Product Concentration, Yield and Productivity in Anaerobic Digestion to Produce Short Chain Organic Acids: A Critical Analysis of Literature Data
Abstract
:1. Introduction
2. Methodology
2.1. Data Collection and Analysis
2.2. Statistical Analysis: Regression Modelling and Pearson’s r Test
3. Results and Discussion
3.1. General Observation and Descriptive Statistics
3.2. Range of Operating Parameters and Performance Variables
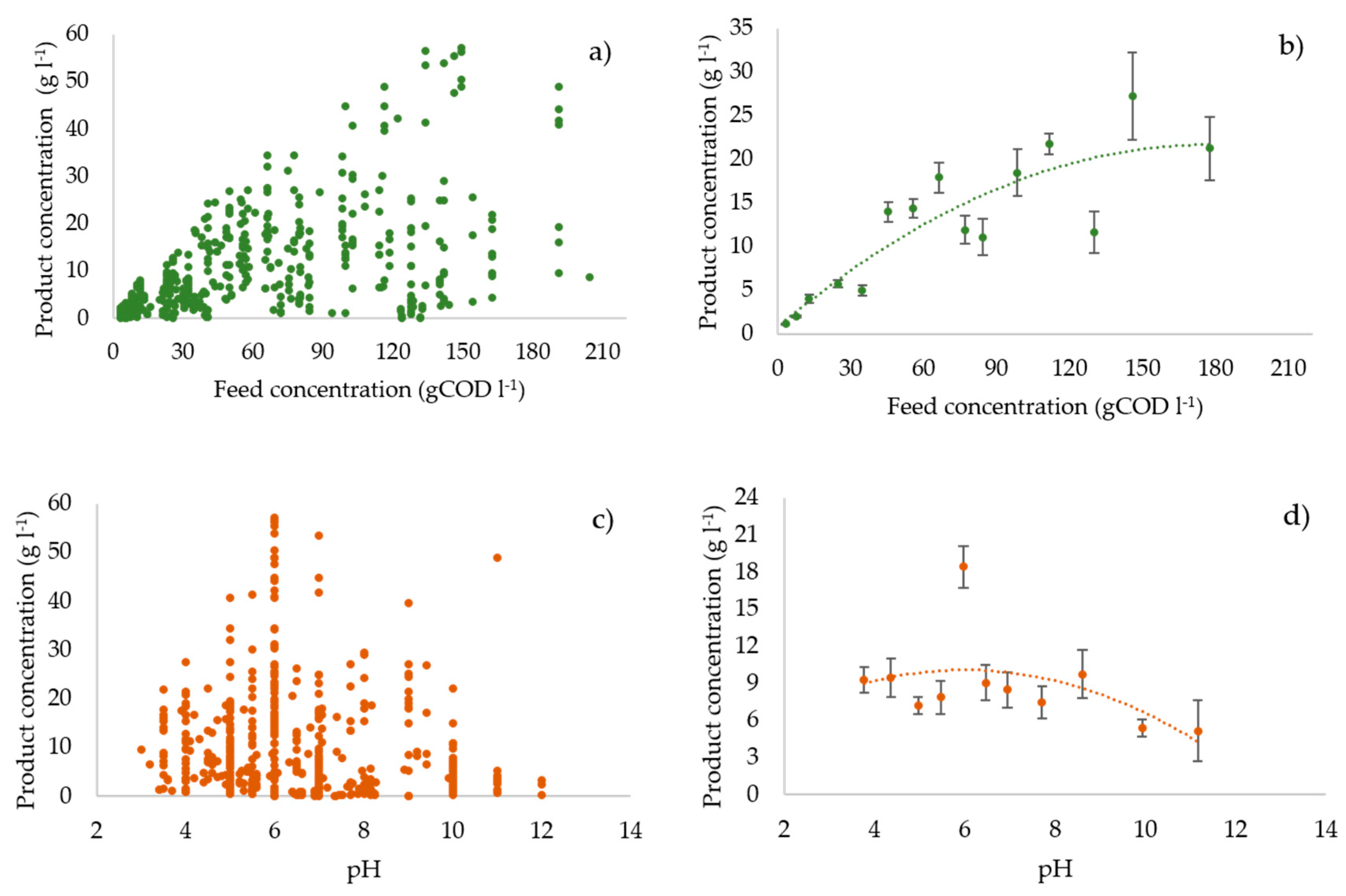
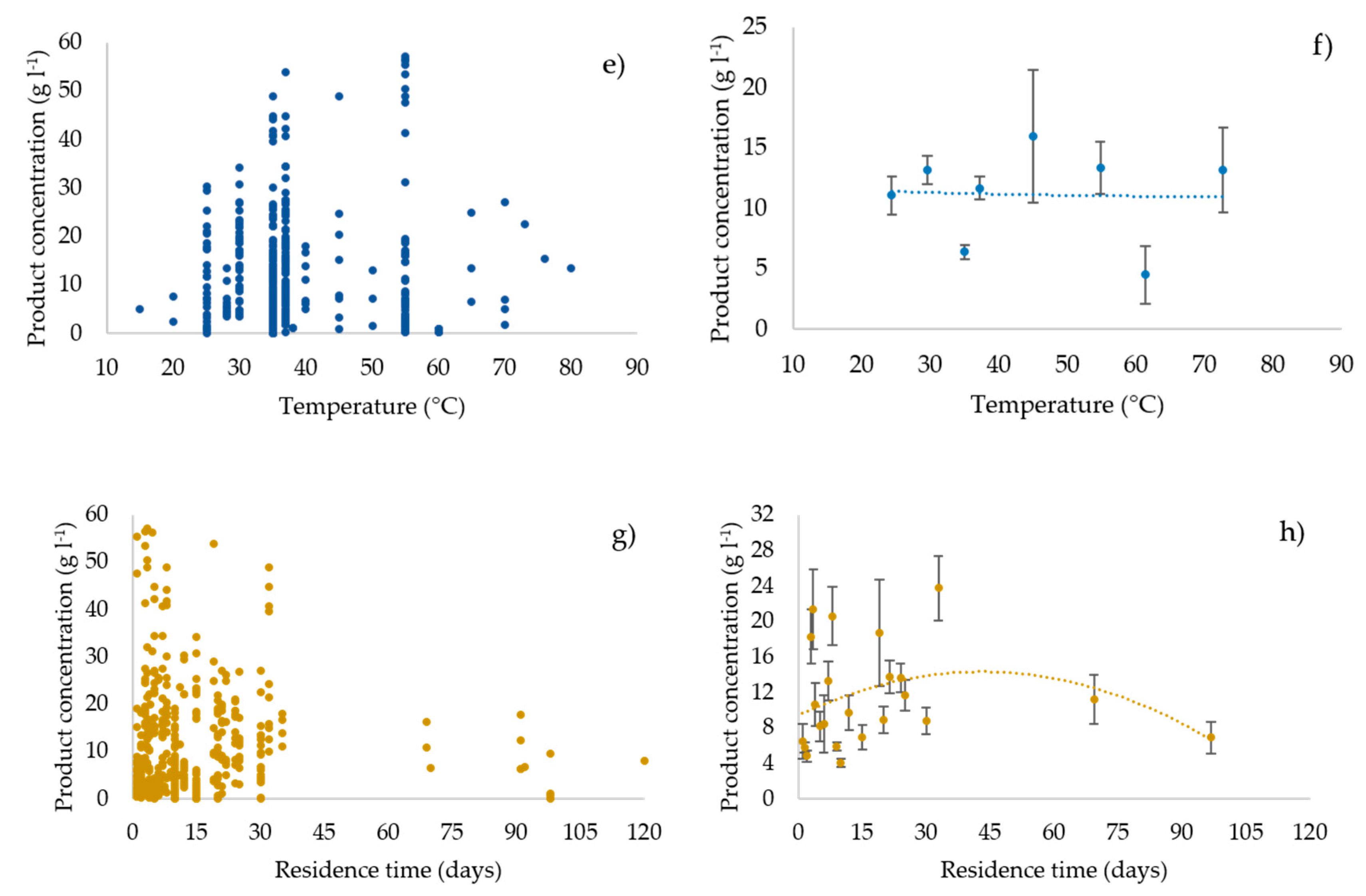
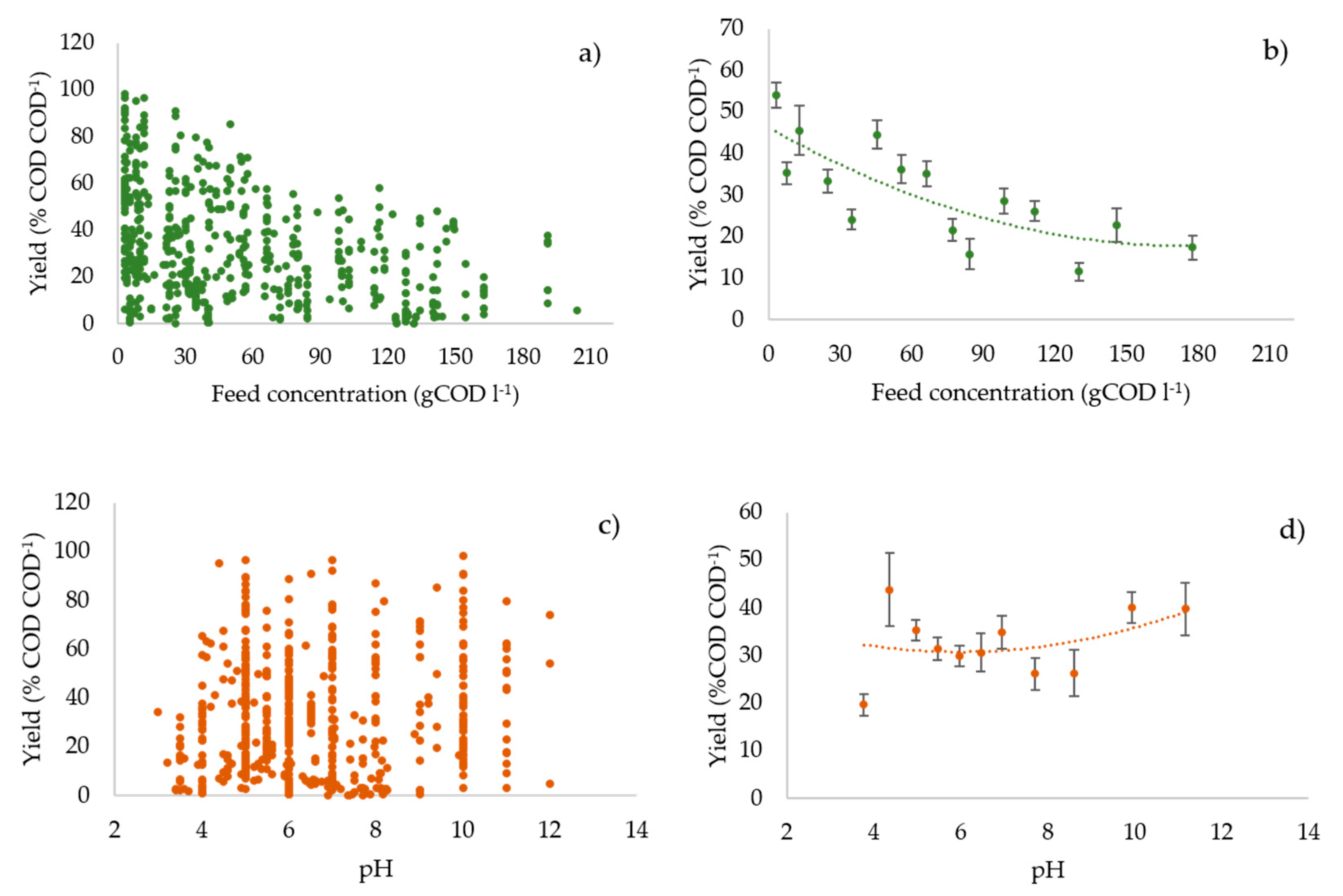
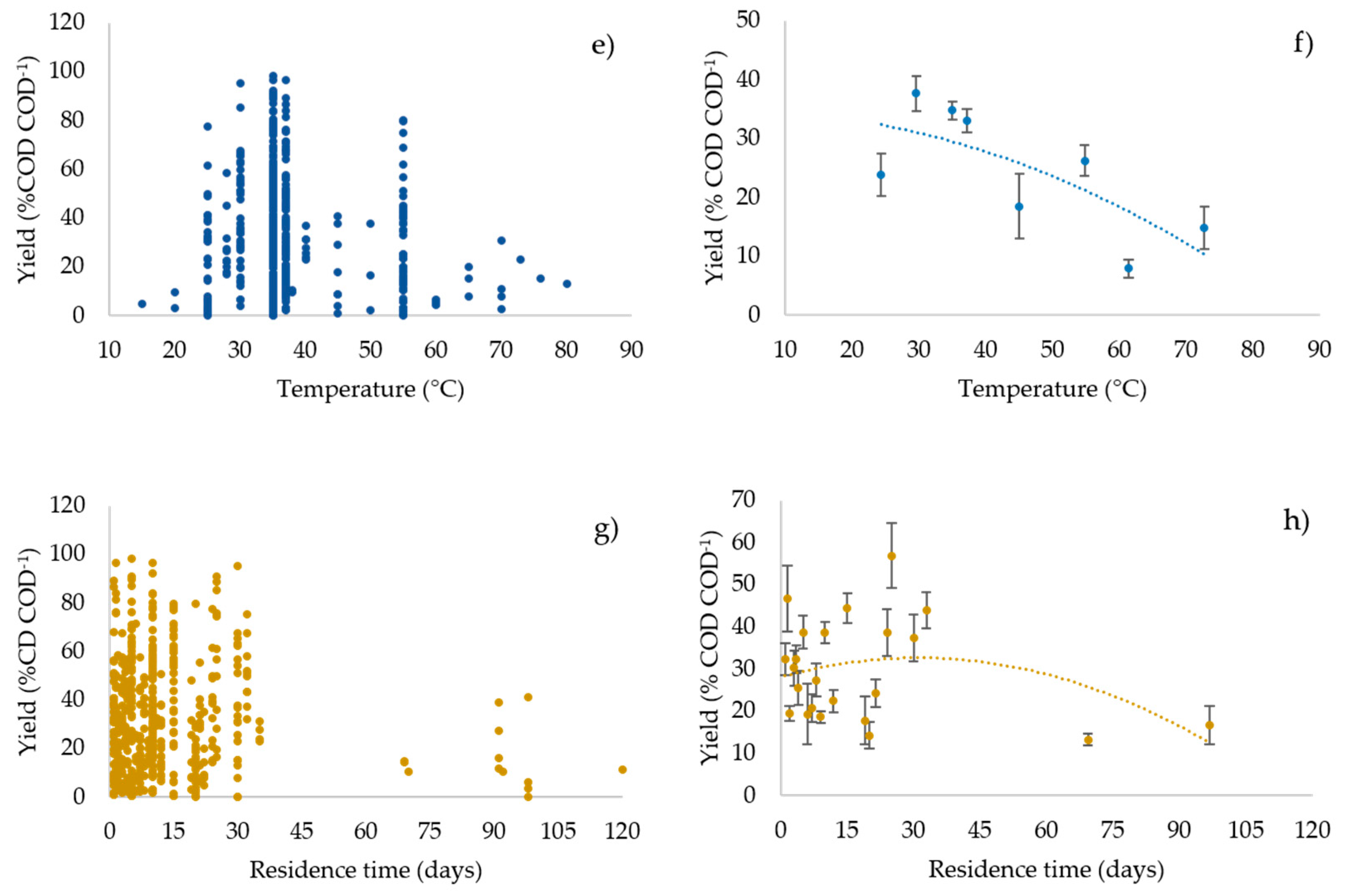
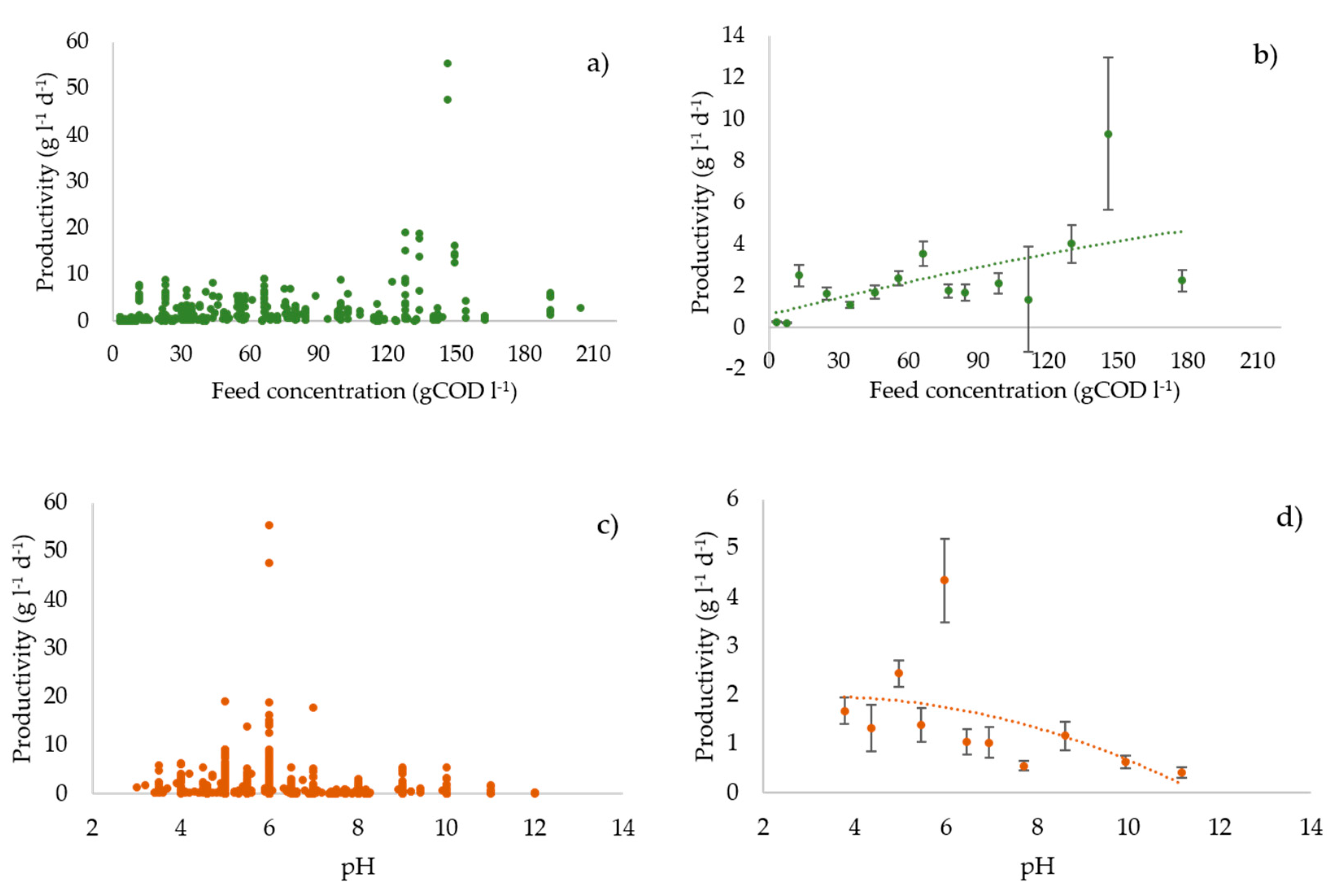
3.3. Profiles of the Performance Variables vs. the Operating Parameters
3.4. Comparison of Operating Modes
3.5. Analysis of Experiments with the Best Performance
3.6. Statistical Analysis: Regression Model and Pearson’s r Test Results
3.7. General Discussion
3.7.1. Comparison with Other Literature Studies
3.7.2. Identification of the Optimum Range of Operating Parameters
3.7.3. Identification of Knowledge Gaps and Recommendations for Further Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kleerebezem, R.; Joosse, B.; Rozendal, R.; Van Loosdrecht, M.C.M. Anaerobic digestion without biogas? Rev. Environ. Sci. Bio Technol. 2015, 14, 787–801. [Google Scholar] [CrossRef] [Green Version]
- Dionisi, D.; Silva, I.M.O. Production of ethanol, organic acids and hydrogen: An opportunity for mixed culture biotechnology? Rev. Environ. Sci. Bio Technol. 2016, 15, 213–242. [Google Scholar] [CrossRef]
- Pal, P.; Nayak, J. Acetic Acid Production and Purification: Critical Review Towards Process Intensification. Sep. Purif. Rev. 2017, 46, 44–61. [Google Scholar] [CrossRef]
- Gonzalez-Garcia, R.A.; McCubbin, T.; Navone, L.; Stowers, C.; Nielsen, L.K.; Marcellin, E. Microbial Propionic Acid Production. Fermentation 2017, 3, 21. [Google Scholar] [CrossRef]
- Komesu, A.; Oliveira, J.A.R.d.; Martins, L.H.d.S.; Wolf Maciel, M.R.; Maciel Filho, R. Lactic acid production to purification: A review. BioResources 2017, 12, 4364–4383. [Google Scholar] [CrossRef]
- Murali, N.; Srinivas, K.; Ahring, B.K. Biochemical Production and Separation of Carboxylic Acids for Biorefinery Applications. Fermentation 2017, 3, 22. [Google Scholar] [CrossRef] [Green Version]
- Alibardi, L.; Cossu, R. Effects of carbohydrate, protein and lipid content of organic waste on hydrogen production and fermentation products. Waste Manag. 2016, 47, 69–77. [Google Scholar] [CrossRef]
- Argelier, S.; Delgenes, J.-P.; Moletta, R. Design of acidogenic reactors for the anaerobic treatment of the organic fraction of solid food waste. Bioprocess. Biosyst. Eng. 1998, 18, 309–315. [Google Scholar] [CrossRef]
- Arras, W.; Hussain, A.; Hausler, R.; Guiot, S.R. Mesophilic, thermophilic and hyperthermophilic acidogenic fermentation of food waste in batch: Effect of inoculum source. Waste Manag. 2019, 87, 279–287. [Google Scholar] [CrossRef]
