of the cold-pressed Japanese quince seed oil: New promising unconventional bio-oil from by-products for the pharmaceutical and cosmetic industry. Ind. Crops Prod. 2013, 48, 178–182. [Google Scholar] [CrossRef]
- Femenia, A.; Rossello, C.; Mulet, A.; Canellas, J. Chemical composition of bitter and sweet apricot kernels. J. Agric. Food Chem. 1995, 43, 356–361. [Google Scholar] [CrossRef]
- Turan, S.; Topcu, A.; Karabulut, I.; Vural, H.; Hayaloglu, A.A. Fatty acid, triacylglycerol, phytosterol, and tocopherol variations in kernel oil of Malatya apricots from Turkey. J. Agric. Food Chem. 2007, 55, 10787–10794. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Gupta, A.; Abrol, G.S.; Joshi, V.K. Value addition of wild apricot fruits grown in North–West Himalayan regions-a review. J. Food Sci. Technol. 2014, 51, 2917–2924. [Google Scholar] [CrossRef] [PubMed]
- Özcan, M. Composition of some apricot (Prunus armeniaca L.) kernels grown in Turkey. Acta Aliment. Hung. 2000, 29, 289–294. [Google Scholar] [CrossRef]
- Blaheta, R.A.; Nelson, K.; Haferkamp, A.; Juengel, E. Amygdalin, quackery or cure? Phytomedicine 2016, 23, 367–376. [Google Scholar] [CrossRef]
- Wang, T.; Lu, S.-M.; Xia, Q.; Fang, Z.-X.; Johnson, S. Separation and purification of amygdalin from thinned bayberry kernels by macroporous adsorption resins. J. Chromatogr. B 2015, 975, 52–58. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.-A.; Song, Y.; Wang, X.; Zhao, W.-Q.; Fan, X.-H. Mathematical modeling of debittered apricot (Prunus armeniaca L.) kernels during thin-layer drying. CyTA J. Food 2016, 14, 509–517. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, Q.-A.; Fan, X.-H.; Zhang, X.-Y. Effect of debitterizing treatment on the quality of the apricot kernels in the industrial processing. J. Food Process. Pres. 2017, 42, 1–8. [Google Scholar] [CrossRef]
- Zhang, Q.-A.; Wei, C.-X.; Fan, X.-H.; Shi, F.-F. Chemical compositions and antioxidant capacity of by-products generated during the apricot kernels processing. CyTA J. Food 2018, 16, 422–428. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.-A.; Fan, X.-H.; Zhang Yang, J.-N.; Zhang, Z.-Q. A Novel Method for Rapid Debitterizing of Apricot Kernels. Chinese Patent ZL 201310376132.X, 2016. [Google Scholar]
- Fan, X.-H.; Zhang, Q.-A.; Liu, M.; Tian, C.-R. Progress in detoxification techniques of apricot kernels. Sci. Technol. Food Ind. 2014, 35, 396–399. (In Chinese) [Google Scholar]
- Jia, G.-T.; Lu, X.-Y. Enrichment and purification of madecassoside and asiaticoside from Centella asiatica extracts with macroporous resins. J. Chromatogr. A 2008, 1193, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Xi, L.-S.; Mu, T.-H.; Sun, H.-N. Preparative purification of polyphenols from sweet potato (Ipomoea batatas L.) leaves by AB-8 macroporous resins. Food Chem. 2015, 172, 166–174. [Google Scholar] [CrossRef]
- Idris, Z.M.; Dzahir, M.I.H.M.; Jamal, P.; Barkat, A.A.; Xian, R.L.W. Purification of bioactive phenolics from Phanerochaete chysosporium biomass extract on selected macroporous resins. IOP Con. Ser. Mater. Sci. Eng. 2017, 206, 012070. [Google Scholar] [CrossRef]
- Liu, P.-W.; Du, Y.-F.; Zhang, X.-W.; Sheng, X.-N.; Shi, X.-W.; Zhao, C.-C.; Zhu, H.; Wang, N.; Wang, Q.; Zhang, L.-T. Rapid analysis of 27 components of Isodon serra by LC-ESI-MS-MS. Chromatographia 2010, 72, 265–273. [Google Scholar] [CrossRef]