- Arslan, D.; Steinbusch, K.; Diels, L.; De Wever, H.; Hamelers, H.; Buisman, C. Selective carboxylate production by controlling hydrogen, carbon dioxide and substrate concentrations in mixed culture fermentation. Bioresour. Technol. 2013, 136, 452–460. [Google Scholar] [CrossRef]
- Atasoy, M.; Eyice, O.; Schnürer, A.; Cetecioglu, Z. Volatile fatty acids production via mixed culture fermentation: Revealing the link between pH, inoculum type and bacterial composition. Bioresour. Technol. 2019, 292, 121889. [Google Scholar] [CrossRef] [PubMed]
- Lakeh, A.B.; Azizi, A.; Koupaie, E.H.; Bekmuradov, V.; Hafez, H.; Elbeshbishy, E. A comprehensive study for characteristics, acidogenic fermentation, and anaerobic digestion of source separated organics. J. Clean. Prod. 2019, 228, 73–85. [Google Scholar] [CrossRef]
- Bermúdez-Penabad, N.; Kennes, C.; Veiga, M.C. Anaerobic digestion of tuna waste for the production of volatile fatty acids. Waste Manag. 2017, 68, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Bi, S.; Qiao, W.; Xiong, L.; Ricci, M.; Adani, F.; Dong, R. Effects of organic loading rate on anaerobic digestion of chicken manure under mesophilic and thermophilic conditions. Renew. Energy 2019, 139, 242–250. [Google Scholar] [CrossRef]
- Bolaji, I.O.; Dionisi, D. Acidogenic fermentation of vegetable and salad waste for chemicals production: Effect of pH buffer and retention time. J. Environ. Chem. Eng. 2017, 5, 5933–5943. [Google Scholar] [CrossRef] [Green Version]
- Calicioglu, O.; Shreve, M.J.; Richard, T.L.; Brennan, R.A. Effect of pH and temperature on microbial community structure and carboxylic acid yield during the acidogenic digestion of duckweed. Biotechnol. Biofuels 2018, 11, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Cavinato, C.; Da Ros, C.; Pavan, P.; Bolzonella, D. Influence of temperature and hydraulic retention on the production of volatile fatty acids during anaerobic fermentation of cow manure and maize silage. Bioresour. Technol. 2017, 223, 59–64. [Google Scholar] [CrossRef]
- Cheah, Y.-K.; Vidal-Antich, C.; Dosta, J.; Mata-Álvarez, J. Volatile fatty acid production from mesophilic acidogenic fermentation of organic fraction of municipal solid waste and food waste under acidic and alkaline pH. Environ. Sci. Pollut. Res. 2019, 26, 35509–35522. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Jiang, X.; Xiao, K.; Shen, N.; Zeng, R.J.; Zhou, Y. Enhanced volatile fatty acids (VFAs) production in a thermophilic fermenter with stepwise pH increase e Investigation on dissolved organic matter transformation and microbial community shift. Water Res. 2017, 112, 261–268. [Google Scholar] [CrossRef]
- Dahiya, S.; Sarkar, O.; Swamy, Y.; Mohan, S.V. Acidogenic fermentation of food waste for volatile fatty acid production with co-generation of biohydrogen. Bioresour. Technol. 2015, 182, 103–113. [Google Scholar] [CrossRef]
- Darvekar, P.; Liang, C.; Karim, M.N.; Holtzapple, M. Effect of headspace gas composition on carboxylates production in open-culture fermentation of corn stover. Biomass Bioenergy 2019, 126, 57–61. [Google Scholar] [CrossRef]
- Demirer, G.N.; Othman, M. Two-Phase Thermophilic Acidification and Mesophilic Methanogenesis Anaerobic Digestion of Waste-Activated Sludge. Environ. Eng. Sci. 2008, 25, 1291–1300. [Google Scholar] [CrossRef]
- Dionisi, D.; Bolaji, I. Biorefinery with open mixed cultures for biofuels and chemicals production from organic waste: Biodegradation of unpretreated cellulose. Chem. Eng. Trans. 2016, 49, 157–162. [Google Scholar]
- Feng, K.; Li, H.; Zheng, C. Shifting product spectrum by pH adjustment during long-term continuous anaerobic fermentation of food waste. Bioresour. Technol. 2018, 270, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Gameiro, T.; Lopes, M.; Marinho, R.; Vergine, P.; Nadais, H.; Capela, I. Hydrolytic-Acidogenic Fermentation of Organic Solid Waste for Volatile Fatty Acids Production at Different Solids Concentrations and Alkalinity Addition. Water Air Soil Pollut. 2016, 227, 391. [Google Scholar] [CrossRef]
- Garcia-Aguirre, J.; Aymerich, E.; De Goñi, J.G.-M.; Esteban-Gutiérrez, M. Selective VFA production potential from organic waste streams: Assessing temperature and pH influence. Bioresour. Technol. 2017, 244, 1081–1088. [Google Scholar] [CrossRef]
- González-Fernández, C.; Mendez, L.; Tomás-Pejó, E.; Ballesteros, M. Biogas and Volatile Fatty Acids Production: Temperature as a Determining Factor in the Anaerobic Digestion of Spirulina platensis. Waste Biomass Valori. 2018, 10, 2507–2515. [Google Scholar] [CrossRef]
- Han, G.; Shin, S.G.; Lee, J.; Lee, C.; Jo, M.; Hwang, S. Mesophilic Acidogenesis of Food Waste-Recycling Wastewater: Effects of Hydraulic Retention Time, pH, and Temperature. Appl. Biochem. Biotechnol. 2016, 180, 980–999. [Google Scholar] [CrossRef]
- Herrero Garcia, N.; Strazzera, G.; Frison, N.; Bolzonella, D. Volatile fatty acids production from household food waste. Chem. Eng. Trans. 2018, 64, 103–108. [Google Scholar]
- Iglesias-Iglesias, R.; Campanaro, S.; Treu, L.; Kennes, C.; Veiga, M.C. Valorization of sewage sludge for volatile fatty acids production and role of microbiome on acidogenic fermentation. Bioresour. Technol. 2019, 291, 121817. [Google Scholar] [CrossRef]
- Jankowska, E.; Chwiałkowska, J.; Stodolny, M.; Oleskowicz-Popiel, P. Effect of pH and retention time on volatile fatty acids production during mixed culture fermentation. Bioresour. Technol. 2015, 190, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Jankowska, E.; Chwiałkowska, J.; Stodolny, M.; Oleskowicz-Popiel, P. Volatile fatty acids production during mixed culture fermentation—The impact of substrate complexity and pH. Chem. Eng. J. 2017, 326, 901–910. [Google Scholar] [CrossRef]