- Dong, Y.; Zhao, M.-M.; Sun-Waterhouse, D.-X.; Zhuang, M.-Z.; Chen, H.-P.; Feng, M.-Y.; Lin, L.-Z. Absorption and desorption behaviour of the flavonoids from Glycyrrhiza glabra L. leaf on macroporous adsorption resins. Food Chem. 2015, 168, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Gökmen, V.; Serpen, A. Equilibrium and kinetic studies on the adsorption of dark colored compounds from apple juice using adsorbent resin. J. Food Eng. 2002, 53, 221–227. [Google Scholar] [CrossRef]
- Allen, S.J.; McKay, G.; Porter, J.F. Adsorption isotherm models for basic dye adsorption by peat in single and binary component systems. J. Colloid Int. Sci. 2004, 280, 322–333. [Google Scholar] [CrossRef]
- Sohn, S.; Kim, D. Modification of Langmuir isotherm in solution systems—Definition and utilization of concentration dependent factor. Chemosphere 2005, 58, 115–123. [Google Scholar] [CrossRef]
- Özcan, A.; Özcan, A.S.; Tunali, S.; Akar, T.; Kiran, I. Determination of the equilibrium, kinetic and thermodynamic parameters of adsorption of copper(ii) ions onto seeds of Capsicum annuum. J. Hazard. Mated. 2005, 124, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-Z.; Zhao, H.-F.; Dong, Y.; Yang, B.; Zhao, M.-M. Macroporous resin purification behavior of phenolics and rosmarinic acid from Rabdosia serra (MAXIM.) HARA leaf. Food Chem. 2012, 130, 417–424. [Google Scholar] [CrossRef]
- Han, R.-P.; Zhang, J.-J.; Han, P.; Wang, Y.-F.; Zhao, Z.-H.; Tang, M.-S. Study of equilibrium, kinetic and thermodynamic parameters about methylene blue adsorption onto natural zeolite. Chem. Eng. J. 2009, 145, 496–504. [Google Scholar] [CrossRef]






| Model | Appearance | Polarity | Aperture/nm | Specific Surface Area/m2·g−1 |
|---|---|---|---|---|
| XDA-1 | Brown opaque globular particles | nonpolar | 8.5–9.5 | 1000–1100 |
| ZGDM11 | White translucent globular particles | nonpolar | 12.0–18.0 | ≥800 |
| H60 | White opaque globular particles | low-pole | 10.0–10.5 | 540–580 |
| ZGDM130 | White opaque globular particles | low-pole | 10.0–12.0 | 450–550 |
| AB-8 | White opaque globular particles | low-pole | 13.0–14.0 | 480–520 |
| LSA-21 | White opaque globular particles | semi-pole | 30 | ≥630 |
| T/K | Fitted Equation | lnKf | n | R2 |
|---|---|---|---|---|
| 298 | lnQe = 0.1914lnCe + 4.6769 | 4.677 | 5.225 | 0.9934 |
| 308 | lnQe = 0.1643lnCe + 4.5893 | 4.589 | 6.086 | 0.9753 |
| 318 | lnQe = 0.1804lnCe + 4.3509 | 4.351 | 5.544 | 0.9844 |
| Qe(mg/g) | ∆H (kJ/mol) | ∆S (kJ·mol−1·K−1) | ||
|---|---|---|---|---|
| 298 K | 308 K | 318 K | ||
| 115 | −4.6017 | 0.0361 | 0.0326 | 0.0262 |
| 160 | −5.4799 | 0.0331 | 0.0298 | 0.0235 |
| 180 | −5.6264 | 0.0326 | 0.0293 | 0.0230 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.-A.; Wu, D.-D.; Wei, C.-X. Purification of Amygdalin from the Concentrated Debitterizing-Water of Apricot Kernelsusing XDA-1 Resin. Processes 2019, 7, 359. https://doi.org/10.3390/pr7060359
Zhang Q-A, Wu D-D, Wei C-X. Purification of Amygdalin from the Concentrated Debitterizing-Water of Apricot Kernelsusing XDA-1 Resin. Processes. 2019; 7(6):359. https://doi.org/10.3390/pr7060359
Chicago/Turabian StyleZhang, Qing-An, Dong-Dong Wu, and Chen-Xi Wei. 2019. "Purification of Amygdalin from the Concentrated Debitterizing-Water of Apricot Kernelsusing XDA-1 Resin" Processes 7, no. 6: 359. https://doi.org/10.3390/pr7060359