- Jankowska, E.; Duber, A.; Chwiałkowska, J.; Stodolny, M.; Oleskowicz-Popiel, P. Conversion of organic waste into volatile fatty acids—The influence of process operating parameters. Chem. Eng. J. 2018, 345, 395–403. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, Y.; Li, K.; Wang, Q.; Gong, C.; Li, M. Volatile fatty acids production from food waste: Effects of pH, temperature, and organic loading rate. Bioresour. Technol. 2013, 143, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, O.P.; Selvam, A.; Wong, J.W. Hydrolysis–acidogenesis of food waste in solid–liquid-separating continuous stirred tank reactor (SLS-CSTR) for volatile organic acid production. Bioresour. Technol. 2016, 200, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Komemoto, K.; Lim, Y.; Nagao, N.; Onoue, Y.; Niwa, C.; Toda, T. Effect of temperature on VFA’s and biogas production in anaerobic solubilization of food waste. Waste Manag. 2009, 29, 2950–2955. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Mohan, S.V. Acidogenic valorization of vegetable waste for short chain carboxylic acids and biohydrogen production: Influence of pretreatment and pH. J. Clean. Prod. 2018, 203, 1055–1066. [Google Scholar] [CrossRef]
- Lee, M.; Hidaka, T.; Tsuno, H. Effect of temperature on performance and microbial diversity in hyperthermophilic digester system fed with kitchen garbage. Bioresour. Technol. 2008, 99, 6852–6860. [Google Scholar] [CrossRef]
- Li, Q.; Li, H.; Wang, G.; Wang, X.C. Effects of loading rate and temperature on anaerobic co-digestion of food waste and waste activated sludge in a high frequency feeding system, looking in particular at stability and efficiency. Bioresour. Technol. 2017, 237, 231–239. [Google Scholar] [CrossRef]
- Li, Y.; Su, D.; Feng, H.; Yan, F.; Liu, H.; Feng, L.; Liu, G. Anaerobic acidogenic fermentation of food waste for mixed-acid production. Energy Sources Part A Recover. Util. Environ. Eff. 2017, 39, 631–635. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Xu, H.; Mu, H.; Hua, D.; Jin, F.; Meng, G. Acidogenic properties of carbohydrate-rich wasted potato and microbial community analysis: Effect of pH. J. Biosci. Bioeng. 2019, 128, 50–55. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y.; Li, Y. Effects of substrate concentration, hydraulic retention time and headspace pressure on acid production of protein by anaerobic fermentation. Bioresour. Technol. 2019, 283, 106–111. [Google Scholar] [CrossRef]
- Lim, S.-J.; Kim, B.J.; Jeong, C.-M.; Choi, J.-D.-R.; Ahn, Y.H.; Chang, H.N. Anaerobic organic acid production of food waste in once-a-day feeding and drawing-off bioreactor. Bioresour. Technol. 2008, 99, 7866–7874. [Google Scholar] [CrossRef]
- Ma, H.; Chen, X.; Liu, H.; Liu, H.; Fu, B. Improved volatile fatty acids anaerobic production from waste activated sludge by pH regulation: Alkaline or neutral pH? Waste Manag. 2016, 48, 397–403. [Google Scholar] [CrossRef]
- Ma, H.; Liu, H.; Zhang, L.; Yang, M.; Fu, B.; Liu, H. Novel insight into the relationship between organic substrate composition and volatile fatty acids distribution in acidogenic co-fermentation. Biotechnol. Biofuels 2017, 10, 137. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Xie, S.; Yu, L.; Zhen, Y.; Zhao, Q.; Frear, C.; Chen, S.; Wang, Z.-W.; Shi, Z. pH shaped kinetic characteristics and microbial community of food waste hydrolysis and acidification. Biochem. Eng. J. 2019, 146, 52–59. [Google Scholar] [CrossRef]
- Moretto, G.; Valentino, F.; Pavan, P.; Majone, M.; Bolzonella, D. Optimization of urban waste fermentation for volatile fatty acids production. Waste Manag. 2019, 92, 21–29. [Google Scholar] [CrossRef]
- Park, Y.-J.; Tsuno, H.; Hidaka, T.; Cheon, J.-H. Evaluation of operational parameters in thermophilic acid fermentation of kitchen waste. J. Mater. Cycles Waste Manag. 2008, 10, 46–52. [Google Scholar] [CrossRef]
- Reddy, M.V.; Hayashi, S.; Choi, D.; Cho, H.; Chang, Y.-C. Short chain and medium chain fatty acids production using food waste under non-augmented and bio-augmented conditions. J. Clean. Prod. 2018, 176, 645–653. [Google Scholar] [CrossRef]
- Shahriari, H.; Warith, M.; Hamoda, M.; Kennedy, K. Evaluation of single vs. staged mesophilic anaerobic digestion of kitchen waste with and without microwave pretreatment. J. Environ. Manag. 2013, 125, 74–84. [Google Scholar] [CrossRef]
- Silva, I.M.O.; Dionisi, D. Anaerobic digestion of wheat grass under mesophilic and thermophilic conditions and different inoculum sources. Chem. Eng. Trans. 2016, 50, 19–24. [Google Scholar]
- Tang, J.; Wang, X.C.; Hu, Y.; Zhang, Y.; Li, Y. Lactic acid fermentation from food waste with indigenous microbiota: Effects of pH, temperature and high OLR. Waste Manag. 2016, 52, 278–285. [Google Scholar] [CrossRef]
- Tang, J.; Wang, X.C.; Hu, Y.; Zhang, Y.; Li, Y. Effect of pH on lactic acid production from acidogenic fermentation of food waste with different types of inocula. Bioresour. Technol. 2017, 224, 544–552. [Google Scholar] [CrossRef]
- Wang, K.; Yin, J.; Shen, D.; Li, N. Anaerobic digestion of food waste for volatile fatty acids (VFAs) production with different types of inoculum: Effect of pH. Bioresour. Technol. 2014, 161, 395–401. [Google Scholar] [CrossRef]
- Wu, Y.; Ma, H.; Zheng, M.; Wang, K. Lactic acid production from acidogenic fermentation of fruit and vegetable wastes. Bioresour. Technol. 2015, 191, 53–58. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, C.; Zheng, M.; Zuo, J.; Wu, J.; Wang, K.; Yang, B. Effect of pH on ethanol-type acidogenic fermentation of fruit and vegetable waste. Waste Manag. 2017, 60, 158–163. [Google Scholar] [CrossRef]
- Yin, J.; Wang, K.; Yang, Y.; Shen, D.; Wang, M.; Mo, H. Improving production of volatile fatty acids from food waste fermentation by hydrothermal pretreatment. Bioresour. Technol. 2014, 171, 323–329. [Google Scholar] [CrossRef]
- Yin, J.; Yu, X.; Zhang, Y.; Shen, D.; Wang, M.; Long, Y.; Chen, T. Enhancement of acidogenic fermentation for volatile fatty acid production from food waste: Effect of redox potential and inoculum. Bioresour. Technol. 2016, 216, 996–1003. [Google Scholar] [CrossRef]
- Yin, J.; Yu, X.; Wang, K.; Shen, D. Acidogenic fermentation of the main substrates of food waste to produce volatile fatty acids. Int. J. Hydrog. Energy 2016, 41, 21713–21720. [Google Scholar] [CrossRef]
- Yu, X.; Yin, J.; Wang, K.; Shen, D.; Long, Y.; Chen, T. Enhancing Food Waste Hydrolysis and the Production Rate of Volatile Fatty Acids by Prefermentation and Hydrothermal Pretreatments. Energy Fuels 2016, 30, 4002–4008. [Google Scholar] [CrossRef]
- Yu, X.; Yin, J.; Shen, D.; Shentu, J.; Long, Y.; Chen, T. Improvement of acidogenic fermentation for volatile fatty acid production from protein-rich substrate in food waste. Waste Manag. 2018, 74, 177–184. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, L.-L.; Zhang, S.-C.; Shi, H.-Z.; Cai, W.-M. The Influence of pH on Hydrolysis and Acidogenesis of Kitchen Wastes in Two-phase Anaerobic Digestion. Environ. Technol. 2005, 26, 329–340. [Google Scholar] [CrossRef]
- Zheng, M.; Zheng, M.; Wu, Y.; Ma, H.; Wang, K. Effect of pH on types of acidogenic fermentation of fruit and vegetable wastes. Biotechnol. Bioprocess. Eng. 2015, 20, 298–303. [Google Scholar] [CrossRef]
- Strazzera, G.; Battista, F.; Garcia, N.H.; Frison, N.; Bolzonella, D. Volatile fatty acids production from food wastes for biorefinery platforms: A review. J. Environ. Manag. 2018, 226, 278–288. [Google Scholar] [CrossRef]
- Zhou, M.; Yan, B.; Wong, J.W.; Zhang, Y. Enhanced volatile fatty acids production from anaerobic fermentation of food waste: A mini-review focusing on acidogenic metabolic pathways. Bioresour. Technol. 2018, 248, 68–78. [Google Scholar] [CrossRef]
- Battista, F.; Frison, N.; Pavan, P.; Cavinato, C.; Gottardo, M.; Fatone, F.; Eusebi, A.L.; Majone, M.; Zeppilli, M.; Valentino, F.; et al. Food wastes and sewage sludge as feedstock for an urban biorefinery producing biofuels and added-value bioproducts. J. Chem. Technol. Biotechnol. 2020, 95, 328–338. [Google Scholar] [CrossRef]
- Dionisi, D.; Bolaji, I.; Nabbanda, D.; Silva, I.M. Calculation of the potential production of methane and chemicals using anaerobic digestion. Biofuels Bioprod. Biorefining 2018, 12, 788–801. [Google Scholar] [CrossRef]
- WRAP. Summary Report—Material Bulk Densities, Report Prepared by Resource Futures; 2009; Available online: http://www.wrap.org.uk/sites/files/wrap/Bulk%20Density%20Summary%20Report%20-%20Jan2010.pdf (accessed on 15 January 2020).
- Komilis, D.; Barrena, R.; Grando, R.L.; Vogiatzi, V.; Sánchez, A.; Font, X. A state of the art literature review on anaerobic digestion of food waste: Influential operating parameters on methane yield. Rev. Environ. Sci. Bio/Technol. 2017, 59, 347–360. [Google Scholar] [CrossRef] [Green Version]
- Bouzas, A.; Gabaldón, C.; Marzal, P.; Penya-Roja, J.; Seco, A. Fermentation of Municipal Primary Sludge: Effect of Srt and Solids Concentration on Volatile Fatty Acid Production. Environ. Technol. 2002, 23, 863–875. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; Liu, X.; Fu, B.; Chen, J.; Yu, H.-Q. Acidogenic fermentation of proteinaceous sewage sludge: Effect of pH. Water Res. 2012, 46, 799–807. [Google Scholar] [CrossRef]
- Eryildiz, B.; Lukitawesa; Taherzadeh, M.J. Effect of pH, substrate loading, oxygen, and methanogens inhibitors on volatile fatty acid (VFA) production from citrus waste by anaerobic digestion. Bioresour. Technol. 2020, 302, 122800. [Google Scholar] [CrossRef]
- Begum, S.; Rao, A.G.; Sridhar, S.; Bhargava, S.K.; Jegatheesan, V.; Eshtiaghi, N. Evaluation of single and two stage anaerobic digestion of landfill leachate: Effect of pH and initial organic loading rate on volatile fatty acid (VFA) and biogas production. Bioresour. Technol. 2018, 251, 364–373. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Q.; Wang, X.; Zhou, X.; Zhu, J. Effect of pH on volatile fatty acid production from anaerobic digestion of potato peel waste. Bioresour. Technol. 2020, 316, 123851. [Google Scholar] [CrossRef]
- Maharaj, I.; Elefsiniotis, P. The role of HRT and low temperature on the acid-phase anaerobic digestion of municipal and industrial wastewaters. Bioresour. Technol. 2001, 76, 191–197. [Google Scholar] [CrossRef]
- Bengtsson, S.; Hallquist, J.; Werker, A.; Welander, T. Acidogenic fermentation of industrial wastewaters: Effects of chemostat retention time and pH on volatile fatty acids production. Biochem. Eng. J. 2008, 40, 492–499. [Google Scholar] [CrossRef]

| Conversion Unit | Conversion Factor | Reference |
|---|---|---|
| VS TS (total solids)−1 food waste | 0.90 | [64,65,66] |
| VS t (total) COD−1 food waste | 0.85 | [64] |
| TS tCOD−1 food waste | 0.90 | [64] |
| gCOD gVS−1 carbohydrates | 1.13 | [67] |
| gCOD gVS−1 proteins | 1.12 | [67] |
| gCOD gVS−1 lipids | 2.90 | [67] |
| Food waste bulk density kg m−3 | 500.00 | [68] |
| tCOD VSS (volatile suspended solids)−1 vegetable waste | 2.49 | [15] |
| % COD TS−1 food waste | 14.01 | [64] |
| VSS tCOD−1 food waste | 0.39 | [64] |
| Substrate Category | Frequency | Percentage % |
|---|---|---|
| Food waste (organic fraction of municipal solid waste, food waste, food waste recycling wastewater, artificial kitchen garbage, microalgae biomass, kitchen waste) | 251 | 45.6 |
| Carbohydrate-rich substrate (potato processing waste stream, vegetable and salad waste, duckweed, crystalline cellulose, paper mill wastewater, winery wastewater, maize silage, vegetable waste, potato waste, wheatgrass powder, fruit and vegetable waste, potato peel waste, simulated vegetable food waste) | 66 | 12.0 |
| Protein-rich substrate (tuna waste, slaughterhouse wastewater, meat and bone meal, Spirulina platensis, cheese whey, whey protein, egg white) | 86 | 15.6 |
| Sludge (primary sludge, secondary sludge, waste-activated sludge, sewage sludge) | 70 | 12.7 |
| Pure substrate (glucose, crude glycerol, peptone) | 63 | 11.5 |
| Manure (chicken manure, cow manure) | 14 | 2.5 |
| Parameter | Min. | Max. | Mean | 90th Percentile |
|---|---|---|---|---|
| Operating parameters | ||||
| Feed concentration (gCOD l−1) | 3 | 204 | 53.08 (2.02) | 127.70 |
| Run length (day) | 1 | 120 | 16.16 (0.94) | 30.00 |
| HRT (d) | 1 | 30 | 6.33 (0.45) | 20.00 |
| SRT (d) | 1 | 50 | 6.89 (0.54) | 20.00 |
| T (°C) | 15 | 80 | 37.81 (0.39) | 55.00 |
| pH | 3 | 12 | 6.50 (0.08) | 10.00 |
| Process performance variables | ||||
| Product concentration (g l−1) | 0.01 | 57.13 | 9.55 (10.92) | 23.14 |
| Yield (% COD COD−1) | 0.01 | 98.43 | 32.06 (23.17) | 63.98 |
| Productivity (g l−1 day−1) | 0.00 | 55.48 | 1.87 (4.04) | 5.05 |
| Operational Mode | Batch | CSTR | SBR |
|---|---|---|---|
| Frequency of experiments (%) | 63 | 34 | 3 |
| Operating parameters | |||
| Feed concentration (gCOD l−1) | 48.48 (2.65) | 58.74 (3.06) | 86.22 (13.04) |
| Run length (day) | 16.16 (0.94) | ||
| HRT (d) | 6.42 (0.47) | 5.25 (1.16) | |
| SRT (d) | 6.42 (0.47) | 12.44 (3.96) | |
| T (°C) | 37.14 (0.49) | 38.36 (0.59) | 46 (3.07) |
| pH | 6.77 (0.11) | 6.00 (0.11) | 6.31 (0.31) |
| Process performance variables | |||
| Product concentration (g l−1) | 9.42 (0.59) | 8.16 (0.60) | 28.79 (5.01) |
| Product yield (% COD COD−1) | 35.41 (1.28) | 25.23 (1.58) | 41.35 (3.55) |
| Productivity (g l−1 day−1) | 1.04 (0.11) | 2.50 (0.20) | 12.12 (4.07) |
| Operating Parameter | Highest Product Concentrations | Highest Product Yields | Highest Productivities |
|---|---|---|---|
| Feed concentration (gCOD l−1) | 108.73 (5.43) | 19.28 (2.42) | 85.92 (7.46) |
| Run length (day) | 14.58 (1.78) | 14.68 (1.48) | 4.62 (0.53) |
| HRT (d) | 5.66 (0.93) | 6.15 (1.54) | 2.46 (0.23) |
| SRT (d) | 6.56 (0.92) | 10.27 (3.86) | 3.02 (0.41) |
| T (°C) | 39.75 (1.37) | 36.31 (0.78) | 40.04 (1.13) |
| pH | 6.48 (0.18) | 7.11 (0.34) | 5.64 (0.14) |
| Product Concentration (g l−1) | Product Yield% (COD COD−1) | Productivity (g l−1 d−1) | ||||
|---|---|---|---|---|---|---|
| Coefficient | p-Value | Coefficient | p-Value | Coefficient | p-Value | |
| Intercept | 3.385 | 0.1188 | 52.768 | 1.14 × 10−22 | 0.321 | 0.71996 |
| Feed concentration (gCOD l−1) | 0.136 | 3 × 10−47 | −0.155 | 1.11 × 10−13 | 0.024 | 1.46 × 10−11 |
| Mode (Batch: 0; CTSR: 1) | −2.600 | 0.0032 | −8.899 | 2.35 × 10−5 | 0.668 | 0.066237 |
| Temperature (°C) | −0.004 | 0.9314 | −0.259 | 0.011862 | 0.042 | 0.018678 |
| pH | 0.061 | 0.7679 | 0.301 | 0.539704 | −0.129 | 0.131204 |
| HRT (day) | −0.039 | 0.1497 | −0.107 | 0.100317 | −0.060 | 1.27 × 10−7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simonetti, S.; Saptoro, A.; Fernández Martín, C.; Dionisi, D. Product Concentration, Yield and Productivity in Anaerobic Digestion to Produce Short Chain Organic Acids: A Critical Analysis of Literature Data. Processes 2020, 8, 1538. https://doi.org/10.3390/pr8121538
Simonetti S, Saptoro A, Fernández Martín C, Dionisi D. Product Concentration, Yield and Productivity in Anaerobic Digestion to Produce Short Chain Organic Acids: A Critical Analysis of Literature Data. Processes. 2020; 8(12):1538. https://doi.org/10.3390/pr8121538
Chicago/Turabian StyleSimonetti, Serena, Agus Saptoro, Claudia Fernández Martín, and Davide Dionisi. 2020. "Product Concentration, Yield and Productivity in Anaerobic Digestion to Produce Short Chain Organic Acids: A Critical Analysis of Literature Data" Processes 8, no. 12: 1538. https://doi.org/10.3390/pr8121538







